Abstract
We previously reported on the emergence of macrolide-resistant pharyngeal isolates of group A streptococci (GAS) in our community. The purpose of the present study was to track longitudinal trends in macrolide resistance in these isolates in southwestern Pennsylvania. Testing for susceptibility to erythromycin and clindamycin was performed for all pharyngeal GAS isolates recovered at the Children's Hospital of Pittsburgh and a local pediatric practice between September 2001 and May 2002. Macrolide resistance phenotypes and genotypes were determined by double-disk diffusion and PCR, respectively. Strain relatedness was determined by field inversion gel electrophoresis and emm gene sequence typing. A total of 708 isolates of GAS were recovered during the study period; 68 (9.6%) were macrolide resistant, while all isolates were sensitive to clindamycin. The monthly prevalence of macrolide resistance ranged from 0 to 41%. Only 21 of 573 (3.7%) strains recovered from September 2001 through March 2002 were macrolide resistant. A sudden increase in the rate of macrolide resistance (47 of 135 isolates [35%]) was seen in April and May 2002. Sixty-two isolates demonstrated the M phenotype (resistance to macrolide antibiotics), and six isolates demonstrated the MLSB phenotype (resistance to most macrolide, lincosamide, and streptogramin B antibiotics); these isolates were confirmed to be mef(A) and erm(A), respectively. Three unique mef(A) clones and four unique erm(A) clones were identified among the resistant isolates. The MIC at which 50% of isolates are inhibited (MIC50) for the mef(A) strains was 16 μg/ml, while the MIC50 for erm(A) strains was 8 μg/ml. The finding of high levels of macrolide resistance among pharyngeal isolates of GAS for a second successive year in our community raises the concern that this problem may be more common in the United States than was previously appreciated. Longitudinal surveillance of isolates from multiple centers is needed to define the prevalence of antimicrobial agent-resistant GAS in the United States.
Sore throat is the most common complaint for which older children and adolescents seek acute medical care (9). Group A streptococci (GAS) are the most frequent and important cause of bacterial pharyngitis in children and adults (7). Penicillin V remains the treatment of choice for GAS pharyngitis (2, 8), and macrolides are recommended only for patients who are allergic to penicillin. Nonetheless, azithromycin is increasingly prescribed because its short course of dosing and single daily dosing are attractive to both parents and practitioners.
A high prevalence of macrolide resistance among GAS has been recognized in Europe and Japan for many years. However, the rates of macrolide resistance among GAS in the United States have remained low (4, 12, 16, 19, 20). In the spring of 2001 we documented the emergence of a high level of macrolide resistance in pharyngeal isolates of GAS in Pittsburgh, Pa., as part of a longitudinal surveillance study of schoolchildren (22). A 38% incidence of macrolide resistance was also found in community isolates of GAS during the same time period. The purpose of this study was to investigate trends in macrolide resistance in pharyngeal GAS over time in our region.
MATERIALS AND METHODS
GAS isolates.
Beginning in September 2001, testing for susceptibility to erythromycin and clindamycin was performed for all pharyngeal GAS isolates recovered from the clinical microbiology laboratory of the Children's Hospital of Pittsburgh. These isolates were obtained from children presenting to the hospital's Emergency Department, Acute Concerns Clinic, or Primary Care Center. No clinical data were available for any of these GAS isolates. However, throat swabs for culture would have been obtained in response to clinical symptoms suggestive of an acute GAS infection. In addition, a smaller number of pharyngeal GAS isolates obtained as part of clinical care at a local pediatric practice underwent susceptibility testing during the same time period. Approval for the use of the pharyngeal GAS isolates for this study was granted by the Human Rights Committee of the Children's Hospital of Pittsburgh.
In vitro susceptibility testing.
Pharyngeal GAS isolates were initially screened for their susceptibilities to erythromycin and clindamycin by using Kirby-Bauer disks (BBL Becton Dickinson, Sparks, Md.) on Mueller-Hinton agar (24). The MICs for isolates that were either intermediate in susceptibility or resistant to erythromycin or clindamycin were then determined by the E-test (AB Biodisk, Piscatawny, N.J.). Isolates of GAS were classified as susceptible, intermediate in susceptibility, or resistant to erythromycin when the MICs were ≤0.25, 0.5, and ≥1.0 μg/ml, respectively. Isolates of GAS were classified as susceptible, intermediate in susceptibility, or resistant to clindamycin when the MICs were ≤0.25, 0.5, and ≥1.0 μg/ml, respectively. The resistant GAS isolates were stored at −70°C for later use.
