Abstract
Fast excitatory synaptic transmission is mediated by AMPA-type glutamate receptors (AMPARs). It is widely accepted that the number of AMPARs in the postsynaptic density (PSD) critically determines the efficiency of synaptic transmission, but an unappreciated aspect of synapse organization is the lateral positioning of AMPARs within the PSD, that is, their distribution across the face of a single synapse. Receptor lateral positioning is important in a number of processes, most notably because alignment with presynaptic release sites heavily influences the probability of receptor activation. In this review, we summarize current understanding of the mechanisms that dynamically control the subsynaptic positioning of AMPARs. This field is still at early stages, but the recent wave of developments in super-resolution microscopy, synapse tomography, and computational modeling now enable the study of lateral protein distribution and dynamics within the nanometer-scale boundaries of the PSD. We discuss data available measuring the lateral distribution of glutamate receptors and scaffold proteins within the PSD, and discuss potential mechanisms that might give rise to these patterns. Elucidating the mechanisms that underlie the lateral organization of the PSD will be critical to improve our understanding of synaptic processes whose disruption may be unexpectedly important in neurological disorders.
1. Introduction
The spatial organization of receptors at individual synapses establishes the efficacy of baseline neurotransmission and permits appropriate forms of neural plasticity that are driven by these levels of activity. If structural organization of the synapse is disrupted, synaptic function is acutely compromised. Over longer periods, the consequently altered regimes of plasticity can cascade to developmental and lifelong pathology. So what forms of synaptic structural organization are central to synapse function? Undoubtedly, the alignment of postsynaptic receptors with sites of neurotransmitter release is the defining feature of synaptic structure. On the presynaptic side of the cleft, mechanisms of vesicle fusion and recycling rates must maintain release at appropriate rates at appropriate sites. On the other side of the cleft, receptors must be concentrated appropriately to maintain their level of activation. At central glutamatergic synapses, the number of AMPA receptors (AMPARs) is tightly controlled, and subtly modulated during numerous forms of synaptic plasticity (Malinow and Malenka, 2002). Indeed, receptors continually enter and leave synapses (Triller and Choquet, 2008), and we know a great deal about the biosynthesis, trafficking, and degradation mechanisms that regulate the supply of receptors able to be incorporated to the synapse (Kennedy and Ehlers, 2006; Shepherd and Huganir, 2007). The labile nature of receptors emphasizes the need to understand the mechanisms at the synapse that counteract receptor diffusion and concentrate receptors at this critical position. These mechanisms are the sine qua non of the postsynaptic density (PSD), the ultimate organizer of postsynaptic function.
The importance of the PSD is clear, as molecular disruption of the PSD causes devastating phenotypes (Bangash et al., 2011; Bayes et al., 2011; Peca et al., 2011; Wang et al., 2011), and achieving an intimate understanding of PSD assembly and interior organization is key to any complete view of synapse function. Accordingly, molecular constituents and biochemical characteristics of the PSD have been intensively investigated and reviewed elsewhere (Okabe, 2007; Sheng and Hoogenraad, 2007). In this review, we examine intrinsic features of the PSD that are less well understood, highlighting that the PSD is not a uniform, regular structure, but contains a great deal of organizational specificity along the plane parallel to the synaptic membrane. We consider this the “lateral” organization of the PSD, to distinguish it from its “vertical” or “laminar” organization in the pre-to-postsynaptic axis orthogonal to the membrane.
Though many synaptic constituents including adhesion molecules, ion channels, and diverse receptor types may be found in unique and important patterns across the lateral extent of the synapse, in this review, we will focus on the lateral distribution of AMPARs because their positioning is expected to have particularly important consequences for synapse function (Newpher and Ehlers, 2009). We discuss specifically those receptors that are present in the synapse within the PSD, and we leave aside perisynaptic or extrasynaptic receptors whose importance is reviewed elsewhere (see Bard and Groc; Gladding and Raymond, this issue). We consider potential mechanisms that can control the distribution of AMPARs within the synapse, including in some detail the PSD scaffold proteins most likely to accomplish this, and then outline recent technical developments that offer hope for understanding roles of dynamic lateral organization of the PSD in health and disease.
