Summary
With aging, there is a decline in bone mass and in osteoblast differentiation of human mesenchymal stem cells (hMSCs) in vitro. Osteoblastogenesis can be stimulated with 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and, in some hMSCs, by the precursor 25-hydroxyvitamin D3 (25OHD3). CYP27B1/1α-hydroxylase activates 25OHD3 and, to a variable degree, hMSCs express CYP27B1. In this study, we tested the hypotheses 1) that age affects responsiveness to 25OHD3 and expression/activity of CYP27B1 in hMSCs, and 2) that parathyroid hormone (PTH) upregulates CYP27B1 in hMSCs, as it does in renal cells. There were age-related declines in osteoblastogenesis (n=8, p=0.0286) and in CYP27B1 gene expression (n=27, r=−0.498; p=0.008) in hMSCs. Unlike hMSCs from young subjects (≤ 50-years), hMSCs from older subjects (≥ 55-years) were resistant to 25OHD3 stimulation of osteoblastogenesis. PTH1-34 (100 nM) provided hMSCs with responsiveness to 25OHD3 (p=0.0313, Wilcoxon matched pairs test) and with two episodes of increased 1,25(OH)2D3 synthesis, of CREB activation, and of CYP27B1 upregulation. Both increases in CYP27B1 expression by PTH were obliterated by CREB-siRNA or KG-501 (which specifically inhibits the downstream binding of activated CREB). Only the second period of CREB signaling was diminished by AG1024, an inhibitor of insulin-like growth factor-I receptor kinase. Thus, PTH stimulated hMSCs from elders with responsiveness to 25OHD3 by upregulating expression/activity of CYP27B1 and did so through CREB and IGF-I pathways.
Keywords: Mesenchymal Stem Cells, Marrow Stromal Cells, CYP27B1, PTH, Vitamin D, Signaling
Introduction
In humans, peak bone mass is attained during the third decade of life. With advancing age, there is a decline in bone mass and an increase in fracture risk (Hui et al. 1988). Human bone marrow contains cells, called human mesenchymal stem cells or marrow stromal cells (hMSCs), that are progenitors of several lineages, including osteoblasts, chondrocytes, and adipocytes (Prockop 1997; Pittenger et al. 1999). We and others, however, showed that there is an age-related decline in osteoblast potential in hMSCs (D'Ippolito et al. 1999; Mueller & Glowacki 2001).
In vitro, the differentiation of hMSCs to osteoblasts is enhanced by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the activated form of vitamin D3 (Liu et al. 1999). We recently reported that osteoblast differentiation was also stimulated by 25-hydroxyvitamin D3 (25OHD3) in some hMSCs (Zhou et al. 2010). That led to the discovery that hMSCs have the capacity to enzymatically activate 25OHD3 to 1,25(OH)2D3 with CYP27B1 (the gene that encodes the enzyme 1α-hydroxylase) (Zhou et al. 2010). We reported that the constitutive level of expression of CYP27B1 in hMSCs was related to the vitamin D status of the subject from whom the cells were obtained and can be upregulated in vitro by the substrate 25OHD3 as well as by insulin-like growth factor-I (IGF-I) (Zhou et al. 2010), but effects of age were not determined. Subsequently, we reported that experimental reduction of CYP27B1 by ketoconazole or CYP27B1-siRNA in hMSCs from young subjects prevented the osteoblastogenic response to 25OHD3, (Geng et al. 2011). Those data provided evidence that 1α-hydroxylation is required for pro-differentiation effects of 25OHD3. Thus, one goal of this study was to assess the effects of age on the expression/activity of CYP27B1 and on stimulation of osteoblast differentiation by 25OHD3.
Parathyroid hormone (PTH) peptides have been used clinically as osteoanabolic therapies for osteoporosis and fracture prevention (Neer et al. 2001; Lane & Silverman 2010). In vivo and in vitro evidence indicates that PTH induces IGF-I (Canalis et al. 1989; Pfeilschifter et al. 1995; Watson et al. 1995; Shinoda et al. 2010). We determined that PTH peptides upregulated both IGF-I and IGF-II in hMSCs (Zhou et al. 2011) and that rhIGF-I induced CYP27B1 expression and 1α-hydroxylase activity in hMSCs (Zhou et al. 2010). Recently, Jilka et al. showed that PTH has greater bone anabolic effects in older mice because in addition to its stimulation of bone formation, it antagonized the age-associated increase in oxidative stress and adverse effects on birth and survival of osteoblasts (Jilka et al. 2010). Further, PTH in vitro (50 nM) protected osteoblasts from acute oxidative-stress-related effects. We recently demonstrated by genetic and pharmacological means that some effects of age on hMSCs were reproduced by experimental blocking of PTH signaling (Zhou et al. 2011). In addition, PTH is the major stimulus for renal production of 1,25(OH)2D3 (Haussler et al. 1976; Brenza et al. 1998; Brenza & DeLuca 2000). This reasoning suggested the possibility that PTH could restore functions of human MSCs.
In this study, we tested the hypotheses 1) that age affects responsiveness to 25OHD3 and expression/activity of CYP27B1 in hMSCs, and 2) that PTH could stimulate hMSCs from older subjects with responsiveness to 25OHD3 by upregulating expression/activity of CYP27B1, as it does in renal cells. Further, we sought to identify the intermediary roles of CREB and IGF-I, and to determine whether effects of age on vitamin D metabolism in hMSCs could be corrected with PTH.
Results
Age-related decline in osteoblastogenesis and CYP27B1 gene expression in hMSCs
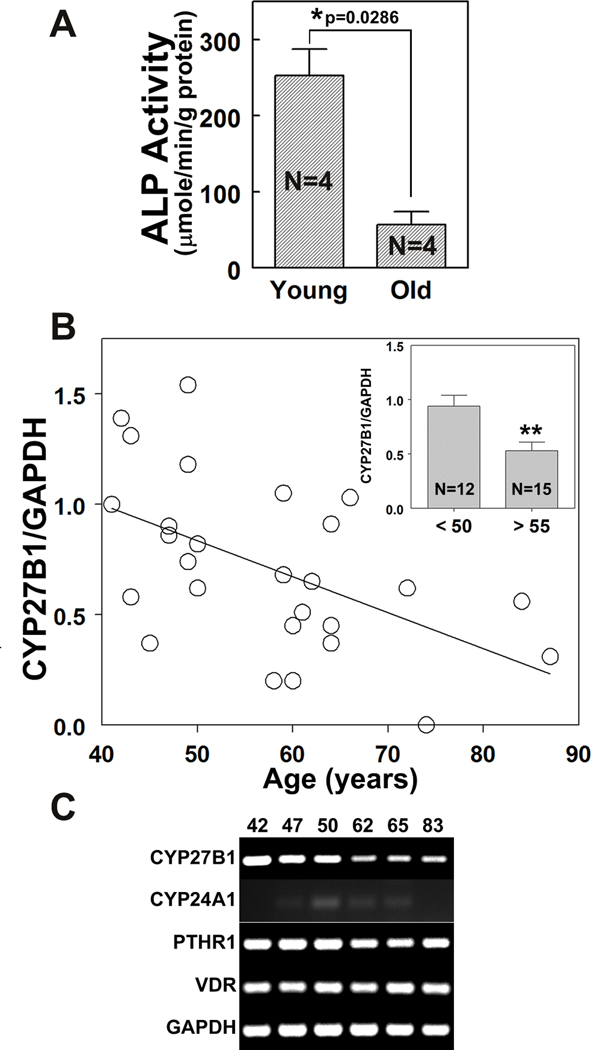
As a test of reproducibility of previous findings, we evaluated osteoblast potential in hMSCs from 4 young (≤ 50 years, mean age 36 ± 14 years) and 4 older (≥ 55 years, mean age 74 ± 4 years) subjects. After 7 days in osteoblastogenic medium, the mean level of alkaline phosphatase enzymatic activity (ALP activity, Fig. 1A) in hMSCs from older subjects (57 ± 17 µmole/min/g protein) was 23% of that for hMSCs from young subjects (253 ± 35 µmole/min/g protein, p=0.0286, Mann-Whitney test).
FIG. 1. Age-related decline in osteoblast potential and in constitutive expression of CYP27B1 in hMSCs.
(A) The development of ALP enzymatic activity in hMSCs from 4 young (<50 years, mean age 36 ± 14 years) and 4 old (>55 years, mean age 74 ± 4 years) subjects was determined. The hMSCs from young and old subjects were incubated in 1% FBS-HI osteogenic medium for 7 days. Results are expressed as Mean ± SEM (*p=0.0286, Mann-Whitney Test).
