SUMMARY
Semi-professional volunteers work in many tertiary care hospitals in China as healthcare assistants. Proper infection control measures are needed to reduce nosocomial transmission involving volunteers. A compartmental model was constructed to describe the transmission characteristics of meticillin-resistant Staphylococcus aureus (MRSA) in the Emergency Ward (EW) and Respiratory Intensive Care Unit (RICU) for volunteers in Beijing Tongren Hospital, Beijing, China. The model consists of components describing uncolonized and colonized patients, uncontaminated and contaminated healthcare workers (HCWs), and uncontaminated and contaminated volunteers. The basic reproduction number (R0) was calculated, and the dependence of R0 on various model parameters was analysed. Moreover, simulations of the model were performed for comparision with the reported data on the numbers of colonized patients in the EW and RICU from 3 March 2009 to 28 February 2010, respectively. Sensitivity analysis of R0 showed that increasing handwashing compliance among HCWs and volunteers would reduce the risk of transmission dramatically. As volunteers care for patients on a one-to-one basis, this study showed that the number of MRSA-positive patients would increase if volunteers were replaced by HCWs. Therefore, in addition to improving hand hygiene among HCWs, the employment of properly trained volunteers is an attractive alternative to decrease MRSA and other multi-drug resistant bacteria infections in the hospital setting.
Keywords: Meticillin-resistant Staphylococcus aureus, Mathematical modelling, Volunteers, Transmission dynamics, Basic reproduction number
Introduction
Meticillin-resistant Staphylococcus aureus (MRSA) infection is associated with considerable morbidity and mortality among inpatients,1,2 and accounts for 35–80% of total staphylococcal infection in China.3 Patients colonized with MRSA are more likely to develop infection.4,5 Behaviours of healthcare workers (HCWs) have been shown to be key determinants in MRSA transmission.6–8 Thus, prevention of MRSA acquisition or transmission mediated by HCWs is critical in reducing MRSA infection.
In China, semi-professional volunteers work in many tertiary care hospitals. These volunteers have less training than HCWs, care for patients on a one-to-one basis, and are known to be less compliant with handwashing than HCWs.9,10 The daily work of volunteers includes taking care of patients' daily life, helping patients transfer from one unit to another, reporting irregular results to doctors, etc. They do not usually share work stations with HCWs. It is not clear whether the current infection control measures are effective to control MRSA transmission on wards with volunteers. Hence, it is important to assess the role of volunteers in MRSA transmission in order to identify reasonable infection control programmes for the whole hospital setting.
Mathematical modelling has provided a means to study the transmission dynamics of nosocomial pathogens in hospitals, including investigations of patient and HCW contact patterns, and HCW- and patient-mediated transmission.11–20 Most studies have used deterministic differential equation models which aggregate patient and HCW populations into compartments such as colonized or uncolonized patients, and contaminated or uncontaminated HCWs, and employ the Ross–Macdonald model structure for vector-borne diseases.14,15 A stochastic version of such models has also been proposed to study the transmission of MRSA in an intensive care unit.21 Following a similar modelling structure, the authors constructed a compartmental model to describe the transmission characteristics of MRSA in the Emergency Ward (EW) and Respiratory Intensive Care Unit (RICU) for volunteers in Beijing Tongren Hospital, Beijing, China. The aim of this study was to analyse the transmission dynamics of MRSA in wards with both professional HCWs and volunteers, and to assess various infection prevention programmes to improve the control of MRSA and other multi-drug-resistant bacteria infections in similar hospitals.
Methods
Patients and procedures
This study was conducted in Beijing Tongren Hospital, a 1600-bed university-affiliated teaching hospital. Seventy volunteers worked at the hospital, distributed over various wards, and all were managed by the Hospital Administration Centre of Nursing Workers. Twenty-three volunteers worked on the EW and two worked on the RICU. As the majority of patients admitted to the EW are critically ill and are usually transferred to other units, the prevention of MRSA transmission is particularly important. There are 23 beds in the EW and seven beds in the RICU. The EW is further divided into two areas; an eight-bed intensive care unit (ICU) and a 15-bed general open-bay ward. Patients on the EW are usually moved frequently between the two areas, depending on the severity and progression of their illness.