Molecular relatedness of erythromycin-resistant strains.
The genetic relatedness of erythromycin-resistant strains of the pharyngeal GAS isolates was investigated by field inversion gel electrophoresis (FIGE) after digestion of bacterial DNA prepared in agarose plugs with ApaI by previously described methods (23). Comparison of banding patterns was performed by visual inspection, and interpretation of clonal relatedness was based on guidelines proposed by Tenover et al. (30). A representative isolate of each FIGE type was sent to the Centers for Disease Control and Prevention (CDC) for emm typing (6).
emm and T typing.
emm and T typing were performed at the Streptococcal Laboratory of CDC as described previously (6). Subtypes were assigned on the basis of the sequences of the first 50 codons encoding the mature M protein, as described previously (21).
Phenotypic detection of resistance mechanisms.
The double-disk diffusion test (29) was performed to identify the mechanism of resistance. Mueller-Hinton agar plates supplemented with 5% sheep blood were inoculated with a suspension of GAS that met a McFarland 0.5 turbidity standard. An erythromycin disk (concentration, 15 μg/ml) and a clindamycin disk (concentration, 2 μg/ml) were placed 16 mm apart (edge to edge) on each plate. Resistance to erythromycin associated with susceptibility to clindamycin with no blunting of the zone of inhibition around the clindamycin disk indicates an M phenotype (resistance to macrolide antibiotics). Resistance to erythromycin with blunting of the zone of inhibition around the clindamycin disk on the side of the erythromycin disk indicates an inducible MLSB phenotype (resistance to most macrolide, lincosamide, and streptogramin B antibiotics); resistance to both erythromycin and clindamycin indicates a constitutive MLSB phenotype. The M phenotype is associated with the mef(A) gene (11), and the MLSB phenotypes are associated with one of several erm genes (28).
PCR-based detection of the resistance genes.
A PCR assay was used to identify the genetic mechanism of resistance of one or two representative isolates of each clone of the macrolide-resistant pharyngeal GAS isolates identified by FIGE. The mef(A), erm(B), and erm(A) resistance genes were detected by PCR amplification with primers described previously (22). The expected sizes of the PCR products were 616, 348, and 206 bp for erm(B), mef(A), and erm(A), respectively.
RESULTS
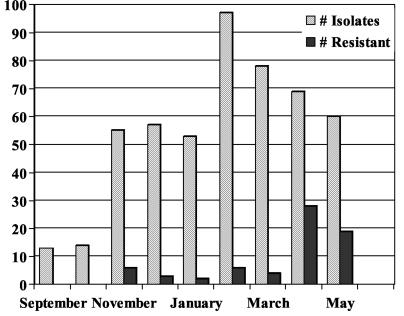
A total of 708 isolates of GAS were recovered during the study period. Sixty-eight (9.6%) were macrolide resistant; all isolates were sensitive to clindamycin. The monthly prevalence of macrolide resistance during this time period ranged from 0 to 41% (Fig. 1). The overall rate of macrolide resistance as well as the monthly prevalence of macrolide resistance among GAS isolates was similar for the isolates recovered from the hospital microbiology laboratory and the pediatric practice. Accordingly, these results were combined for the analysis. Of interest, only 21 of 573 (3.7%) strains recovered between September 2001 and March 2002 were macrolide resistant; all were sensitive to clindamycin. Fifteen isolates demonstrated the M phenotype, while six demonstrated the inducible MLSB phenotype by double-disk diffusion. The overall MIC at which 50% of isolates are inhibited (MIC50) for resistant strains was 12 μg/ml (range, 2 to 24 μg/ml). The MIC50 for strains of the M phenotype was 16 μg/ml (range, 8 to 24 μg/ml), while the MIC50 for isolates with the inducible MLSB phenotype was 8 μg/ml (range, 2 to 12 μg/ml).