2. Lateral distribution of synaptic glutamate receptors
2.1. Functional consequences of subsynaptic receptor distribution
When glutamate is released from a presynaptic vesicle, glutamate concentration within the synaptic cleft rises to millimolar levels (Clements et al., 1992). Though the peak of this spike is very high (1-3 mM), the transient is narrow (<200 nm) and dissipates substantially from diffusion and buffering within ~100 μsec (Bergles et al., 1999; Diamond and Jahr, 1997). Importantly, the EC50 of glutamate for activating AMPARs is remarkably low, ranging from ~0.5 mM to nearly 2 mM for different subtypes (Traynelis et al., 2010), and thus receptors displaced from the site of release likely experience concentrations of glutamate insufficient to open them effectively. Just as critically, the probability of channel opening is steeply dependent on the number of bound agonists (Rosenmund et al., 1998), and on the kinetics of transitions to open or desensitized states (Robert and Howe, 2003). Because of these biophysical factors, numerical modeling suggests that while AMPA receptors directly across from the site of release open with fairly high probability (~0.6), receptors laterally displaced by even 50 nm are much less likely to open (Franks et al., 2003; Raghavachari and Lisman, 2004; Xie et al., 1997). In fact, single release events may activate just a fraction of the total number of AMPARs at the synapse: at relatively large synapses, the activated hotspot may cover as little as 25% of the PSD (Lisman and Raghavachari, 2006; Lisman et al., 2007). Thus, not just the total number of AMPARs in a synapse, but also the local density of AMPARs within synapse subdomains may determine the efficiency of synaptic transmission (Fig. 1).
Figure 1.
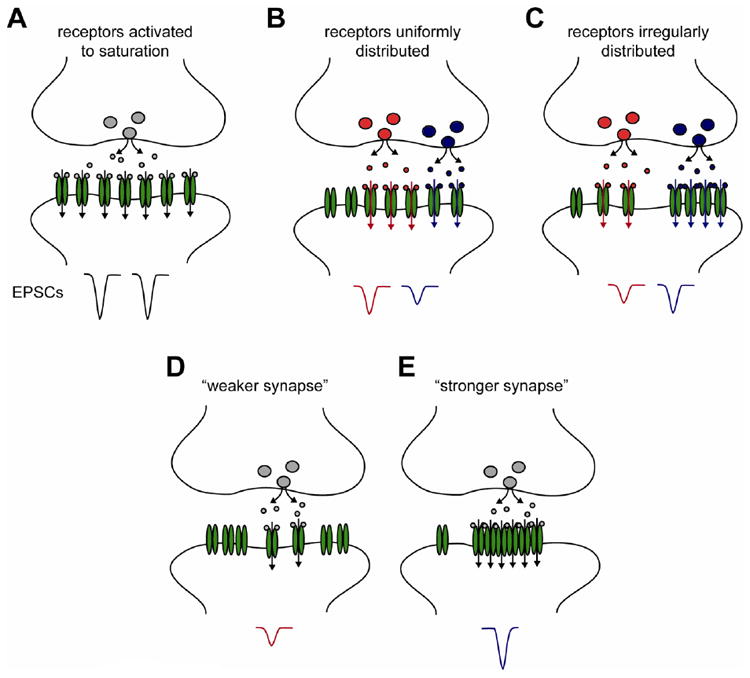
(A) Side-view of a synapse where a single release event activates all postsynaptic AMPARs (green), which are shown as randomly positioned. Vertical arrows indicate opened channels. Release from sites anywhere in the presynaptic active zone will elicit EPSCs (below) with little variance. (B) A synapse for which a single release event activates only a subset of randomly positioned receptors. A release event in the center of the synapse (in red) is likely to activate more receptors and trigger a more robust postsynaptic current than a release site located more peripherally (blue). (C) In contrast, if postsynaptic AMPARs distribution is not random, the local density of receptors at the release sites, not simply their number, will determine the amplitude of the EPSC elicited by a release event. Glutamate release aligned with a receptor-sparse region (red), will trigger a smaller EPSC than release aligned with denser region of the synapse (blue). Modulating the alignment of presynaptic release machinery with postsynaptic AMPARs, potentially via transsynaptic mechanisms involving the receptors themselves, is thus a potential mechanism to decrease (D) or increase (E) synaptic strength.