(B) The constitutive expression of CYP27B1 in hMSCs from 27 subjects (from 41 to 87 years, mean age 57 ± 12 years) was correlated with age (Spearman r=−0.498; p=0.008). (Inset) the expression of CYP27B1 in hMSCs from young (<50 years, n=12) and old (>55 years, n=15) subjects was determined, with each value normalized to GAPDH. Results are expressed as Mean ± SEM (t-test, **p=0.007).
(C) Gel electrophoretogram shows RT-PCR products of CYP27B1, CYP24A1, PTHR1, VDR and GAPDH in 6 hMSCs from 3 young (42, 47, 50 years of age) and 3 old (62, 65, 83 years of age) subjects.
A larger cohort of hMSCs obtained from 27 subjects (41 to 87 years, mean age 57±12 years) was used to determine the effect of age on constitutive expression of CYP27B1/1α-hydroxylase. There was an inverse correlation between CYP27B1 expression and age (r=−0.498; p=0.008; Fig. 1B). The mean level of expression of CYP27B1 in the older group (≥ 55 years, n=15, p=0.007) was 56% of that for the younger group (≤ 50 years, n=12, Fig. 1B inset). Another series of hMSCs was available from 14 subjects (64 ± 12 years) for whom serum 25OHD levels had been determined. Trios of young and old hMSCs from subjects known to be vitamin D-sufficient (serum 25OHD >32 ng/ml) were selected for further studies (Fig. 1C). There was lower constitutive expression of CYP27B1 in hMSCs from the older than the young subjects, with similar expression of 24-hydroxylase/CYP24A1, parathyroid hormone receptor type 1 (PTHR1), and vitamin D receptor (VDR).
PTH1-34 stimulated the effects of 25OHD3 on osteoblastogenesis in hMSCs from elders
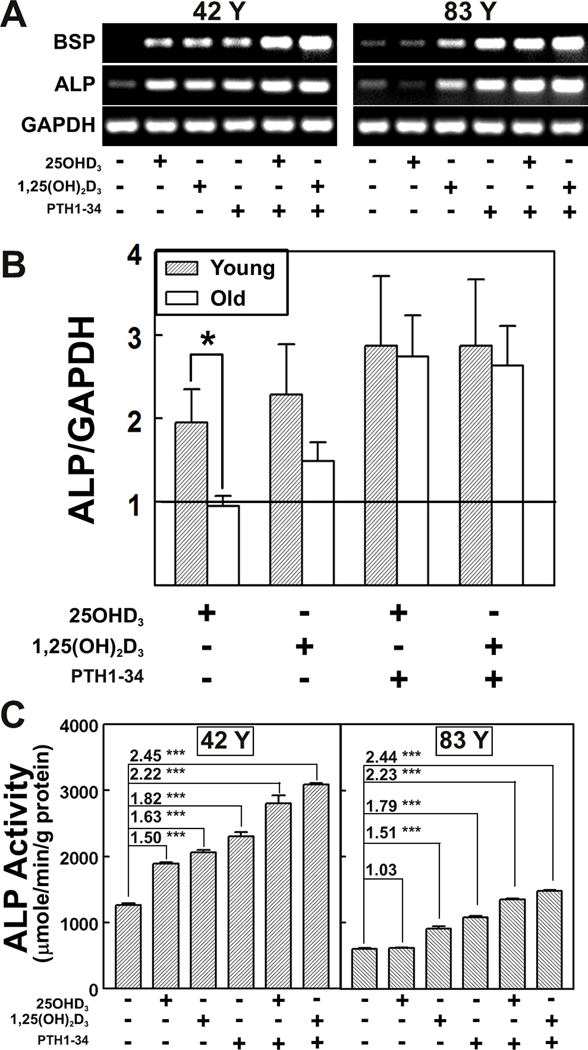
In hMSCs from young subjects (≤ 50 years), osteoblast differentiation, as assessed by osteoblast signature genes, bone sialoprotein (BSP) and alkaline phosphatase (ALP), was stimulated by 25OHD3, 1,25(OH)2D3, PTH1-34, and the combination treatment (Fig. 2A). In contrast, hMSCs from elders (≥ 55 years) were refractory to 25OHD3 and were stimulated by 1,25(OH)2D3, PTH1-34, and the combination treatment. Three samples from elders were not responsive to 25OHD3 (Fig. 2B; ALP/GAPDH, 0.95 ± 0.12, fold relative to control), compared with three samples from young subjects (1.95 ± 0.40, p = 0.05). With the numbers available, the effects of 1,25(OH)2D3 (p=0.40) and combination treatments (p>0.999) were statistically similar for the two age groups. For the hMSCs from elders, the combination of PTH and 25OHD resulted in greater ALP expression (2.74 ± 0.50) than for 25OH alone (0.95 ± 0.12, p=0.050). Results for BSP expression were similar.
FIG. 2. Age-related effects of vitamin D metabolites and PTH on osteoblast differentiation.
(A) Gel electrophoretograms of RT-PCR products for representative hMSCs from a 42-year-old (42 Y) and an 83-year-old subject (83 Y) show Bone Sialoprotein (BSP), Alkaline Phosphatase (ALP), and GAPDH in the absence or presence of 25OHD3 (10 nM), 1,25(OH)2D3 (10 nM), PTH1-34 (100 nM) or preincubated with PTH1-34 for 12 hours followed by 25OHD3 or 1,25(OH)2D3 treatment in osteoblastogenic medium (1% FBS-HI) for 3 days.
(B) Comparison of expression of Alkaline Phosphatase (ALP/GAPDH) in hMSCs from Young (n=3) and Old (n=3) subjects in the absence or presence of 25OHD3 (10 nM), 1,25(OH)2D3 (10 nM), or preincubated with PTH1-34 for 12 hours followed by 25OHD3 or 1,25(OH)2D3 treatment in osteoblastogenic medium (1% FBS-HI) for 3 days. Results are expressed as Mean ± SEM for each treatment compared with control (*p=0.05).
(C) Alkaline phosphatase enzymatic activity (ALP Activity, 3 replicate wells) was measured after 7 days in osteoblastogenic medium (1% FBS-HI) in two hMSCs (42 Y and 83 Y) in the absence or presence of 25OHD3 (10 nM), 1,25(OH)2D3 (10 nM), PTH1-34 (100 nM) or preincubated with PTH1-34 for 12 hours followed by 25OHD3 or 1,25(OH)2D3 treatment. Results are expressed as Mean ± SEM. The fold differences of treatments compared with corresponding control level (in 0.1% BSA in 1% FBS-HI osteogenic medium) are shown (ANOVA, *** p< 0.001).
Osteoblast differentiation was also quantified by ALP enzymatic activity 7 days after transfer to osteoblastogenic medium (Fig. 2C). The ALP activity in hMSCs from an older subject (83 Y) was 48% of that for hMSCs from a young subject (42 Y) (p<0.0001). In hMSCs from a young subject (42 Y), there was equivalent stimulation of osteoblast differentiation by 25OHD3 (1.50-fold, compared with control level, p<0.001) and by 1,25(OH)2D3 (1.63-fold, p<0.001). In striking contrast, hMSCs (83 Y) were not stimulated by 25OHD3 (1.03-fold), yet they were stimulated by 1,25(OH)2D3 (1.51 fold, p<0.001). PTH1-34 stimulated osteoblast differentiation in both specimens (42 Y: 1.82-fold, p<0.001; and 83 Y: 1.79-fold, p<0.001). Pre-treatment of the hMSCs (42 Y) with PTH1-34 stimulated differentiation with 25OHD3 (2.22-fold, p<0.001) and with 1,25(OH)2D3 (2.45-fold, p<0.001). There was 48% more ALP with 25OHD3 and PTH pre-treatment than without (p<0.001), and 50% more ALP with 1,25(OH)2D3 and PTH pre-treatment than without (p<0.001). It was striking that ALP in hMSCs (83 Y) was stimulated by 25OHD3 after pre-treatment with PTH1-34 (2.23-fold, p<0.001), with a magnitude equivalent to stimulation by 1,25(OH)2D3 (2.44-fold, p<0.001). In these cells, there was 118% more ALP stimulated by 25OHD3 with PTH pre-treatment than without (p<0.001), and there was 62% more ALP stimulated by 1,25(OH)2D3 with PTH pre-treatment than without (p<0.001).