FIG. 1.
Recovery of pharyngeal isolates of GAS by month during 2001 and 2002.
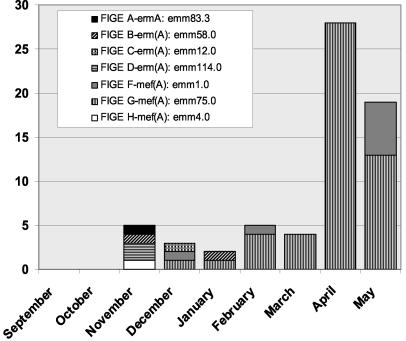
Three unique clones displaying the M phenotype (including one clone accounting for 10 of 13 strains tested) and four unique clones with the inducible MLSB phenotype (accounting for all 6 strains with the MLSB phenotype) were identified among 19 resistant isolates analyzed by FIGE and emm typing from the initial time period. Two of the resistant isolates were not available for testing. The results of emm and T-type testing for each of the clones initially detected by FIGE are shown in Table 1. Results of PCR testing identified mef(A) and erm(A) in the 17 clones with the M phenotype available for testing and 4 clones with the inducible MLSB phenotype clones, respectively. erm(B) was not identified in any macrolide-resistant isolate in this series.
TABLE 1.
Results of molecular typing and resistance genotyping of macrolide-resistant pharyngeal isolates of GAS from Pittsburgh, 2001 and 2002a
| FIGE pattern | emm type | T type | Resistance genotype | No. of isolates |
|---|---|---|---|---|
| A | emm83.3 | 3/13/B3264 | erm(A) | 1 |
| B | emm58.0 | 2/25 | erm(A) | 2 |
| C | emm12.0 | 12 | erm(A) | 1 |
| D | emm114.0 | Nontypeable | erm(A) | 2 |
| F | emm1.0 | 1 | mef(A) | 8 |
| G | emm75.0 | 25 | mef(A) | 50 |
| H | emm4.0 | 4 | mef(A) | 1 |
Two strains were not available for performance of molecular typing.
A marked increase in the prevalence of macrolide resistance in pharyngeal GAS isolates was observed in April and May 2002 (Fig. 1). The increased prevalence of resistance was observed for isolates from both the hospital microbiology laboratory and the pediatric practice. Forty-seven of 135 (35%) isolates recovered during these 2 months were macrolide resistant. The MIC50 for these isolates was 12 μg/ml (range, 4 to 24 μg/ml). All of the resistant isolates recovered during the later time period demonstrated the M phenotype. The prevalences in April and May were 41 and 28%, respectively. The results of FIGE and emm typing for the 47 macrolide-resistant isolates recovered during the later time period identified two clones, both of which had been observed earlier in the study. The dominant type emm75.0 clone accounted for 41 of 47 (87%) of the macrolide-resistant isolates of GAS recovered during this time period. The second type emm1.0 clone accounted for the remaining six (13%) resistant isolates. The emm gene sequences of both resistant clones were identical to those obtained from the CDC prototype strains of these two emm types. The recovery of macrolide-resistant pharyngeal GAS isolates by FIGE pattern, emm type, and resistance phenotype during the entire study is shown in Fig. 2.
FIG. 2.
Recovery of macrolide-resistant pharyngeal isolates of GAS by month and FIGE group.