Physiological evidence consistent with this notion of limited receptor activation has been obtained at a variety of synapses (Frerking and Wilson, 1996). Notably, single release events fail to activate all synaptic AMPARs in cultured hippocampal neurons (Liu et al., 1999; McAllister and Stevens, 2000), as indicated by a larger amplitude and lower variance of responses to glutamate applied iontophoretically at the synapse. At the calyx of Held in acute brainstem slices, the concentration of glutamate in the presynaptic terminal is limiting, such that adding exogenous glutamate via a presynaptic patch pipette increases the response to spontaneous vesicle fusion (Yamashita et al., 2009). Likewise, at cerebellar mossy fiber–granule cell connections composed of a single release site, receptors appear well below saturation, and the quantal variance is high (Sargent et al., 2005).
Given the computational and experimental support for the idea of receptor subsaturation, much of this review is concerned with whether AMPARs are uniformly distributed across the PSD. Importantly, if at a given synapse receptors are not spatially uniform, then the impact of receptor subsaturation will likely be much higher. Consider first a synapse in which sites of vesicular fusion are randomly distributed across the presynaptic membrane. At this synapse, if AMPARs are maximally activated to open following fusion of a single vesicle, then the EPSC amplitude will depend simply on the number of receptors in the PSD; response variance event to event will be quite low, and both the release location and receptor subsynaptic pattern will matter little for response amplitude (Fig. 1a). On the other hand, if receptors in only a subregion of the synapse are activated to open after release, then the distribution of receptors matters quite a bit. If receptors are uniformly distributed, then EPSCs resulting from release events at the center or edge of the synapse will potentially differ in amplitude, for instance, because the number of receptors experiencing high glutamate concentrations will differ (Fig. 1b). Conversely, if receptors are not uniformly distributed (Fig. 1c), then the position of release will have an additional impact on EPSC amplitude: the EPSC amplitude will be large for events aligned over receptor-dense regions of the PSD, but smaller for events occurring over receptor-sparse regions. Presumably, if considered over a large number of responses, the average EPSC amplitude of synapses in Figs. 1b and 1c will be nearly the same, though each will be smaller than synapse in Fig. 1a by a factor corresponding to the average fraction of synaptic receptors that are activated; however, the variance at the synapse in Fig. 1c will be higher.
At some synapses, physiological recordings indicate that the distribution of receptors may in fact play little functional role. For instance, at the massive and complex cerebellar climbing fiber synapse onto Purkinje neurons, response variance is very low apparently in part because of nearly saturated receptors (Foster et al., 2002). Indeed, even at large synapses where single vesicle responses may be subsaturating, the release of more than one vesicle following an action potential can serve to activate additional receptors (Meyer et al., 2001; Tong and Jahr, 1994). Interestingly, other parameters of the synapse, such as the cleft geometry (Cathala et al., 2005) or the extracellular factors influencing glutamate diffusion (Nielsen et al., 2004) can additionally influence whether all AMPA receptors are activated following release from a single vesicle. This hints at the possibility of even greater complexity.
Perhaps the most important addition to consider is if we adapt our hypothetical synapse to include the feature that sites of vesicle fusion are not randomly distributed, for instance at sparse but nearly immobile protein ensembles in the presynaptic membrane (Schoch and Gundelfinger, 2006; Ziv and Garner, 2004). Presuming receptor non-saturation, EPSC amplitude will then depend strongly on whether release is biased to occur more frequently over dense or sparse regions (Figs. 1d, 1e). Indeed, the average EPSC amplitude (or its variance) may in fact be either larger or smaller than synapses in Figs. 1b and 1c. Thus, it is apparent that transsynaptically linking the release machinery to postsynaptic sites of receptor abundance could be a very effective means of assuring a strong and low-variance synaptic connection. Indeed, modification of such linkage would seem to be a molecularly straightforward and potentially rapid means to raise or lower synaptic strength.