Collection of data on MRSA
For the EW, MRSA screening was performed selectively for patients at admission if any of the following criteria were met: transferred from other wards within the hospital, transferred from another hospital, received home health care, used antibiotics within the previous year, or had a history of an antibiotic-resistant organism.22–24 Thereafter, patients in the general open-bay wards had nasal swabs every three days when MRSA carriers were on the ward, and every seven days when no MRSA carriers were on the ward. For all patients in the ICU, screening was performed at admission and every two days thereafter. For the RICU, all patients were screened for MRSA carriage at admission and were sampled every two days thereafter until discharge.
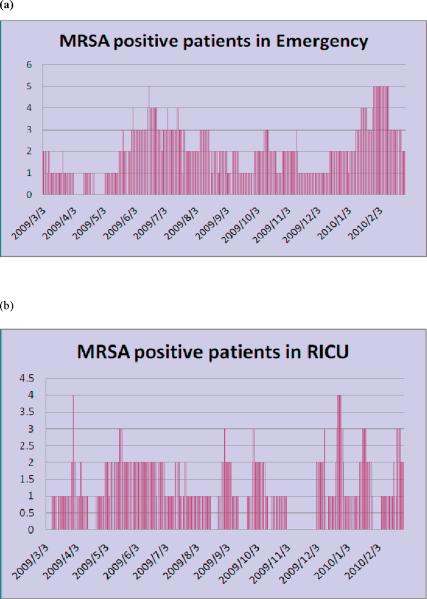
Before sampling started, a consent form was obtained for each patient and the study was approved by the hospital ethics committee. Clinical cultures were obtained as indicated at the discretion of the attending physician. Specimens were obtained from the nares, axilla, groin, any wound or lesion, and surgical incision (where appropriate). Specimens for detection of MRSA were performed using standard laboratory methods. Automated repetitive-sequence-based polymerase chain reaction (Diversilab System, bioMérieux) was used for molecular typing to confirm the strain relatedness between cases. The numbers of MSRA-positive patients in the EW and RICU from 3 March 2009 to 28 February 2010 are shown in Figure 1, which demonstrates that MRSA is endemic on these two wards.
Figure 1.
Numbers of meticillin-resistant Staphylococcus aureus positive patients from (a) the Emergency Ward (23 beds) and (b) the Respiratory Intensive Care Unit (seven beds) in Beijing Tongren Hospital from 3 March 2009 to 28 February 2010.
Basic model
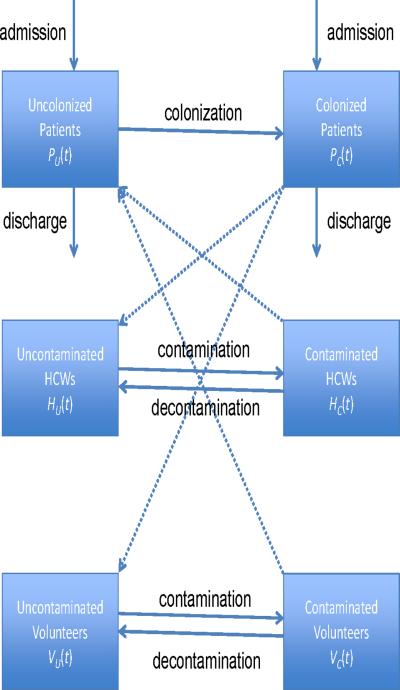
To describe the transmission dynamics of MRSA in patients, HCWs and volunteers in the EW and RICU in Beijing Tongren Hospital, the patients were divided into two groups: uncolonized [PU(t)] and colonized [PC(t)]. No distinction was made between colonized and infected patients. The HCWs and volunteers were also grouped into two subpopulations: uncontaminated HCWs [HU(t)] and contaminated HCWs [HC(t)], and uncontaminated volunteers [VU(t)] and contaminated volunteers [VC(t)], respectively.