DISCUSSION
During the first 7 months of this investigation, a low level of macrolide resistance (mean, 3.7%; range 0 to 10.9%) consistent with those from previous reports from the United States (3, 11, 15, 18, 19) was observed. However, during April and May 2002, the rate of macrolide resistance climbed to 35%, a level similar to our findings in the spring of 2002 (22). The overall rate of macrolide resistance among GAS was 9.6% for the entire 2001-2002 GAS infection season. Previous studies that have identified low prevalence rates of macrolide resistance in the United States may have underestimated the rate of resistance since surveillance was not conducted longitudinally. The rates may also have been underestimated because previous reports combined results from multiple surveillance sites; thus, they may have failed to account for areas of regional variation where the prevalence of macrolide resistance might be markedly higher than the national average. Such regional variations in the prevalence of resistance within individual countries (1, 13) and even between counties within a given region of a state within the United States (33) have been reported previously. Such variation may be a prodrome to more general increases within a country, as was seen in Italy in the 1990s (13). However, data from a 1999 national surveillance study carried out within the United States found that the prevalence of macrolide resistance in GAS varied only from 3.0 to 7.7% (15). The presence of episodic increases and a geographic focus of higher rates of resistance in two successive years in our region warrant caution in the use of macrolides for the treatment of GAS pharyngitis in the United States. While it is possible that these higher rates of resistance were due to an epidemic clone in a single region, the fact that the dominant emm type accounting for the resistance in our region differed (emm6 versus emm75) in each of the 2 years adds to the concern that this may not be a self-limited local phenomenon. Longitudinal data from multiple centers are needed to fully describe the contributions made by temporal and geographic variations on the true prevalence and impact of macrolide resistance in the United States.
Two major mechanisms account for nearly all macrolide resistance in GAS: target site modification and active efflux of erythromycin. The first mechanism, target site modification, occurs through methylation of the bacterial ribosome (28). The erm(B) or erm(A) gene codes for related methylase enzymes, which account for both the constitutive and the inducible MLSB resistance phenotypes in GAS. The second major mechanism of macrolide resistance in GAS is the presence of a macrolide efflux pump, which is encoded by the mef(A) gene (28). Expression of this protein results in the M phenotype, isolates of which demonstrate resistance to 14- and 15-member macrolides but which are always susceptible to clindamycin. Regional variations in the relative prevalence of the two resistance phenotypes have been observed. Recent data from Taiwan, Italy, Spain, and Chile suggest that macrolide-resistant GAS expressing the M phenotype now account for the majority of resistant isolates observed in those countries (1, 5, 25, 32). This is similar to the results from our study, in which greater than 90% of the macrolide-resistant isolates recovered from Pittsburgh in 2001-2002 expressed the M phenotype. Nearly 85% of our M-phenotype isolates were accounted for by a single emm75 clone; an emm1 clone accounted for 14% of the remaining M-phenotype strains. Of interest, we did not identify any isolate of the emm6 macrolide-resistant clone which was observed in a longitudinal study of schoolchildren in Pittsburgh in 2001 (22) and 2002.
The rate of occurrence of multiple clones of M-phenotype GAS circulating simultaneously within a given geographic region is similar to data from most other centers (14, 32). This might be explained by the transfer of the mef(A) gene between bacterial strains on a conjugative transposon (18, 26). Thus, the increasing prevalence of macrolide-resistant GAS expressing the M phenotype may be due to both the dissemination of resistant clones and the spread of resistance via a transposon to macrolide-susceptible strains. Our experience is consistent with either or both of these mechanisms since we have one dominant clone and we have also determined that our emm1, emm4, and emm75 M-phenotype isolates share identical M-phenotype-determining regions and T-agglutination patterns with the corresponding CDC reference strains. Since these 30- to 60-year-old reference strains are susceptible to macrolides, the presence of resistance in our isolates is most consistent with acquisition of such a transposon. Support for this hypothesis is provided by the demonstration of the presence of an unusual chimeric genetic element containing DNA identical to Tn1207.1, a transposable element reported to encode mef(A) in macrolide-resistant Streptococcus pneumoniae, in seven different emm types of GAS expressing the M phenotype of macrolide resistance (3).