Overall, these theoretical and physiological observations make clear that the lateral distribution of AMPARs within the PSD provides a potent mechanism for modulating synaptic strength. While this receptor pattern may be just one of many factors that control synapse response amplitude and variability, detailed knowledge of the lateral distribution of receptors—and whether it is able to be modified—is thus important for understanding synapse functional organization. We note that the lateral organization of other components of the PSD dictate numerous other functional characteristics worthy of review, for instance controlling adjacency of kinases or phosphatases and their effectors, positioning proteins within calcium nanodomains, and modulating near-neighbor relationships of oligomerizing proteins. For reasons of brevity here, we will focus strictly on the lateral distribution of AMPARs, and we hope that the principles we discuss will generalize to the study of other synaptic proteins
2.2 Subsynaptic pattern of AMPA receptor distribution
Measuring the pattern of receptors at a synapse has been a difficult task, as it requires spatial resolution well beyond traditional light microscopy as well as statistically high probability of identifying the receptors. The most broadly used tool for the task has been EM immunogold labeling. This approach has suggested that at least under some circumstances, AMPARs are enriched in the periphery of the PSD. In neocortex, GluA2/3 AMPARs were found to be localized peripherally (Bernard et al., 1997; Kharazia and Weinberg, 1997). Similarly, in striatal medium spiny neurons, GluA1 and 2/3 subunits were found to be enriched away from the center of the synapse (Bernard et al., 1997; Kharazia and Weinberg, 1997). Functionally, this is intriguing, because vesicular fusion events at the center of the synapse would align with a region of low receptor abundance. In addition, it has been suggested that receptors at the PSD edge may be more easily released from the synapse. Such a peripheral pattern may not be universal, however. In spines of hippocampal CA1 neurons, Somogyi et al. measured equally dense GluA2/3 immunoreactivity at the center and edges of PSDs (Somogyi et al., 1998).
Attempting to reduce the 2-dimensional synaptic face to a 1-dimensional measure (such as “distance from the center”) may gloss over important complexity in the receptor distribution. Traditional immunogold EM faces a critical limit in this respect, because the typically sparse labeling necessitates averaging distribution measurements over multiple synapses. Three new approaches offer the possibility of a complete 2-dimensional receptor map. Using immunolabeling of SDS-digested freeze-fracture replicas, the Shigemoto lab has achieved nearly one-to-one antibody labeling of both NMDA and AMPA receptors in the synapse (Tanaka et al., 2005). At parallel fiber synapses onto Purkinje neurons, AMPARs were seen to be highly irregularly distributed within single synapses, frequently forming clusters of receptors within small regions and leaving relatively large areas of the synapse devoid of receptors (Masugi-Tokita et al., 2007). Importantly, such clusters could be found either laterally or centrally at individual parallel fiber synapses, confirming the importance of understanding 2D rather than just radial positioning. This finding has been confirmed in the dorsal lateral geniculate nucleus, where AMPARs in both retinogeniculate and corticogeniculate synapses form very similarly sized clusters (Tarusawa et al., 2009) despite the synapses themselves being greatly different in area. Interestingly, though, the tendency to form clusters is not universal: the receptor pattern at climbing fiber synapses onto Purkinje neurons showed no evidence of this patterning (Masugi-Tokita et al., 2007).
EM tomography offers a second approach to mapping receptors at single synapses. In virtual slices of the tomographic image series through PSDs of cultured neurons, Chen et al. (Chen et al., 2011; Chen et al., 2008b) matched the size of electron-dense features in the synaptic membrane to the AMPAR crystal structure. Though not an unequivocal identification of the receptors, it provided a means to examine the distribution of these “AMPAR-like” structures. In the synapses examined, these structures were more peripherally located than the structures presumed to be NMDARs, consistent with immunogold reports of centrally positioned NMDARs (Kharazia and Weinberg, 1997; Perez-Otano et al., 2006; Somogyi et al., 1998). In addition, the distance between receptors varied over a wide range within a synapse, consistent with a clustered distribution. Future refinement of EM tomographic methods (Chen et al., 2008c; Rostaing et al., 2006) should permit unprecedented detail of the distribution of receptors and associated proteins at single synapses.
A third mapping approach avoids the use of the EM at all, by taking advantage of recent super-resolution imaging methods. Using Stochastic Optical Reconstruction Microscopy (STORM; (Huang et al., 2008; Rust et al., 2006) to map the location of individual antibody molecules with ~10 nm precision, Dani et al. analysed the spatial organization of molecules at synapses in perfusion-fixed slices of the accessory olfactory bulb (Dani et al., 2010). The dense labeling achieved in cryostat sections rather than ultrathin EM sections made it possible to examine the protein distribution within individual synapses. Using three-color STORM immunocytochemistry, AMPAR and NMDAR radial displacement from the center of the synapse was mapped in relationship to postsynaptic scaffold markers Homer or Shank. Surprisingly, at different individual synapses within the accessory olfactory bulb, either type of receptor could be found enriched either in the central or peripheral regions of the PSD.