It was assumed that patients are admitted to the hospital at a rate λ, and a portion φ are colonized with MRSA. δU and δC represent the discharge rates for uncolonized and colonized patients, respectively. Patients can become colonized through contact with contaminated HCWs and volunteers, respectively, according to mass-action law: (1-η)βPHPU (t)HC (t) and (1-ξ)βPV PU (t)VC (t), where η describes the hand hygiene of HCWs, and ξ describes the hand hygiene of volunteers. It was assumed that the transmission rate for volunteers (βPV) was lower than the transmission rate for HCWs (βPH) as volunteers only care for one patient at a time. HCWs can become contaminated through contact with colonized patients, βPHPC (t)HU (t), as can volunteers, βPVPC (t)VU (t). 1/γH and 1/γV represent the durations of contamination of HCWs and volunteers, respectively. Figure 2 shows a schematic illustration of the mathematical compartments. Baseline parameters were obtained from the literature,15,20 and estimated through computerized records from Tongren Hospital from March 2009 to February 2010 (see Table I).
Figure 2.
A compartmental model of transmission dynamics of meticillin-resistant Staphylococcus aureus among patients, healthcare workers (HCWs) and volunteers in Beijing Tongren Hospital.
Table I.
Baseline parameters and estimates for the transmission of meticillin-resistant Staphylococcus aureus for volunteers in the Emergency Ward (EW) and Respiratory Intensive Care Unit (RICU) at Beijing Tongren Hospital
| Parameter | Symbol | Baseline value |
Source | |
|---|---|---|---|---|
| EW | RICU | |||
| Total number of beds | N | 23 | 7 | Tongren |
|
| ||||
| Total number of HCWs | H | 23 | 14 | Tongren |
|
| ||||
| Total admissions per day | X | 0.79 | 0.33 | Tongren |
|
| ||||
| Fraction of admissions per day | ||||
| Colonized patients | φ | 0.067 | 0.165 | Tongren |
|
| ||||
| Length of stay | ||||
| Uncolonized patients | 1/ δU | 13 | 7 | Tongren |
| Colonized patients | 1/δC | 20 | 13 | Tongren |
|
| ||||
| Hand hygiene compliance | ||||
| HCWs | η | 0.41 | 0.46 | Observed |
| Volunteers | ξ | 0.2 | 0.23 | Observed |
|
| ||||
| Transmission probability per contact | δ | 0.09 | Observed | |
|
| ||||
| Transmission rate | ||||
| Colonized patients to HCWs | β PH | 0.72 | Estimated | |
| Colonized patients to volunteers | β PV | 0.20 | Estimated | |
|
| ||||
| Duration of contamination | ||||
| HCWs | 1γH | 1/24 | 1/24 | Austin et al.13 |
| Volunteers | 1/γV | 1/12 | 1/12 | Tongren |
HCW, healthcare worker.
Parameter estimate
An investigator observed the daily care activities of HCWs and volunteers over three 1-h periods (9:00–10:00am, 12:30–13:30pm, 23:00–24:00pm), repeated on three different days, including compliance with handwashing and other infection control measures, and patient care activities. Contact plate sampling was employed to detect MRSA colonies on the hands of HCWs/volunteers before and after medical care of MRSA-positive patients. HCWs/volunteers were asked to wash their hands thoroughly before contact with patients. MRSA was transferred to the hands of HCWs/volunteers in 19 out of 207 observed contacts with patients or their local environment (19/207, 9.2%). Therefore, δ was estimated to be 0.09. Handwashing compliance rates of volunteers were 20.7% (25/121) on the EW and 23.1% (30/130) on the RICU. Corresponding figures for HCWs were 41.0% (50/122) and 45.6% (57/125). Note that βPH =θ×ϕPH (contact rate, patients–HCWs), where θPH=number of patients×(total number of contacts between HCWs and patients per day)/(total number of patients×total number of HCWs in the ward). βPV =ω×θPVH (contact rate, patients–volunteers), where θPH=number of patients×(total number of contacts between volunteers and patients per day)/(total number of patients×total number of volunteers in the ward). Only `effective contacts' between patients and volunteers were calculated, which were composed of two parts: contacts that occurred when a volunteer had contacts with other patients in the ward, and effective contacts between a volunteer and his/her dedicated patient. Baseline parameters and their estimates are listed in Table I.