In addition, osteoblast differentiation with combined treatments was greater than with PTH1-34 alone. With hMSCs (42 Y), ALP levels with PTH1-34 and 25OHD3 (122%, p<0.001) and with PTH1-34 and 1,25(OH)2D3 (134%, p<0.001) were greater than PTH1-34 alone. Likewise, with hMSCs (83 Y), PTH1-34 with 25OHD3 (125%, p<0.001) and PTH1-34 with 1,25(OH)2D3 (136%, p<0.001) were greater than PTH1-34 alone. For the hMSCs from the elder (83 Y), the combined PTH and 25OHD3 treatment resulted in ALP activity (1,350 ± 17 µmole/min/g protein) similar to control level of hMSCs (42 Y) (1,262 ± 25 µmole/min/g protein, 0.1% BSA in 1% FBS-HI osteogenic medium).
These unexpected results indicate that PTH pre-treatment provided hMSCs from older subjects with responsiveness to 25OHD3. These cells were used in subsequent experiments to determine the mechanism by which PTH "restores" responsiveness to 25OHD3 in hMSCs.
PTH1-34 upregulated 1α-hydroxylase activity and expression in hMSCs from elders
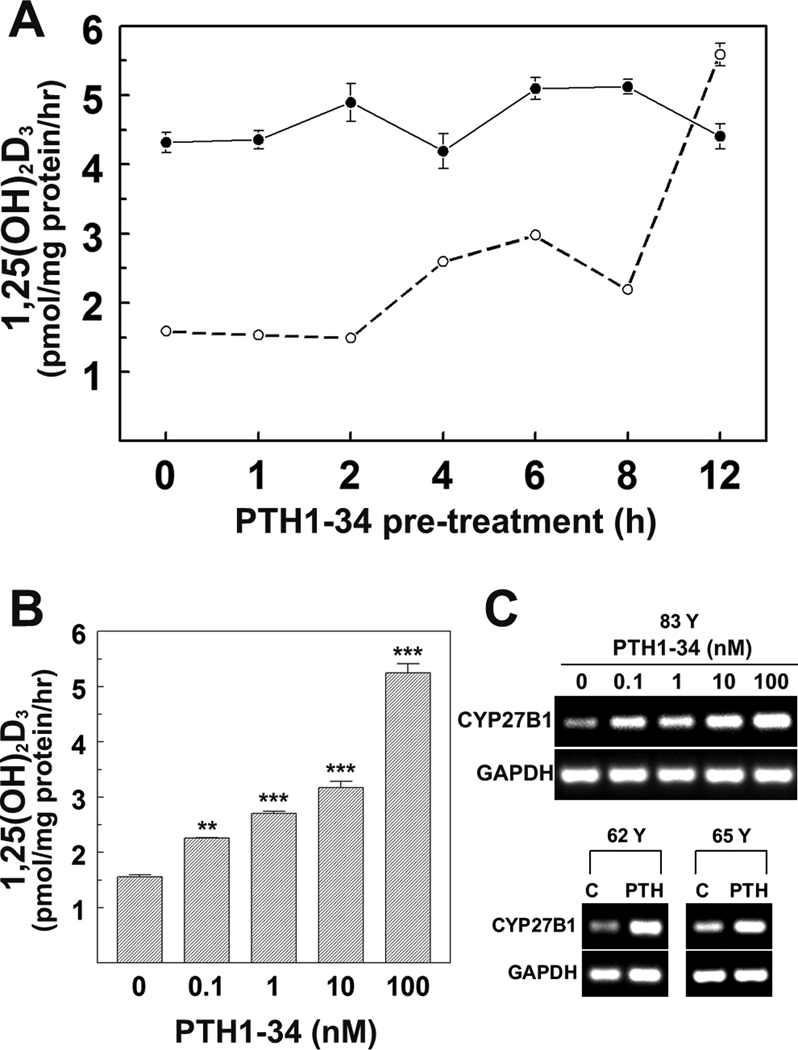
We tested the effects of pretreatment with PTH1-34 on 1α-hydroxylase enzymatic activity (in vitro synthesis of 1,25(OH)2D3) in hMSCs from young and old subjects. In baseline conditions, there was greater synthesis of 1,25(OH)2D3 in hMSCs (42 Y: 4,320 ± 146 fmol/mg protein/hr) than in hMSCs from an older subject (83 Y: 1,593 ± 52, p=0.0011, t-test with Welch correction, Fig. 3A). Pre-treatment with 100 nM PTH1-34 for 1 to 12 hours had little effect on synthesis of 1,25(OH)2D3 in hMSCs (42 Y), but for hMSCs (83 Y) there was 63% more at 4 hours (p<0.001) and 251% more at 12 hours (p<0.001, Fig. 3A). Synthesis of 1,25(OH)2D3 in hMSCs (83 Y) after 12 hours exposure to PTH (5,589 ± 162 fmol/mg protein/hr) was similar to that synthesized by hMSCs from a younger subject (42 Y; 4,407 ± 181, p=0.0011, t-test with Welch correction, Fig. 3A). There was the appearance of two episodes of stimulation, with an early (4–6 hours) and a later (12 hours) greater increase in synthesis of 1,25(OH)2D3. In hMSCs (83 Y), there was a dose-dependent increase in PTH stimulation of synthesis of 1,25(OH)2D3 (Fig. 3B). Analysis of gene expression showed that there was a dose-dependent effect of PTH on expression of CYP27B1 in hMSCs (83 Y) (Fig. 3C). The effect of 100 nM PTH to increase expression of CYP27B1 was reproduced in two additional hMSCs (Fig. 3C; 62 Y and 65 Y). Subsequent studies were carried out to determine the mechanisms by which PTH upregulates CYP27B1 in hMSCs from older subjects.
FIG. 3. Dose- and time-dependent effects of PTH1-34 on vitamin D 1α-hydroxylase activity and expression in hMSCs.
(A) Comparison time-course of PTH1-34 effects on 1,25(OH)2D3 production in hMSCs from two subjects (42 Y and 83 Y). Cells were treated with 100 nM PTH1-34 for 0, 1, 2, 4, 6, 8, 12 hours in serum-free α-MEM. For synthesis of 1,25(OH)2D3, they were changed to serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1,2-Dianilinoethane (N,N’-diphenylethylene-diamine) with or without 1,000 nM 25OHD3 for another 24 hours. Cellular 1,25(OH)2D3 production was determined by EIA. Results are presented as Mean ± SEM (3 replicate wells).
(B) Dose-response of PTH1-34 on 1,25(OH)2D3 production in hMSCs (83 Y). Cells were treated with 0, 0.1, 1, 10, or 100 nM PTH1-34 for 12 hours in serum-free α-MEM, then changed to serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1,2-Dianilinoethane (N,N’-diphenylethylene-diamine) with or without 1,000 nM 25OHD3 for another 24 hours. Cellular 1,25(OH)2D3 production was determined by EIA. Results are presented as Mean ± SEM (3 replicate wells, ANOVA, **p<0.01; ***p<0.001).
(C) Gel electrophoretograms show RT-PCR products of CYP27B1 and GAPDH in hMSCs (83 Y) with 0, 0.1, 1, 10, or 100 nM PTH1-34 and hMSCs from 62, 65 years old subjects (62 Y and 65 Y) with 100 nM PTH1-34 for 8 hours in standard growth medium (10% FBS-HI).