Notably, all three of these methods also offer the hope to map more rigorously the alignment between presynaptic release sites and postsynaptic receptors. Immunolabeled glutamate receptors have been observed located across the cleft from presynaptic dense projections suggestive of release apparatus (Kharazia and Weinberg, 1999). However, systematic analysis of these sites with respect to gradations of receptor density will likely necessitate both very fine-scale receptor mapping and, perhaps even more difficult, a means to identify whether the location of release sites at a single synapse are persistent or transient. As suggested below, however (see section 3.1.2), the identification of transsynaptic molecular linkages may provide additional clues as to whether subsynaptic receptor distribution is related to active zone structure.
The diversity of reported AMPAR distributions suggests that multiple modes of AMPAR positioning are possible. It is intriguing to think that even at a single synapse the receptor pattern may be modulated. Though the methods used to date to map receptor distribution at single synapses (STORM, EM, tomography) rely on tissue fixation and give a static picture, much evidence indicates that net receptor position at a synapse likely varies over time. Indeed, at individual synapses imaged over minutes, the PSD undergoes continuous, actin-driven changes in morphology and internal density (Blanpied et al., 2008) likely to drive changes in subsynaptic AMPAR placement. On the molecular level, recovery after photobleaching of GFP-tagged AMPAR subunits demonstrates that receptors exchange in and out of synapses on the time scale of seconds to minutes (Ashby et al., 2006; Makino and Malinow, 2009; Sharma et al., 2006). Further, single-molecule tracking of antibody-labeled receptors has made clear that even within the synapse, the lateral position of at least some receptors is constantly shifting (Tardin et al., 2003; Triller and Choquet, 2008). Intriguingly, the proportion of the synapse covered by mobile receptors is typically rather small, but can be regulated by synaptic activity (Ehlers et al., 2007; Triller and Choquet, 2008). However, the proportion of receptors so immobilized is difficult to quantify based on antibody labeling because antibodies might be presumed to have easier access to extrasynaptic receptors. Thus, it will be important to develop methods to measure receptor patterning at living synapses. Because this will likely at first require the use of neurons grown in culture, additional effort will eventually be required to synthesize information from more intact preparations.
In summary, immunocytochemical evidence has made clear that AMPARs within a single synapse can be found in distinctive non-random distributions, and that this pattern can vary between synapses and between synapse types. Subdomains of high and low receptor density are very likely to mediate large and small postsynaptic responses to glutamate release aligned with them, at synapses where postsynaptic receptors are not saturated by quantal glutamate release. Importantly, however, it is unclear whether the distribution pattern of receptors at a single synapse is maintained for long periods, or whether it is actively modified. Dynamic modulation of postsynaptic receptor pattern would offer a potentially powerful level of regulation over synaptic strength.
3. Possible mechanisms controlling subsynaptic AMPAR distribution
What are the mechanisms that could give rise to a distinctive spatial distribution of AMPARs within the PSD? In the following sections, we will discuss several possibilities. As depicted in Fig. 2, we most importantly differentiate between mechanisms that rely on protein-protein interactions between receptors and PSD scaffolding molecules (section 3.1), and mechanisms that confine receptor position without direct protein-protein interactions (section 3.2). These are not mutually exclusive, and we favor the idea that synapse organization and plasticity likely incorporate both types of mechanism.
Figure 2.
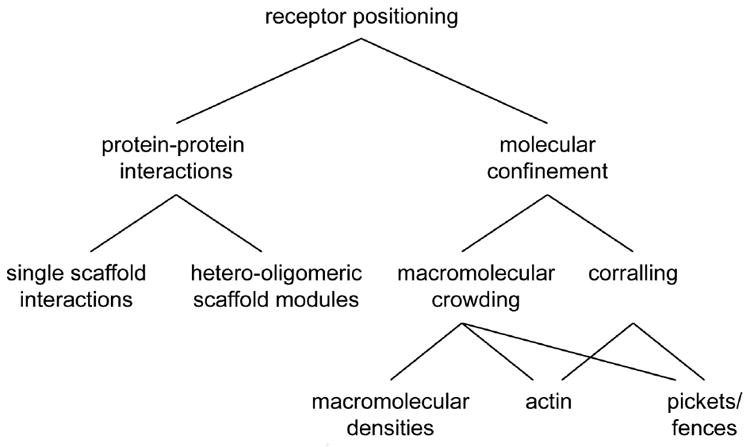
A scheme outlining different mechanisms that can control glutamate receptor positioning, as discussed in this review.