Results
Model simulations
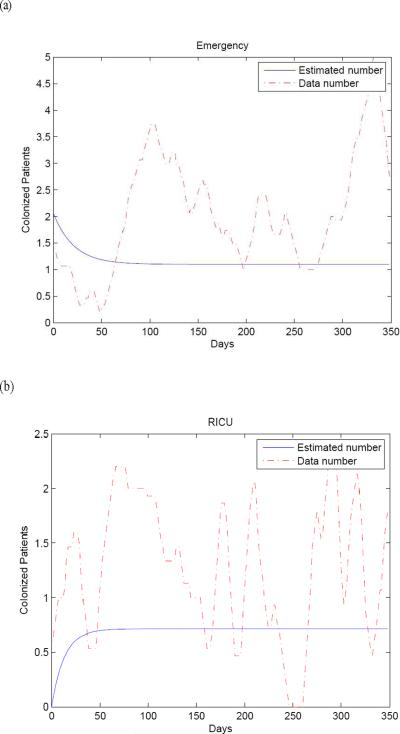
Model simulations using the parameter values given in Table I were performed for comparision with the reported data on the numbers of colonized patients in the EW and RICU in Beijing Tongren Hospital from 3 March 2009 to 28 February 2010. The results are compared in Figure 3.
Figure 3.
Model simulations using baseline parameters from Table I for the numbers of meticillin-resistant Staphylococcus aureus colonized patients (solid line) compared with the actual data (dotted line) for (a) the Emergency Ward and (b) the Respiratory Intensive Care Unit at Beijing Tongren Hospital. Fifteen day averages were used to plot the data.
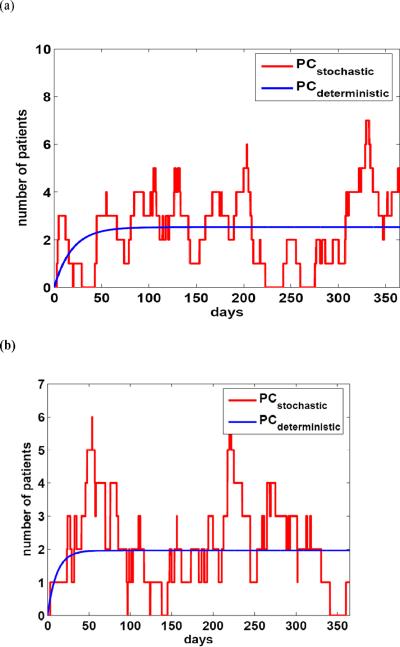
Since the populations in the EW and RICU were small, the numerical simulations were performed according to the stochastic model, and compared with the simulations using the deterministic model for both the EW and RICU (Figure 4).
Figure 4.
Numerical simulations for both the deterministic and stochastic models over one year corresponding to (a) the Emergency Ward and (b) the Respiratory Intensive Care Unit, using the parameters in Table I. Solid lines correspond to the deterministic model and dotted lines correspond to the stochastic model.
Simulations using the stochastic model appeared to provide a better explanation of the transmission dynamics for small populations. However, when using the deterministic model, the basic reproduction number (R0) can be expressed in terms of model parameters, and various control and prevention measures can be proposed and evaluated by sensitivity analysis of R0.
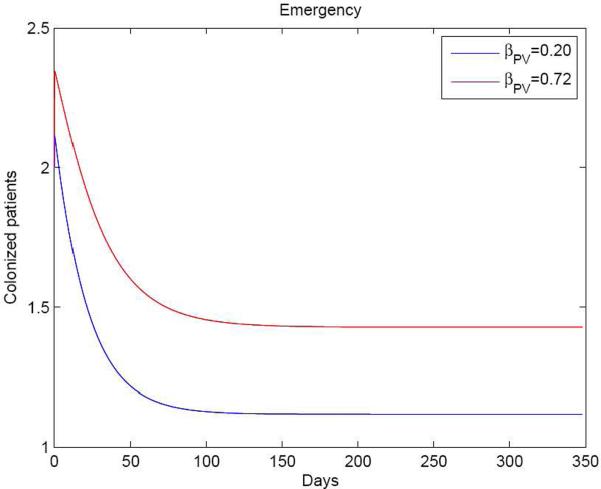
Since βPV represents the transmission rate between patients and volunteers, it is possible to analyse the effect of using volunteers on MRSA transmission. The transmission rate is the proportion of contacts between colonized and uncolonized individuals that result in infection. Thus, in numerical terms, it is transmission probability per contact × contact rate. As volunteers interact with patients on a one-to-one basis, the transmission rate from volunteers to patients is less than that from HCWs to patients. If there were no volunteers, an approximately equal number of HCWs would have to replace these volunteers. Equal transmission rates were used for both HCWs and volunteers to describe the situation if there were no volunteers. Figure 5 shows that if volunteers were replaced by HCWs (i.e. βPV =βPH), the number of colonized patients would increase; this was confirmed by analysis of R0.