Two episodes of PTH upregulation of CYP27B1/1α-hydroxylase and CREB phosphorylation
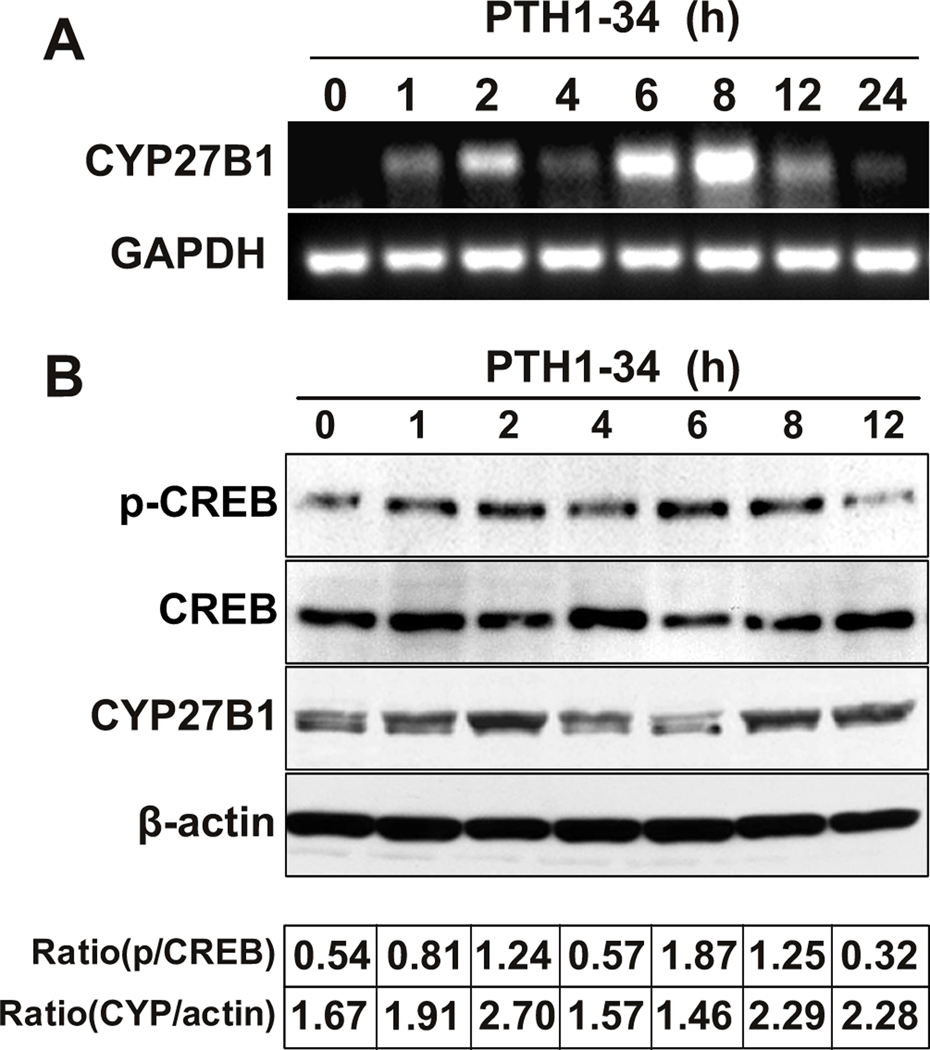
Similar to the appearance of two periods of PTH stimulation of 1,25(OH)2D3 synthesis, there were two increases in CYP27B1 gene expression, one peaking at 1–2 hours and one at 6–8 hours (Fig. 4A) and two periods of increases in CYP27B1 protein levels, at 2 and 8–12 hours (Fig. 4B). We investigated whether there were distinct mechanisms by which PTH had two episodes of upregulation of CYP27B1. The kinetics of phosphorylation of the PTH signaling molecule, the nuclear transcription factor, cAMP response element binding protein (CREB), were assessed. Similar to effects on its target gene CYP27B1, PTH1-34 stimulated two increases in CREB phosphorylation, one at 2 hours and the other at 6–8 hours.
FIG. 4. Role of CREB signaling in mediating PTH upregulation of CYP27B1/1α-hydroxylase.
(A) Gel electrophoretogram shows RT-PCR products of CYP27B1 and GAPDH in hMSCs from 83-year-old subject after pre-treatment with 100 nM PTH1-34 for 0, 1, 2, 4, 6, 8, 12, and 24 hours in standard growth medium (10% FBS-HI). Results are representative of 3 independent experiments.
(B) Western immunoblot shows phospho-CREB (p-CREB), total CREB (CREB), CYP27B1, and β-actin in hMSCs from 83-year-old subject after 0, 1, 2, 4, 6, 8, and 12 hours with 100 nM PTH1-34 in standard growth medium (10% FBS-HI). The ratios of p-CREB relative to total CREB (p/CREB) and of CYP27B1 relative to β-actin (Ratio CYP/actin) are shown beneath the image of immunoblot. Results are representative of 3 independent experiments.
CREB signaling was required for PTH upregulation of CYP27B1/1α-hydroxylase
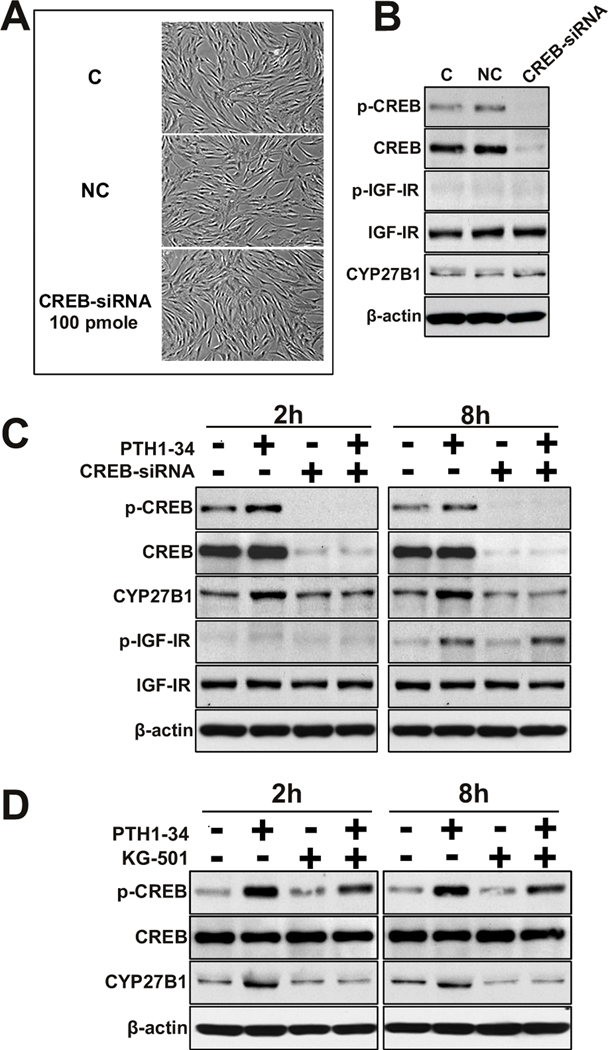
The hMSCs from an older subject (83 Y) were engineered to have reduced constitutive expression of CREB. There were no noticeable differences in cell density or appearance of control cells (electroporated with PBS, C), cells treated with non-silencing control siRNA (NC), and cells treated with 100 pmole CREB-siRNA (Fig. 5A). Transient transfection of CREB-siRNA into hMSCs resulted in reductions in basal levels of phosphorylated CREB (p-CREB) and total CREB protein (ratio of p-CREB/total CREB, 1% of control), but did not affect insulin-like growth factor I receptor (IGF-IR) or CYP27B1 expression (Fig. 5B). No effect was shown with a non-silencing, scrambled siRNA sequence (lane NC in Fig. 5B). After transfection, hMSCs were treated with PTH1-34. At both 2 hours and 8 hours, PTH stimulation of p-CREB and CYP27B1 was abrogated by CREB-silencing. At 8 hours, phosphorylation of IGF-IR (p-IGF-IR) and total IGF-IR were not affected (Fig. 5C). Similar results were obtained from a replicate experiment with cells from a 65-year-old subject (data not shown).
FIG. 5. Role of CREB signaling in mediating PTH upregulation of CYP27B1/1α-hydroxylase.
Groups of hMSCs were treated by electroporation with PBS (C, control), with non-silencing control siRNA (NC), or with 100 pmole of CREB-siRNA (CREB-siRNA).
(A) Photomicrographs show cultures of control (C), non-silencing control (NC) and hMSCs transfected with 100 pmole of CREB-siRNA (CREB-siRNA 100 pmole) at 200 × magnification.
(B) Western immunoblot shows p-CREB, CREB, phospho-IGF-IR (p-IGF-IR), total IGF-IR (IGF-IR), CYP27B1, and β-actin protein levels in controls and in transfected cells.
(C) Western immunoblot shows p-CREB, CREB, CYP27B1, p-IGF-IR, IGF-IR, and β-actin in transfected hMSCs after 2 hours or 8 hours in the absence or presence of 100 nM PTH1-34.
(D) Western immunoblot shows p-CREB, CREB, CYP27B1, and β-actin in hMSCs after 2 hours or 8 hours in the absence or presence of 100 nM PTH1-34 ± 30 µM KG-501. Human MSCs were incubated with the indicated concentration of KG-501 for 1 hour before stimulation with PTH1-34. Results are representative of 3 independent experiments.
Other hMSCs were treated with KG-501, an inhibitor of the downstream binding of activated phospho-CREB to CBP/p300. KG-501 abrogated PTH1-34 upregulation of CYP27B1 at both 2 hours and 8 hours, and, as expected, had small effects on CREB phosphorylation (Fig. 5D).