3.1 Protein-protein interaction mechanisms
3.1.1 Can AMPARs be positioned by a single scaffold protein
The most straightforward mechanism is that a single PSD anchoring molecule determines the position of AMPARs within the PSD directly via protein-protein interactions. A number of candidate scaffold proteins for this mechanism have been proposed, including the directly AMPAR-interacting PDZ-containing proteins GRIP/ABP, SAP97 and PICK1 (Dong et al., 1997; Leonard et al., 1998; Xia et al., 1999). Indeed, mutations that disrupt receptor interaction with these proteins reduce the synaptic content of AMPARs; however, these effects are likely explained by defects in synapse delivery, rather than retention (Osten et al., 2000). By far the best candidate is PSD-95, the prototypical membrane-associated guanylate kinase (MAGUK) family member. PSD-95 interacts with AMPARs via their auxiliary TARP subunits (Transmembrane AMPAR Regulatory Proteins (Chen et al., 2000), and although TARP subunits promote surface expression of AMPARs, TARP interaction with PSD-95 is further required for the synaptic retention of AMPARs (Bats et al., 2007; Schnell et al., 2002). Moreover, overexpression of PSD-95 increases, while acute knockdown decreases synaptic AMPAR content and AMPAR-mediated currents (Ehrlich et al., 2007; El-Husseini et al., 2000b; Elias et al., 2006; Schluter et al., 2006). These results have been interpreted to imply that alterations in synaptic AMPAR numbers during synaptic plasticity are determined by changes in PSD-95 content. The notion of ‘slots’ was suggested to indicate that a PSD contains a limited capacity for holding receptors, and that if the number of slots is altered (i.e. by adding or subtracting proteins that make up the slots), then the number of synaptic receptors is consequently varied (Lisman and Raghavachari, 2006; Malinow and Malenka, 2002; Shi et al., 2001). Indeed, activity-dependent depalmitoylation or ubiquitination reduce levels of both PSD-95 and AMPARs at the synapse (Colledge et al., 2003a; El-Husseini et al., 2002). However, preventing dissociation of PSD-95 from the membrane does not necessarily block the induction of long-term depression (Xu et al., 2008). Similarly, though increases in PSD-95 might be expected to precipitate receptor accumulation during LTP, there is no clear evidence for an increase in synaptic PSD-95 content during LTP induction. Alternatively, it may not be the absolute number of PSD-95 molecules that is modified during induction of LTP or LTD, but instead their affinity for receptors or TARPs. In fact, such affinity can be regulated by post-translational modifications. For instance, activity-dependent phosphorylation of the TARP stargazin by CaMKIIα controls its interaction with PSD-95 (Opazo et al., 2010; Sumioka et al., 2010). It is thus clear that PSD-95 has a critical role in retaining AMPARs within the PSD, though may not be a stand-alone “slot” or the sole determinant of receptor numbers.
Aside from any effect on receptor number, does PSD-95 position receptors when they are at the synapse? If this were the case, its lateral distribution would be expected to match the observed receptor distribution. Several studies have examined the distribution of PSD-95 across the face of the PSD, and asked whether it is uniform or clustered, as with the distribution of AMPARs. Using immunogold EM, PSD-95 has in general been found fairly uniformly across the synapse, with of course a steep decrease at the border of the PSD (Sassoe-Pognetto et al., 2003; Valtschanoff and Weinberg, 2001; Zhang and Diamond, 2009). However, these studies average the sparse immuno-labeling densities over multiple synapses, and substantial but irregular variations in the distribution can easily be missed. From analysis of isolated PSDs following biochemical fractionation, MAGUK proteins PSD-95 and PSD-93 appear to be non-uniform as judged by statistical analysis of immunogold labeling (DeGiorgis et al., 2008; Swulius et al., 2010), suggesting a clustered assembly of PSD constituents. So, although low labeling densities can make such analyses difficult (and the effects of fractionation are yet unknown), it is intriguing to consider that at least a subpopulation of PSD-95 in the PSD is assembled in clusters that can retain high concentrations of AMPARs
With EM tomography, some proteins can be identified based upon their structural characteristics rather than antibody labeling, so the technique may serve as a powerful means to map protein location in single synapses. As reported by EM tomography, PSD-95 in the PSDs of cultured hippocampal neurons adopts a distinctive, extended structure, orientated “vertically” in the PSD (Chen et al., 2011; Chen et al., 2008b) with its N-terminal cysteines palmitoylated and engaged with the membrane (El-Husseini et al., 2000a). Virtually every AMPAR-like structure in the PSD is closely associated with one of these vertical filaments likely to be PSD-95. Moreover, RNAi-mediated reduction of PSD-95 protein levels results in a “patchy” loss of the protein from individual synapses, and the areas of these synapses with sparser PSD-95 have fewer AMPARs (Chen et al., 2011). This provides strong evidence that the distribution of PSD-95 is central to determining the pattern of AMPARs. To a first approximation, the vertical filaments themselves appear rather uniformly spaced (with a low variance of interprotein distance), though this has not been statistically tested in tomography data. It will be of interest to develop assays that can measure simultaneously the distribution of scaffold and receptor at high labeling density and high resolution in living synapses.