Figure 5.
Numbers of meticillin-resistant Staphylococcus aureus positive patients on the Emergency Ward with volunteers and healthcare workers (HCWs) (βPV =0.2, solid line) and HCWs alone (βPH=0.72, dotted line). This indicates that the number of colonized patients would increase if the volunteers were replaced by HCWs.
Basic reproduction number
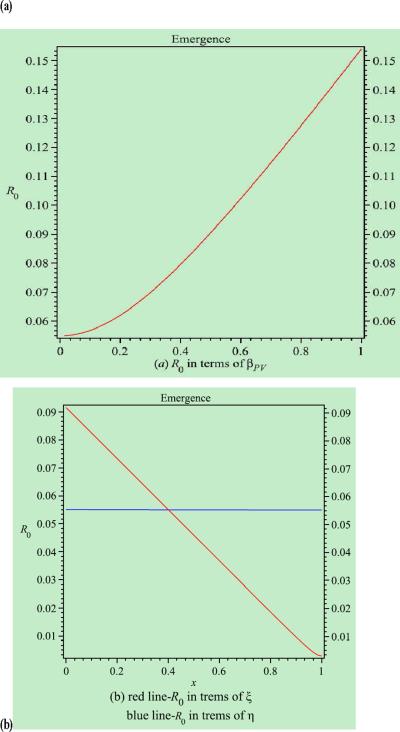
R0 is a very useful and important concept in modelling and studying the transmission dynamics of infectious diseases,25,26 that represents the number of patients who are colonized with MRSA by an index case patient at the beginning of the epidemic. From the point of view of infection control, the infection and spread of MRSA can be controlled in a hospital if R0 <1, whereas if R0 >1, MRSA has become endemic in the hospital. This study aimed to determine factors that affect the spread and control of MRSA in the hospital by analysing the dependence of R0 on various model parameters (i.e. sensitivity analysis). The explicit expression of R0 is derived in the Appendix in the case where ϕ = 0 (i.e. when no colonized patients are admitted to hospital). Sensitivity analyses of R0 were undertaken in terms of the model parameters, such as the transmission rate between patients and volunteers, hand hygiene constants of HCWs and volunteers, length of hospital stay for uncolonized and colonized patients, and duration of contamination of HCWs and volunteers (see Figure 6).
Figure 6.
Simulations of the basic reproduction number (R0) in terms of the model parameters. (a) Contact rate between patients and volunteers. (b) Hand hygiene compliance of healthcare workers (solid line) and volunteers (dotted line).
Using parameter values for the EW, Figure 6a shows that R0 will increase when the transmission rate between patients and volunteers (βPV) increases. Figure 6b shows that increasing the hand hygiene constant of HCWs or volunteers will reduce the risk of transmission; however, the effect of hand hygiene among volunteers is more dramatic. According to the model, if the length of hospital stay of patients (both uncolonized and colonized) is shortened or the duration of cross-transmission (for both HCWs and volunteers) is reduced, R0 will also decrease (figures not shown). The simulations in R0 based on parameter values for the RICU show similar results and are not given here.
This study had limitations. In the process of calculating R0, it was assumed that ϕ = 0. Therefore, the possibility of other values of ϕ was excluded, leading to imprecision of R0.
Discussion
The control of antibiotic-resistant bacterial infections in China is very difficult due to the huge population size, the large number of patients in hospitals, and the high transmission rate between HCWs and patients,. Mathematical modelling can improve understanding of the current situation, and help in the development of effective strategies to reduce prevalence rates.