PTH induced IGF-I and IGF-I signaling, and treatment with rhIGF-I upregulated CYP27B1/1α-hydroxylase
Because IGF-I is a known target of PTH, its role in upregulating CYP27B1 was investigated. PTH1-34 upregulated IGF-I expression at 6 hours, but had no effect at 2 hours, distinct from PTH's induction of CYP27B1 gene expression at both 2 and 6 hours (Fig 6A). There was a dose-dependent upregulation of IGF-I expression by PTH1-34, evaluated at 6 hours (Fig. 6B).
FIG. 6. Effects of PTH on IGF-I and of rhIGF-I on CREB and CYP27B1/1α-hydroxylase.
(A) Gel electrophoretogram shows RT-PCR products of CYP27B1, IGF-I, and GAPDH in hMSCs after 0, 2, and 6 hours with 100 nM PTH1-34 in standard growth medium (10% FBS-HI).
(B) Gel electrophoretogram shows RT-PCR products of IGF-I and GAPDH in hMSCs after 6 hours with 0, 0.1, 1, 10, or 100 nM PTH1-34 in standard growth medium (10% FBS-HI).
(C) Western immunoblot shows p-IGF-IR, IGF-IR, p-CREB, CREB, CYP27B1, and β-actin in hMSCs after 0, 10, 30, 60, and 120 minutes with 100 ng/ml rhIGF-I in standard growth medium (10% FBS-HI). Results are representative of 3 independent experiments.
We examined the direct effects of rhIGF-I on IGF-IR, CREB, and CYP27B1. Ten minutes exposure to rhIGF-I resulted in a rapid 3.1-fold increase in IGF-IR phosphorylation and a 1.8-fold increase in CREB phosphorylation (Fig 6C). There was a more gradual increase in CYP27B1 protein to 2.7-fold at 120 min (Fig. 6C). These results suggest that the second episode of CREB signaling stimulated by PTH1-34 may be mediated by IGF-I.
IGF-I signaling of CREB mediated PTH’s second upregulation of CYP27B1/1α-hydroxylase
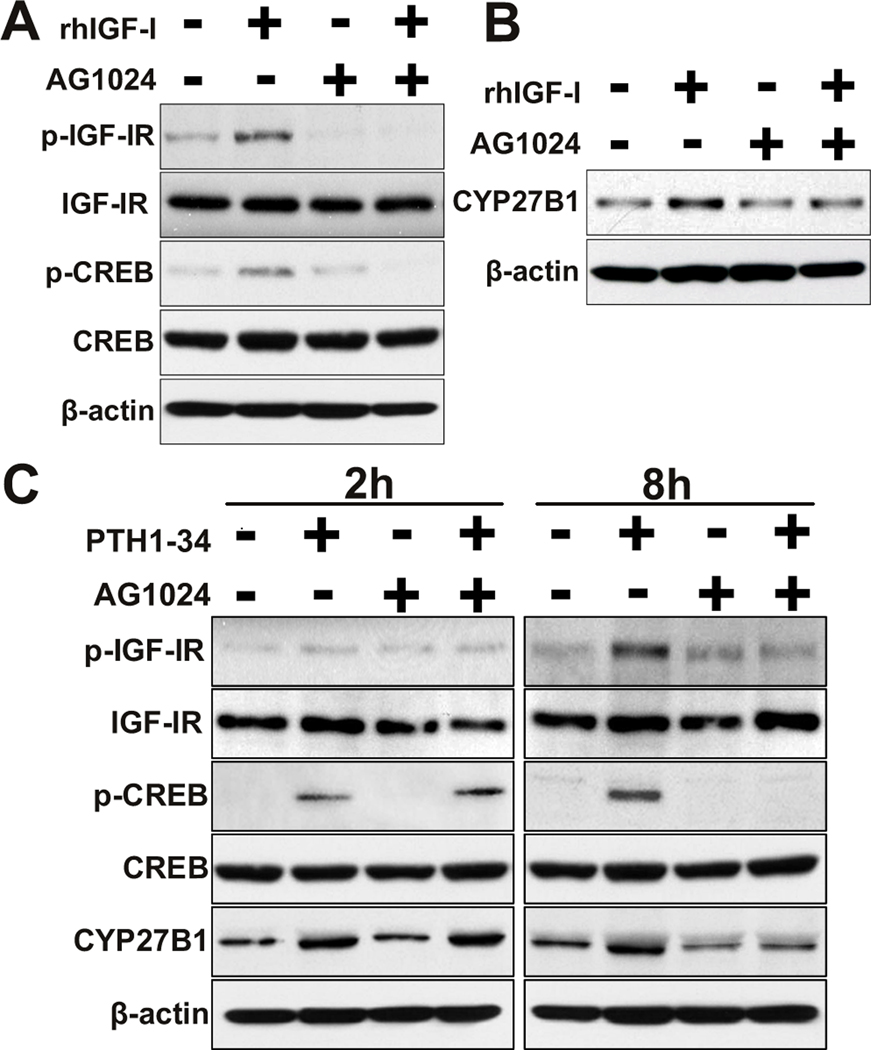
In the presence of AG1024, a specific inhibitor of IGF-IR kinase, IGF-I's rapid effects on IGF-IR phosphorylation and CREB activation were abrogated (Fig. 7A). In the presence of AG-1024, IGF-I's later upregulation of CYP27B1 (174%, relative to control) was blocked (112%, Fig. 7B).
FIG. 7. Role of IGF-I signaling in mediating second episode of PTH1-34 upregulation of CREB and CYP27B1/1α-hydroxylase.
Human MSCs were incubated with AG1024 for 1 hour before stimulation with IGF-I or PTH1-34.
(A) Western immunoblot shows p-IGF-IR, IGF-IR, p-CREB, CREB, and β-actin in hMSCs after 10 minutes in the absence or presence of 100 ng/ml rhIGF-I ± 3 µM AG1024.
(B) Western immunoblot shows CYP27B1 and β-actin in hMSCs from old subjects after 120 minutes in the absence or presence of 100 ng/ml rhIGF-I ± 3 µM AG1024.
(C) Western immunoblot shows p-IGF-IR, IGF-IR, p-CREB, CREB, CYP27B1, and β-actin in hMSCs from old subjects after 2 hours or 8 hours in the absence or presence of 100 nM PTH1-34 ± 3 µM AG1024. Results are representative of 3 independent experiments.
In the presence of AG1024, PTH1-34's induction of IGF-IR phosphorylation at 8 hours was abolished. AG1024 had no effect on PTH stimulation of IGF-IR phosphorylation, CREB phosphorylation, and CYP27B1 at 2 hours, but abolished those effects of PTH at 8 hours (Fig. 7C).
Discussion
The recent discovery that human marrow stromal cells, which include osteoblast progenitors, have the molecular machinery for regulated vitamin D metabolism (Zhou et al. 2010) suggested that vitamin D metabolites may serve autocrine/paracrine roles in osteoblast differentiation. These studies provide new evidence that in hMSCs there is an age-related decline in expression of CYP27B1, the gene that encodes the vitamin D-activating 1α-hydroxylase. Diminished synthesis of 1,25(OH)2D3 can explain the resistance of hMSCs from older subjects to 25OHD3 stimulation of osteoblast differentiation. This hypothesis is supported by our recent report that experimental silencing or inhibition of CYP27B1 in hMSCs from young subjects rendered them no longer responsive to 25OHD3 (Geng et al. 2011). The studies herein present evidence that PTH1-34 stimulated CYP27B1 expression and enzymatic activity; this provided hMSCs from old subjects with responsiveness to 25OHD3. The effects of PTH were mediated directly by CREB signaling and indirectly by IGF-I signaling. Thus, the regulation of CYP27B1 by PTH in hMSCs is similar to PTH stimulation of CYP27B1 in renal cells (Haussler et al. 1976; Brenza et al. 1998; Brenza & DeLuca 2000).
A decline in the numbers of or differentiation potential of stem cell populations in adult organs could contribute to human age and age-related disease (Rao & Mattson 2001; Carrington 2005). A loss of progenitor cell functionality may in turn contribute to a number of age-related musculoskeletal pathologies such as osteoporosis, arthritis, and tendinosis (Stolzing & Scutt 2006). Although there has been research to define the pathophysiology of bone loss associated with sex steroid deficiency and development of osteoporosis, there is less information about the mechanism(s) by which aging influences bone loss. Data in this report confirm other studies that show an age-related decline in osteoblast differentiation (Mueller & Glowacki 2001; Zhou et al. 2008). Data from studies with colony assays are variable, with some evidence for an age-related decline in colony number (D'Ippolito et al. 1999; Nishida et al. 1999), while others found no effects of age (Justesen et al. 2002). Kassem and Marie recently declared that "impaired differentiation of MSC to osteoblasts may contribute to the age-related bone loss" (Kassem & Marie 2011). A better understanding of intrinsic age-related changes is needed to mitigate or avoid loss of bone with age.