Nevertheless, despite the clear participation of PSD-95 in AMPAR positioning, it remains unlikely that PSD-95 alone directly determine the spatial distribution of receptors within the PSD. A means of establishing the distribution of PSD-95 at the synapse in absence of other proteins has not been described, and PSD-95 is certainly assembled into macromolecular scaffold complexes with other proteins that are reciprocally dependent on PSD-95 expression levels (Okabe, 2007). Indeed, following PSD-95 RNAi, patches of the synapse with sparse PSD-95 show loss of other proteins as well, including those that typically appear in the tomographic images as “horizontal filaments” likely to interact with and cross-link the vertical filaments (Chen et al., 2011). In addition, PSD-95 does not exhibit free lateral motion within the PSD (Blanpied et al., 2008) whereas synaptic AMPARs can be quite mobile, so it seems inevitable that receptors do not remain bound to PSD-95 at all times within the synapse. For these reasons, we next consider higher-order interactions in the PSD that might govern receptor distribution.
3.1.2 Hetero-oligomeric scaffold modules
PSD-95 and the other MAGUKs contain multiple protein interaction domains, each of which can engage in several potential interactions, suggesting that they may assemble oligomeric complexes (Okabe, 2007; Sheng and Sala, 2001; Xu, 2011). Such putative assemblies of multiple scaffold proteins, here referred to as modules, would provide a high local density of AMPAR interaction sites, and thus facilitate clustering of receptors (Fig. 3A). Some evidence for modular structural organization within the synapse, beyond biochemical and molecular characterization studies, has already emerged. The PSD contains a prominent laminar organization parallel to the plane of the synaptic membrane (i.e. along the axo-dendritic axis). PSD-95 is situated closest to the synapse (Petersen et al., 2003; Valtschanoff and Weinberg, 2001) in a vertical orientation (Chen et al., 2008a). In a deeper layer are found Shank and GKAP (Petersen et al., 2003; Valtschanoff and Weinberg, 2001), potentially oriented in an extended, horizontal conformation (Chen et al., 2008a). The relative axo-dendritic positions of PSD-95 and Shank has recently been confirmed by 3D STORM (Dani et al., 2010).
Figure 3. Two classes of mechanisms that may control AMPAR positioning in the synapse.