Traditional strategies include reducing transmission rates between patients and nurses. In this study, the introduction of volunteers provides more options in designing control intervention strategies. Meanwhile, decreasing the transmission rates between patients and HCWs and between patients and volunteers, while increasing hand hygiene compliance of HCWs and volunteers, are all important in controlling infection based on the sensitivity analysis of R0 for these parameters. However, among these parameters, an increase in handwashing compliance for HCWs and volunteers would decrease R0 most dramatically, as the dependence is almost linear. If volunteers were replaced by HCWs and handwashing compliance was not 100%, MRSA transmission would increase as HCWs care for more than one patient. The simulation results can be explained by previous studies, which showed that increasing the workload of HCWs was closely associated with higher nosocomial infection rates.27 Employment of volunteers would decrease the workload of HCWs dramatically. Cohort nursing is known to be one of the most effective ways to prevent cross-transmission.21,28 The volunteer system in the study hospital is similar to cohort nursing, and volunteers can conduct many nursing tasks, thus reducing multi-patient contact by HCWs. Cohort nursing by fully trained HCWs would be even more effective.
This study also investigated the behaviours of volunteers during the study period. Compared with HCWs, more frequent contacts were observed between volunteers and environmental surfaces, and this may increase the probability of transmission of MRSA from the surrounding environment of MRSA patients to other environmental surfaces and vice versa. Volunteers' behaviours may be partly influenced by their long-term assignment to a ward. However, a recent study revealed that proper education for volunteers in infection control can decrease the nosocomial infection rate,29 which suggests that employment of volunteers undergoing nursing training is an alternative approach to nursing care.
In conclusion, improving the handwashing compliance of volunteers, who cohort nurse patients, will decrease MRSA transmission dramatically.
Supplementary Material
Acknowledgements
The authors are grateful to the reviewer for his/her careful reading, helpful comments and suggestions which helped to improve the presentation of the paper significantly.
Funding sources This work was partially supported by National Natural Science Foundation of China Grant 30970126, National Institutes of Health Grant R01GM083607, and National Science Foundation Grant DMS-1022728.
Appendix
The deterministic model is a system of six ordinary differential equations:
When ϕ = 0 (i.e. no colonized patients admitted to hospital), the disease-free steady state is obtained:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None declared.
References
- 1.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Qi Y, Kaye KS, et al. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Liu Y, Sun H, Xu Y, Xie X, Chen M. In vitro activity of ceftobiprole, linezolid, tigecycline, and 23 other antimicrobial agents against Staphylococcus aureus isolates in China. Diagn Microbiol Infect Dis. 2008;62:226–229. doi: 10.1016/j.diagmicrobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Niven DJ, Laupland KB, Gregson DB, Church DL, S. aureus Screening Initiative Group Epidemiology of Staphylococcus aureus nasal colonization and influence on outcome in the critically ill. J Crit Care. 2009;24:583–589. doi: 10.1016/j.jcrc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Reilly JS, Stewart S, Christie P, et al. Universal screening for meticillin-resistant Staphylococcus aureus: interim results from the NHS Scotland Pathfinder Project. J Hosp Infect. 2010;74:35–41. doi: 10.1016/j.jhin.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Creamer E, Dorrian S, Dolan A, et al. When are the hands of healthcare workers positive for meticillin-resistant Staphylococcus aureus? J Hosp Infect. 2010;75:107–111. doi: 10.1016/j.jhin.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Echanove J, Edwards JR, Richards MJ, et al. Effect of nurse staffing and antimicrobial-impregnated central venous catheters on the risk for blood stream infections in intensive care units. Infect Control Hosp Epidemiol. 2003;24:916–925. doi: 10.1086/502160. [DOI] [PubMed] [Google Scholar]