This study showed an age-related decline in CYP27B1 gene expression in hMSCs. Previously, we reported that the level of expression of CYP27B1 in hMSCs was related to the vitamin D status of the subject from whom the cells were obtained (Zhou et al. 2010), but there was insufficient power to assess the influence of age. In a series of hMSCs from vitamin D-sufficient subjects evaluated herein, there was lower constitutive expression of CYP27B1 in the specimens from the older than the young subjects. A larger study and multiple regression analysis will be needed to resolve the relative effects of age and serum 25OHD on constitutive expression of CYP27B1.
It is known that PTH is a major stimulus of renal CYP27B1 (Haussler et al. 1976; Brenza et al. 1998; Brenza & DeLuca 2000) and that PTH1-34 positively regulates renal CYP27B1 gene expression through a PKA-dependent pathway (Murayama et al. 1999). Thus, we tested whether PTH regulates CYP27B1 in hMSCs. Detecting lower expression of PTHR1 in hMSCs from older than younger subjects in this series is consistent with our previous report of age-related declines in PTHR1 expression and signaling with 10 nM PTH1-34 (Zhou et al. 2011). In this project, however, a higher concentration (100 nM) of PTH1-34 was used and was shown to be effective in upregulating CYP27B1 in cells from elders. Compared with cells from young subjects, osteoblast differentiation of hMSCs from older subjects was resistant to stimulation by 25OHD3, but responsiveness to 25OHD3 became evident after pre-treatment with PTH1-34. Stimulation of 1α-hydroxylation of 25OHD3 by PTH1-34 pre-treatment explains the increase in osteoblast differentiation with the combined treatments. These data indicate that PTH1-34 “restored” hMSCs from old subjects with responsiveness to 25OHD3 by upregulation of CYP27B1 expression and enzymatic activity. Samadfam et al. recently showed that intermittently administered PTH increased bone density in 1α-hydroxylase−/− mice, but that there was a greater effect in mice with an active 1,25(OH)2D-synthesizing system (Samadfam et al. 2008). They concluded that PTH and vitamin D may interact to potentiate osteoblast differentiation. This concept is also supported by an analysis of factors associated with heterogeneity in skeletal response to clinical PTH therapy for osteoporosis (Sellmeyer et al., 2007). Of all the variables tested, only the change in serum 1,25(OH)2D explained larger gains in bone density in response to PTH.
Kinetic analysis of synthesis of 1,25(OH)2D3 in hMSCs from an older subject revealed two waves of stimulation by PTH1-34, such that 1,25(OH)2D3 production following 12 hours exposure to PTH1-34 was similar to the level synthesized by hMSCs from a young subject. The levels of synthesis of 1,25(OH)2D3 by these cells were similar to those reported for osteoblast-like cells (Atkins et al. 2007). Our studies do not shed light on whether 1,25(OH)2D that is synthesized in marrow enters the circulation.
To determine the mechanisms by which PTH1-34 stimulated two episodes of increased CYP27B1 gene expression and protein levels, we monitored CREB activation, a well characterized pathway for PTH action (Johannessen et al. 2004). Upon binding to its receptor, PTH1-34 induces gene expression by its second messenger cAMP activating protein kinase A (PKA), which subsequently phosphorylates CREB at Ser133. That phosphorylation alters the affinity of the transactivation domain of CREB to the acceptor domain of the CREB-binding protein (CBP) and p300, and eventually results in enhancing transcription of CRE-dependent genes. In C-21 human kidney cells, three CRE-like sequences were identified within the PTH-sensitive area of the CYP27B1 promoter; their deletion reduced induction by 50%–95% (Flanagan et al. 2003). Thus, CYP27B1 is a CRE-dependent gene in kidney cells. We used two experimental approaches to determine the role(s) of CREB in PTH upregulation of CYP27B1 in hMSCs: targeted CREB silencing and use of small molecule inhibitor of CREB signaling. In a previous analysis of the age-related decline in PTH signaling in hMSCs, we showed that CREB-siRNA completely obliterated PTH stimulation of osteoblast differentiation that was typical for hMSCs from young subjects (Zhou et al. 2011). On the other hand, in this study with hMSCs from older subjects, transfection with CREB-siRNA blocked PTH1-34 upregulation of CYP27B1 at both 2 hours and 8 hours. The compound KG-501, which disrupts the downstream interaction between phospho-CREB and CBP/p300, abrogated PTH1-34 upregulation of CYP27B1 at both time periods. It is noted, however, that KG-501 also interferes with the interaction with NF-κB (Best et al. 2004). Our studies indicate that intact CREB signaling is necessary for PTH stimulation of osteoblast differentiation (Zhou et al. 2011) and, as shown herein, for PTH upregulation of CYP27B1 which is required for responsiveness to 25OHD3. Thus, for these cellular effects of PTH, experimental inhibition of CREB signaling can be viewed as a model for the natural age-related decline in CREB signaling in hMSCs. The CREB pathway is evolutionarily conserved and regulates many, diverse genes (Johannessen et al. 2004) and is likely to be involved in aging. There is other literature on the central importance of CREB-related genes in aging, in lifespan prediction, and in mediating lifespan extension by dietary restriction (Zhang et al. 2009). Recent research shows the critical role of the C. elegans orthologue of CREB in extension of longevity (Mair et al. 2011). The hMSCs may be useful to test other strategies for rejuvenating CREB pathway status and bone formation.
Evidence from a variety of systems indicates that osteoanabolic actions of PTH are mediated through IGF-I (McCarthy et al. 1989; Watson et al. 1995; Bikle et al. 2002; Shinoda et al. 2010). We previously reported that PTH peptides upregulate both IGF-I and IGF-II in hMSCs (Zhou et al. 2011) and that rhIGF-I upregulates CYP27B1 expression and 1α-hydroxylase enzymatic activity in hMSCs from old subjects (Zhou et al. 2010). AG1024 is a tyrphostin with specificity to inhibit activation of the IGF-I receptor (Parrizas et al., 1997). Evidence in this study shows that the second episode of CREB phosphorylation and upregulation of CYP27B1 at 6–8 hours is mediated by IGF-I. Other in vivo and in vitro studies indicate that IGF-I regulates the renal production of 1,25(OH)2D3 (Nesbitt & Drezner 1993; Menaa et al. 1995; Wong et al. 2000; Gomez 2006). Another important concept that has emerged recently is the requirement of IGF-I and its receptor for bone-building effects of PTH therapies (Bikle et al. 2002; Yamaguchi et al. 2005). Cao et al. showed in mice that aging is associated with skeletal resistance to IGF-I signaling (Cao et al. 2007). Furthermore, IGF activation of the IGF-IR is believed to stimulate CREB phosphorylation and regulate the expression of CRE-target genes for growth and survival in malignant and normal cell types (Pugazhenthi et al. 1999; Liu et al. 2002; Zheng & Quirion 2006; Kim et al. 2010).
These studies begin to elucidate the role of IGF-I in PTH1-34 effects on differentiation of hMSCs, but more information is needed. The regulation of IGF-I is important for many cell functions; for example, in bone cultures, PTH stimulation of collagen synthesis requires IGF-I, whereas its mitogenic effects do not (Canalis et al. 1989). Moreover, in osteoblastic cells, there is crosstalk whereby PTH1-34 potentates effects of 1,25(OH)2D3 on induction of c/EBPβ and target genes (Dhawan et al. 2005). Although the samples studied were obtained from enrolled subjects known to be vitamin D-sufficient, it is possible that factors other than age may contribute to the biology of the hMSCs.
In conclusion, there are age-related declines in osteoblastogenesis and expression/activity of CYP27B1 in hMSCs, and an age-related resistance to 25OHD3. In vitro, PTH1-34 provided hMSCs from old subjects with responsiveness to 25OHD3 by upregulation of CYP27B1 expression and activity through CREB and IGF-I mediated pathways. The stimulation of CYP27B1 by PTH in hMSCs is similar to that in renal cells. Vitamin D sufficiency is thought important to ensure proper mineralization of bone, but these studies indicate further that PTH and vitamin D may interact to potentiate osteoblast differentiation in elders. It will be clinically important to determine the proper vitamin D status needed to optimize PTH osteoanabolic therapy, especially in elders.