The importance of identifying macrolide resistance in GAS is dependent upon whether these antimicrobials are used for treatment and if in vitro resistance results in clinical failure. At present, erythromycin is the recommended alternative treatment for patients with GAS pharyngitis who are allergic to penicillin (2, 8). Beyond this recommendation, the newer macrolides, particularly azithromycin, have increasingly been used for the treatment of GAS pharyngitis because of their ease of administration. Several studies have shown that patients infected with macrolide-resistant GAS and treated with the newer macrolides experience a significantly lower bacteriologic cure rate compared to the rate observed when the same agents are used to treat patients infected with strains that are susceptible to macrolides (5, 31). Microbiologic cure rates after treatment of GAS pharyngitis with macrolides have been shown to fall from a range of 80 to 88% to a range of 60 to 63% for macrolide-susceptible and macrolide-resistant strains, respectively (5, 31). In addition, a 100% bacteriologic failure rate was observed when clarithromycin was used to treat patients infected with isolates expressing the constitutive MLS phenotype, for all of which the clarithromycin MIC was >128 μg/ml (5). The use of a macrolide for the treatment of macrolide-resistant GAS pharyngitis was also associated with a significantly lower clinical cure rate compared to that achieved by treatment with amoxicillin, amoxicillin-clavulanate, or cefaclor (5). Thus, predicting the likelihood of a successful treatment outcome may require knowledge of both the MIC and the phenotype of resistance for a given isolate. In the absence of such data, the use of macrolides for the treatment of GAS infections may be associated with an increased risk of both bacteriologic failure and the sequelae of GAS pharyngitis, including acute rheumatic fever.
Previous studies have documented a correlation between increased rates of use of macrolides and increased rates of resistance to this class of antibiotics among GAS (10, 28). Recent national and local pharmacy data substantiate impressive increases in the rates of prescription of azithromycin (22). The use and overuse of this agent go well beyond its use for the treatment of GAS pharyngitis. Azithromycin is frequently prescribed for both nonspecific upper respiratory tract infections and acute bronchitis, which are likely of viral origin. Limiting the use of macrolides has been associated with decreased rates of resistance in both Japan and Finland (17, 27) and should be encouraged in the United States.
Acknowledgments
We thank Richard Facklam for the T-typing results obtained in his laboratory.
REFERENCES
- 1.Alos, J. I., B. Aracil, J. Oteo, C. Torres, J. L. Gomez-Garces, et al. 2000. High prevalence of erythromycin-resistant, clindamycin/miocamycin-susceptible (M phenotype) Streptococcus pyogenes: results of a Spanish multicentre study in 1998. J. Antimicrob. Chemother. 45:605-609. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. 2003. Group A streptococcal infections, p. 526-536. In Redbook: report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, Ill.
- 3.Banks, B. J., S. Porcella, K. Barbian, J. Martin, and J. Musser. J. Infect. Dis., in press. [DOI] [PubMed]
- 4.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti, M., G. Manno, A. Collida, A. Ferrando, G. Gatti, E. Ugolotti, M. Cruciani, and D. Bassetti. 2000. Erythromycin resistance in Streptococcus pyogenes in Italy. Emerg. Infect. Dis. 6:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisno, A. L. 1991. Group A streptococcal infections and acute rheumatic fever. N. Engl. J. Med. 325:783-793. [DOI] [PubMed] [Google Scholar]
- 8.Bisno, A. L., M. A. Gerber, J. M. Gwaltney, Jr., E. L. Kaplan, and R. H. Schwartz. 1997. Diagnosis and management of group A streptococcal pharyngitis: a practice guideline. Clin. Infect. Dis. 25:574-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2001. National Center for Health Statistics, National Ambulatory Medical Care Survey, 1999 Summary. Centers for Disease Control and Prevention, Hyattsville, Md. [Online.] www.cdc.gov/nchs/data/ad/ad322.pdf. (Accessed 9 July 2003.)