- 8.Beggs CB, Shepherd SJ, Kerr KG. How does healthcare worker hand hygiene behaviour impact upon the transmission of MRSA between patients? An analysis using a Monte Carlo model. BMC Infect Dis. 2009;9:64–72. doi: 10.1186/1471-2334-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su Y. Knowledge and awareness of nosocomial infection among care workers: investigations and counter measures. Chin J Nosocomiol. 2010;20:1132–1133. [Google Scholar]
- 10.Yong LR. Analysis of the Influencing factors of hand-washing compliance of hospital cleaners and nursing workers. Nurs J Chin PLA. 2010;27:60–61. [Google Scholar]
- 11.Cooper BS. Confronting models with data. J Hosp Infect. 2007;65(Suppl. 2):88–92. doi: 10.1016/S0195-6701(07)60022-X. [DOI] [PubMed] [Google Scholar]
- 12.Robotham JV, Scarff CA, Jenkins DR, Medley GF. Meticillin-resistant Staphylococcus aureus (MRSA) in hospitals and the community: model predictions based on the UK situation. J Hosp Infect. 2007;65(Suppl. 2):93–99. doi: 10.1016/S0195-6701(07)60023-1. [DOI] [PubMed] [Google Scholar]
- 13.Bonten MJM, Austin DJ, Lipsitch M. Understanding the spread of antibiotic resistant pathogens in hospitals: mathematical models as tools for control. Clin Infect Dis. 2001;33:1739–1746. doi: 10.1086/323761. [DOI] [PubMed] [Google Scholar]
- 14.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Agata EMC, Horn MA, Webb GF. The impact of persistent gastrointestinal colonization on the transmission dynamics of vancomycin-resistant Enterococci. J Infect Dis. 2002;185:766–773. doi: 10.1086/339293. [DOI] [PubMed] [Google Scholar]
- 16.D'Agata EMC, Webb GF, Horn MA, Moellering RC, Jr., Ruan S. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into the hospital setting. Clin Infect Dis. 2009;48:274–284. doi: 10.1086/595844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundmann H, Hellriegel B. Mathematical modelling: a tool for hospital infection control. Lancet Infect Dis. 2006;6:39–45. doi: 10.1016/S1473-3099(05)70325-X. [DOI] [PubMed] [Google Scholar]
- 18.Grundmann H, Hori S, Winter B, Tami A, Austin DJ. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Infect Dis. 2002;185:481–488. doi: 10.1086/338568. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitch M, Bergstrom CT, Levin BR. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc Natl Acad Sci USA. 2000;97:1938–1943. doi: 10.1073/pnas.97.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb GF, Horn MA, D'Agata EMC, Moellering RC, Jr., Ruan S. Competition of hospital-acquired and community-acquired methicillin-resistant Staphylococcus aureus strains in hospitals. J Biol Dynam. 2010;4:115–129. doi: 10.1080/17513750903026411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBryde ES, Pettitt AN, McElwain DLS. A stochastic mathematical model of methicillin resistant Staphylococcus aureus transmission in an intensive care unit: predicting the impact of interventions. J Theor Biol. 2007;245:470–481. doi: 10.1016/j.jtbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Moore C, Dhaliwal J, Tong A, Eden S, Wigston C, Willey B, McGeer A. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital. Infect Control Hosp Epidemiol. 2008;29:600–606. doi: 10.1086/588567. [DOI] [PubMed] [Google Scholar]
- 23.Gopal Rao G, Michalczyk P, Nayeem N, Walker G, Wigmore L. Prevalence and risk factors for meticillin-resistant Staphylococcus aureus in adult emergency admissions – a case for screening all patients? J Hosp Infect. 2007;66:15–21. doi: 10.1016/j.jhin.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multi-drug resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 25.den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 26.Diekmann O, Heesterbeek JAP, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugonnet S, Chevrolet JC, Pittet D. The effect of workload on infection risk in critically ill patients. Crit Care Med. 2007;35:76–81. doi: 10.1097/01.CCM.0000251125.08629.3F. [DOI] [PubMed] [Google Scholar]
- 28.Beggs CB, Noakes CJ, Shepherd SJ, Kerr KG, Sleigh PA, Banfield K. The influence of nurse cohorting on hand hygiene effectiveness. Am J Infect Control. 2006;34:621–626. doi: 10.1016/j.ajic.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Peng MD. Effect evaluation of hand washing education for nursing workers. Nurs J Chin PLA. 2010;27:60–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.