Experimental procedures
Reagents
Recombinant human parathyroid hormone peptide 1–34 (PTH1-34) was purchased from Bachem Americas, Inc. (Torrance, CA, USA) and recombinant human insulin-like growth factor (rhIGF-I) was purchased from R&D Systems (Minneapolis, MN, USA); other reagents such as 25OHD3, 1,25(OH)2D3, KG-501, AG1024 were purchased from Sigma (St. Louis, MO, USA). PTH1-34 and rhIGF-I were prepared in 0.1% BSA; 25OHD3, 1,25(OH)2D3 were prepared in 0.1% ethanol; others was prepared as stock solutions at 10−3 M in DMSO and stored at −80°C.
Preparation of Human Marrow Stromal cells (hMSCs)
Bone marrow samples were obtained with IRB approval as femoral tissue discarded during primary hip arthroplasty for osteoarthritis. A set of subjects scheduled for hip arthroplasty was consented for research studies, including measurement of serum 25OHD. Criteria for exclusion are rheumatoid arthritis, cancer, and other comorbid conditions that may influence skeletal metabolism, i.e. renal disease, alcoholism, active liver disease, malabsorption, hyperthyroidism, ankylosing spondylitis, aseptic necrosis, hyperparathyroidism, morbid obesity, and diabetes. Also excluded were patients who were taking medications that may influence skeletal metabolism (e.g. thyroid hormone, glucocorticoids, non-steroidal anti-inflammatory drugs, bisphosphonates, and osteoanabolic drugs). Low-density marrow mononuclear cells were isolated by centrifugation on Ficoll/Histopaque 1077 (Sigma, St. Louis, MO, USA) (Zhou et al. 2005). This procedure removes differentiated cells and enriches for undifferentiated, low-density marrow mononuclear cells that include a population of non-adherent hematopoietic cells and a fraction capable of adherence and differentiation into musculoskeletal cells. The non-adherent hematopoietic stem cells were rinsed away 24 hours after seeding and the adherent hMSCs were expanded in monolayer culture with standard growth medium, phenol red-free α-MEM, 10% fetal bovine serum-heat inactivated (FBS-HI), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). For osteoblastogenic differentiation experiments, we lowered the serum of standard osteogenic medium (10% FBS-HI) to 1% FBS-HI to reduce possible differences in proliferation and cell numbers for cells from young and old subjects (Zhou et al. 2008). After transfection with siRNA, all medium used were without 100 U/ml penicillin, 100 µg/ml streptomycin. We used constant seeding density and constant splitting protocol, regardless of the time to proliferate. Initially, 30×106 bone marrow mononuclear cells were seeded per 100-mm dish. We define passage 0 as when the initial hMSCs proliferate to ~80% confluence. In each experiment, standardized conditions were used: same passage (passage 2–3), identical seeding density, and identical reagents. This approach avoids changes in cell behaviors that are associated with prolonged culture, such as in vitro senescence or culture stress (Zhou et al. 2008). Like other normal mammalian cells, when cultured for many passages, MSCs display what is termed “in vitro senescence”, i.e. decreased proliferation, replicative quiescence, enlargement, increase in SA-β-gal activity, and erosion of telomeres (Fehrer & Lepperdinger 2005; Sethe et al. 2006). Further, there is a link between the accumulation of DNA damage and loss of multipotency of human MSCs with time of culture (Alves et al. 2009). Cellular senescence can contribute to the physiological processes of normal organismal age (Jeyapalan & Sedivy 2008). The results obtained herein, however, should reflect the effects of in vivo age because cells from young and old individuals were treated the same way and evaluated upon isolation or at early passage.
RNA isolation and RT–PCR
Total RNA was isolated from human MSCs with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). For RT–PCR, 2 µg of total RNA was reverse transcribed into cDNA with SuperScript II (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. Concentrations of cDNA and amplification conditions were optimized for each gene product to reflect the exponential phase of amplification. One-twentieth of the cDNA was used in each 50 µl PCR reaction (30–40 cycles of 94 °C for 1 min, 55–60 °C for 1 min, and 72 °C for 2 min) as described (Zhou et al. 2005). Gene-specific primer pairs for CYP27B1 (Zhou et al. 2010), CYP24A1 (Zhou et al. 2010), PTHR1 (Winn et al. 1999), VDR (Zhou et al. 2010), ALP (Zhou et al. 2008), BSP (Zhou et al. 2008), IGF-I (Zhou et al. 2010), GAPDH (Zhou et al. 2008) were used for amplification. Polymerase chain reaction products were separated by agarose gel electrophoresis and were quantified by densitometry of captured gel images with KODAK Gel Logic 200 Imaging System and KODAK Molecular Imaging Software, following the manufacturer’s instructions (KODAK, Molecular Imaging Systems, New Haven, CT, USA). Data were expressed by normalizing the densitometric units to GAPDH (internal control) and, in some cases, as treated relative to control.
Alkaline phosphatase (ALP) enzymatic activity assay
For ALP enzymatic activity assay, the concentration of serum in standard osteogenic medium was reduced to 1% FBS-HI to minimize possible subsequent differences in proliferation that could confound interpretation of effects of vitamin D metabolites on osteoblastogenesis (Gregory et al. 2005; Zhou et al. 2008). ALP enzyme activity was measured spectrophotometrically, as previously described (Zhou et al. 2008). Protein concentration was determined with the BCA system (Thermo Fisher Scientific Inc., Rockford, IL, USA). The ALP enzyme activity was expressed as µmole/min/g protein and calculated the fold change (treated relative to control). The standard curves are linear, but values differ from experiment to experiment; we always compare the results within an experiment due to the limitation of the assay. For the pre-treatment experiments, 100 nM PTH1-34 was added to cells 12 hours prior to 10 nM 25OHD3 or 1,25(OH)2D3. Culture medium was changed every 2 days.
In vitro biosynthesis of 1, 25(OH)2D3 by hMSCs
In order to generate detectable levels of 1,25(OH)2D3, hMSCs were cultivated in 12-well plates until confluence, preincubated for duration 0, 1, 2, 4, 6, 8 and 12 hours with 100 nM PTH1-34 or for 12 hours with or without 0.1, 1, 10, 100 nM PTH1-34 in serum-free α-MEM (Sigma, St. Louis, MO, USA), and then changed the medium to serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1, 2-Dianilinoethane (N, N’ -diphenylethylene-diamine) (Sigma, St. Louis, MO, USA) and treated with or without 1,000 nM 25OHD3 for another 24 hours. 1, 2-Dianilinoethane was added into the cultures as an antioxidant as described (Zehnder et al. 2002). Supernatants were harvested and stored at −20 °C prior to analysis for 1,25(OH)2D3 content. The 1, 25(OH)2D3 levels in medium were quantitatively determined with a 1, 25(OH)2D3 ELISA kit (Immunodiagnostic systems Ltd., Scottsdale, AZ, USA), according to the manufacturer’s instructions. The hMSCs were lysed with a buffer containing 150 mM NaCl, 3 mM NaHCO3, 0.1% Triton X-100, and a mixture of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Protein concentration was determined with the BCA system (Thermo Fisher Scientific Inc., Rockford, IL, USA). The CYP27B1 activity was expressed as biosynthesized 1,25(OH)2D3 in medium per milligram protein per hour of 25OHD3 treatment (femtomoles per milligram protein per hour).
RNA interference with CREB-siRNA
Transient transfection of siRNA into hMSCs from old subjects were performed by electroporation with the Human MSC Nucleofector Kit® (Lonza/Amaxa Biosystems) with either CREB-siRNA (Stealth RNAi duplex siRNA, Invitrogen, Carlsbad, CA, USA), non-silencing control siRNA (a non-homologous, scrambled sequence equivalent) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), or PBS according to the manufacturer’s instructions and as our previous described (Zhou 2011; Shen et al., 2011). In brief, hMSCs were harvested by trypsinization, and resuspended at 106 cells in 100 µl of Nucleofector Solution with 100 pmole of CREB-siRNA. Electroporation was performed in Nucleofector II® device with program U-23 (Lonza/Amaxa Biosystems, Walkersville, MD, USA). Immediately after electroporation, the cells were transferred to 60-mm dishes in phenol red-free α-MEM, 10% FBS-HI. Some cells were collected at 80% confluence for Western immunoblot to determine the effect of CREB-siRNA. 24 hours after electroporation, some cells were treated with either PTH1-34 (100 nM) or vehicle control (0.1% BSA) at 2 or 8 hours in standard growth medium (10% FBS-HI) for Western immunoblot.