- 10.Cizman, M., M. Pokorn, K. Seme, A. Orazem, and M. Paragi. 2001. The relationship between trends in macrolide use and resistance to macrolides of common respiratory pathogens. J. Antimicrob. Chemother. 47:475-477. [DOI] [PubMed] [Google Scholar]
- 11.Clancy, J., J. Petitpas, F. Dib-Hajj, W. Yuan, M. Cronan, A. V. Kamath, J. Bergeron, and J. A. Retsema. 1996. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 22:867-879. [DOI] [PubMed] [Google Scholar]
- 12.Coonan, K. M., and E. L. Kaplan. 1994. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr. Infect. Dis. J. 13:630-635. [DOI] [PubMed] [Google Scholar]
- 13.Cornaglia, G., M. Ligozzi, A. Mazzariol, M. Valentini, G. Orefici, R. Fontana, et al. 1996. Rapid increase of resistance to erythromycin and clindamycin in Streptococcus pyogenes in Italy, 1993-1995. Emerg. Infect. Dis. 2:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cresti, S., M. Lattanzi, A. Zanchi, F. Montagnani, S. Pollini, C. Cellesi, and G. M. Rossolini. 2002. Resistance determinants and clonal diversity in group A streptococci collected during a period of increasing macrolide resistance. Antimicrob. Agents Chemother. 46:1816-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Critchley, I. A., D. F. Sahm, C. Thornsberry, R. S. Blosser-Middleton, M. E. Jones, and J. A. Karlowsky. 2002. Antimicrobial susceptibilities of Streptococcus pyogenes isolated from respiratory and skin and soft tissue infections: United States LIBRA surveillance data from 1999. Diagn. Microbiol Infect. Dis. 42:129-135. [DOI] [PubMed] [Google Scholar]
- 16.Freeman, A. F., and S. T. Shulman. 2002. Macrolide resistance in group A streptococcus. Pediatr. Infect. Dis. J. 21:1158-1160. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, K., K. Murono, M. Yoshikawa, and T. Murai. 1994. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr. Infect. Dis. J. 13:1075-1078. [DOI] [PubMed] [Google Scholar]
- 18.Gay, K., and D. S. Stephens. 2001. Structure and dissemination of a chromosomal insertion element encoding macrolide efflux in Streptococcus pneumoniae. J. Infect. Dis. 184:56-65. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, E. L. 1997. Recent evaluation of antimicrobial resistance in beta-hemolytic streptococci. Clin. Infect. Dis. 24(Suppl. 1):S89-S92. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan, E. L., D. R. Johnson, M. C. Del Rosario, and D. L. Horn. 1999. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr. Infect. Dis. J. 18:1069-1072. [DOI] [PubMed] [Google Scholar]
- 21.Li, Z., V. Akota, D. Jackson, A. R. Franklin, B. Beall, et al. 2003. Array of M protein gene subtypes in 1064 recent invasive group A streptococcus isolates recovered from the active bacterial core surveillance. J. Infect. Dis. 188:1587-1592. [DOI] [PubMed] [Google Scholar]
- 22.Martin, J. M., M. Green, K. A. Barbadora, and E. R. Wald. 2002. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N. Engl. J. Med. 346:1200-1206. [DOI] [PubMed] [Google Scholar]
- 23.Martin, J. M., E. R. Wald, and M. Green. 1998. Field inversion gel electrophoresis as a typing system for group A streptococcus. J. Infect. Dis. 177:504-507. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2000. Performance standards for susceptibility testing: tenth informational supplement. M100-S10 (M7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Palavecino, E. L., I. Riedel, X. Berrios, S. Bajaksouzian, D. Johnson, E. Kaplan, and M. R. Jacobs. 2001. Prevalence and mechanisms of macrolide resistance in Streptococcus pyogenes in Santiago, Chile. Antimicrob. Agents Chemother. 45:339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santagati, M., F. Iannelli, M. R. Oggioni, S. Stefani, and G. Pozzi. 2000. Characterization of a genetic element carrying the macrolide efflux gene mef(A) in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppala, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, et al. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]
- 28.Seppala, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Huovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob. Agents Chemother. 40:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varaldo, P. E., E. A. Debbia, G. Nicoletti, D. Pavesio, S. Ripa, G. C. Schito, G. Tempera, et al. 1999. Nationwide survey in Italy of treatment of Streptococcus pyogenes pharyngitis in children: influence of macrolide resistance on clinical and microbiological outcomes. Clin. Infect. Dis. 29:869-873. [DOI] [PubMed] [Google Scholar]
- 32.Yan, J. J., H. M. Wu, A. H. Huang, H. M. Fu, C. T. Lee, and J. J. Wu. 2000. Prevalence of polyclonal mefA-containing isolates among erythromycin-resistant group A streptococci in southern Taiwan. J. Clin. Microbiol. 38:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.York, M. K., L. Gibbs, F. Perdreau-Remington, and G. F. Brooks. 1999. Characterization of antimicrobial resistance in Streptococcus pyogenes isolates from the San Francisco Bay area of northern California. J. Clin. Microbiol. 37:1727-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]