Western immunoblot
Human MSCs were cultured in 100-mm dishes in standard growth medium (10% FBS-HI). At 80% confluence, the cells were treated with 100 nM PTH1-34 for different time. The whole-cell lysates were prepared with lysis buffer (150 mM NaCl, 3 mM NaHCO3, 2 mM Na3VO4, 5 mM NaF, 0.1% Triton X-100, and a mixture of protease inhibitors, Roche Diagnostics, Indianapolis, IN, USA) and were homogenized with a pestle (Kontes) and centrifuged at 16,000 × g (Eppendorf centrifuge). Protein concentration was determined (BCA system, Thermo Fisher Scientific Inc., Rockford, IL, USA). The Western blotting was performed as previously described (Zhou et al. 2004). In brief, proteins were resolved on 4–12% SDS-PAGE (NuPAGE Bis-Tris gel; Invitrogen, Carlsbad, CA, USA) and transferred onto polyvinylidene fluoride membranes (PVDF, Amersham Biosciences, Piscataway, NJ, USA). The membranes were blocked with 5% nonfat milk in PBS buffer containing 0.1% Tween-20 (PBST) for 2–3 hours at room temperature and incubated at 4°C for overnight with primary antibodies with 1:1000 Phospho-CREB (p-CREB), 1:3000 total CREB (CREB), 1:1000 Phospho-IGF-I Receptor (p-IGF-IR) and 1:1000 IGF-I Receptor β (IGF-IR) (Cell signaling Technology, Beverly, MA, USA); 1:1000 CYP27B1 and 1:8000 β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After removal of the unbound primary antibodies by three 5-minute washes with PBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 1 hour at room temperature and washed 3 times for 5 minutes with PBST. The antibody-associated protein bands were revealed with the ECL-plus Western blotting system (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
All experiments were performed at least in triplicate. Group data are presented as Mean values ± SEM. Unless otherwise indicated, quantitative data were analyzed by GraphPad Instat® (GraphPad Software, Inc.) with non-parametric tools, either the Mann-Whitney test for group comparisons or Spearman correlation test, or with parametric tools, ether group comparisons by two-tailed unpaired t test with Welch correction or multiple comparisons by one-way ANOVA followed by Tukey’s Post Hoc analysis. A value of p < 0.05 was considered significant.
Acknowledgments
The authors greatly appreciate Drs. Zhenggang Bi, Regina O'Sullivan, Shuichi Mizuno, and Ms. Sara Anderson for guidance and assistance. This work is based on a thesis by Shuo Geng for the M.D./Ph.D. degrees from Harbin Medical University, China. Shuo Geng was supported by the China Scholarship Council (CSC). This project was supported by NIH grants AG025015 and AG028114.
Footnotes
This study was presented in part at the ASBMR annual meeting in Toronto, ON, Canada, 2010 and ASBMR Forum on Aging and Skeletal Health, Bethesda, MD, USA, 2011.
Author contributions
Shuo Geng, Shuanhu Zhou, and Julie Glowacki designed the studies, analyzed, and interpreted the data. Shuo Geng acquired the data and drafted the manuscript. All authors critically edited each draft.
Contributor Information
Shuo Geng, Email: shuogeng.hmu@gmail.com.
Shuanhu Zhou, Email: szhou@rics.bwh.harvard.edu.
References
- Alves H, Munoz-Najar U, De Wit J, Renard AJ, Hoeijmakers JH, Sedivy JM, Van Blitterswijk C, De Boer J. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med. 2009;14:2729–2738. doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O'Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Best JL, Amezcua CA, Mayr B, Flechner L, Murawsky CM, Emerson B, Zor T, Gardner KH, Montminy M. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proc Natl Acad Sci U S A. 2004;101:17622–17627. doi: 10.1073/pnas.0406374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1α-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381:143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca HF. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A. 1998;95:1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83:60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;22:1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328:700–708. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol. 2005;25:472–487. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Flanagan JN, Wang L, Tangpricha V, Reichrath J, Chen TC, Holick MF. Regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene and its splice variant. Recent Results Cancer Res. 2003;164:157–167. doi: 10.1007/978-3-642-55580-0_12. [DOI] [PubMed] [Google Scholar]
- Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Miner Res. 2011;26:1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7:125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental "niches" in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Baylink DJ, Hughes MR, Brumbaugh PF, Wergedal JE, Shen FH, Nielsen RL, Counts SJ, Bursac KM, McCain TA. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 Suppl:151S–165S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Hui SL, Slemenda CW, Johnston CC., Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9:851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191–197. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- Kim HB, Kim WH, Han KL, Park JH, Lee J, Yeo J, Jung MH. cAMP-response element binding protein (CREB) positively regulates mouse adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2010;391:634–639. doi: 10.1016/j.bbrc.2009.11.111. [DOI] [PubMed] [Google Scholar]
- Lane NE, Silverman SL. Anabolic therapies. Curr Osteoporos Rep. 2010;8:23–27. doi: 10.1007/s11914-010-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- Liu W, Chin-Chance C, Lee EJ, Lowe WL., Jr. Activation of phosphatidylinositol 3-kinase contributes to insulin-like growth factor I-mediated inhibition of pancreatic beta-cell death. Endocrinology. 2002;143:3802–3812. doi: 10.1210/en.2002-220058. [DOI] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabedian M. Insulin-like growth factor I, a unique calcium-dependent stimulator of 1,25-dihydroxyvitamin D3 production. Studies in cultured mouse kidney cells. J Biol Chem. 1995;270:25461–25467. doi: 10.1074/jbc.270.43.25461. [DOI] [PubMed] [Google Scholar]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, Kato S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1alpha,25(OH)2D3 in intact animals. Endocrinology. 1999;140:2224–2231. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nesbitt T, Drezner MK. Insulin-like growth factor-I regulation of renal 25-hydroxyvitamin D-1-hydroxylase activity. Endocrinology. 1993;132:133–138. doi: 10.1210/endo.132.1.8419119. [DOI] [PubMed] [Google Scholar]
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Laukhuf F, Muller-Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J Clin Invest. 1995;96:767–774. doi: 10.1172/JCI118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Boras T, O'Connor D, Meintzer MK, Heidenreich KA, Reusch JE. Insulin-like growth factor I-mediated activation of the transcription factor cAMP response element-binding protein in PC12 cells. Involvement of p38 mitogen-activated protein kinase-mediated pathway. J Biol Chem. 1999;274:2829–2837. doi: 10.1074/jbc.274.5.2829. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Samadfam R, Xia Q, Miao D, Hendy GN, Goltzman D. Exogenous PTH and endogenous 1,25-dihydroxyvitamin D are complementary in inducing an anabolic effect on bone. J Bone Miner Res. 2008;23:1257–1266. doi: 10.1359/jbmr.080318. [DOI] [PubMed] [Google Scholar]
- Sellmeyer DE, Black DM, Palermo L, Greenspan S, Ensrud K, Bilezikian J, Rosen CJ. Hetereogeneity in skeletal response to full-length parathyroid hormone in the treatment of osteoporosis. Osteoporos Int. 2007;18:973–979. doi: 10.1007/s00198-007-0336-x. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Shen L, Glowacki J, Zhou S. Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp Cell Res. 2011;317:1796–1803. doi: 10.1016/j.yexcr.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Kawaguchi H, Higashikawa A, Hirata M, Miura T, Saito T, Nakamura K, Chung UI, Ogata N. Mechanisms underlying catabolic and anabolic functions of parathyroid hormone on bone by combination of culture systems of mouse cells. J Cell Biochem. 2010;109:755–763. doi: 10.1002/jcb.22454. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone. 1995;16:357–365. doi: 10.1016/8756-3282(94)00051-4. [DOI] [PubMed] [Google Scholar]
- Winn SR, Randolph G, Uludag H, Wong SC, Hair GA, Hollinger JO. Establishing an immortalized human osteoprecursor cell line: OPC1. J Bone Miner Res. 1999;14:1721–1733. doi: 10.1359/jbmr.1999.14.10.1721. [DOI] [PubMed] [Google Scholar]
- Wong MS, Tembe VA, Favus MJ. Insulin-like growth factor-I stimulates renal 1, 25-dihydroxycholecalciferol synthesis in old rats fed a low calcium diet. J Nutr. 2000;130:1147–1152. doi: 10.1093/jn/130.5.1147. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- Zhang M, Poplawski M, Yen K, Cheng H, Bloss E, Zhu X, Patel H, Mobbs CV. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000245. e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006;7:51. doi: 10.1186/1471-2202-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Bueno EM, Kim SW, Amato I, Shen L, Hahne J, Bleiberg I, Morley P, Glowacki J. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00717.x. 2011 Apr 25. doi: 10.1111/j.1474-9726.2011.00717.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. J Biol Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]