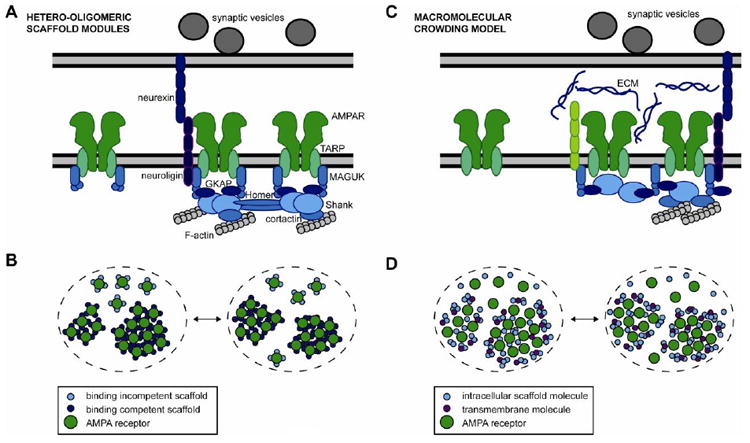
(A) Side view of a synapse, showing hetero-oligomeric scaffold modules controlling the position of AMPARs by direct protein-protein interactions. In the binding model, AMPAR/TARP complexes are stably anchored by interacting with MAGUK proteins such as PSD-95. The multimeric MAGUK proteins and other scaffold molecules can engage in multiple interactions with each other, and can assemble into hetero-oligomeric scaffold modules. PSD-95 forms complexes with GKAP and Shank molecules that interact with the actin cytoskeleton via cortactin and other intermediates. Intramolecular interactions between the SH3 and GK domains of MAGUKs potentially control oligomerization of numerous components of scaffold modules, as well as signaling molecules (not shown). Drawings are not to scale. (B) Top view of a synapse, showing hetero-oligomeric scaffold modules organizing the lateral distribution of AMPARs in the synapse. Modification of the binding competency of scaffolding molecules can change their oligomerization capability (light vs. dark blue small circles) and regulate interactions with AMPARs over time. (C) Side view of a synapse, showing different sources of macromolecular crowding. Scaffolding molecules close to the membrane can reduce AMPAR mobility, even if binding to receptors is rare. Postsynaptic transmembrane proteins such as neuroligin and cadherins may serve as obstacles to receptor motion, or alternatively may form structures more reminiscent of fences that subdivide the PSD. Transmembrane or extracellular proteins contribute to macromolecular crowding in the synaptic cleft, where the bulky extracellular domain of the AMPAR may increase obstruction of receptor motion. (D) Macromolecular crowding by various proteins (small circles) as in C can retain receptors within the synapse and within synapse subdomains over time. Note that the same distribution of receptors as in B could be produced by a differential distribution of obstructions. Both mechanisms shown in B and D, macromolecular crowding and binding to hetero-oligomeric scaffold modules, likely operate in tandem to determine receptor lateral distribution.
The extreme cytosolic face of the PSD is complex and irregular, much moreso than its membrane-directed face (Petersen et al., 2003). This topography partly reflects the association of diverse molecules such as CaMKII (Petersen et al., 2003), but likely also groups of scaffold molecules (Rostaing et al., 2006). Interestingly, it may be that through these modules, connections to the actin cytoskeleton (Capani et al., 2001; Fifkova and Delay, 1982) are established. Indeed, recent tomography confirms that the irregular cytoplasmic side of the PSD comprises cytoplasmic-facing ‘spikes’ that are frequently in contact with filamentous actin (RJ Weinberg, personal communication).
Given these apparent links to actin at specific points in the PSD, the cytoskeleton is well positioned to regulate the assembly or maintenance of PSD modules. Indeed, actin destabilization with latrunculin causes the rapid loss of a substantial fraction of synaptic GKAP, Shank and Homer molecules (Kuriu et al., 2006). Interestingly, the remaining fraction of these molecules (as well as the complete population of synaptic PSD-95 molecules) is unaffected in terms of their molecular exchange rate measured by recovery after photobleaching. This indicates that different subpopulations of scaffolds exist in the PSD that are differentially controlled by actin, though whether these molecular subsets are organized in any spatial manner has not been determined. It is also possible that an actin-based mechanism would actively mix or segregate synapse components, if actin filament turnover applies force directly to modules within the PSD. Consistent with this idea, actin adjacent to the synapse has notably high polymerization-driven dynamics when compared with other regions of the spine (Frost et al., 2010b). This polymerization apparently produces considerable force, because individual PSDs undergo rapid, constitutive changes in morphology as measured by time-lapse imaging of PSD-95-GFP, and these changes are sensitive to disruption of actin treadmilling (Blanpied et al., 2008). This reinforces the idea that actin may directly regulate protein modules within the PSD, raising the possibility that the dynamics of actin filaments, rather than just their presence, may be required to maintain these domains.
The location of PSD-95 at the plasma membrane facilitates its binding to a complement of adhesion molecules such as neuroligins, LRRTMs, and SALMs (de Wit et al., 2009; Han and Kim, 2008; Irie et al., 1997). While these proteins have a clearly demonstrated role in synaptogenesis (Linhoff et al., 2009; Scheiffele et al., 2000) or development of proper balance of excitatory and inhibitory synaptic inputs (Chih et al., 2005), the adhesion complexes continue to be expressed beyond development and may play additional roles. For example, it has been shown that the neuroligin-PSD-95 complex promotes synaptic efficacy by a transsynaptic mechanism, apparently via neurexin (Futai et al., 2007). As a result, an important role for adhesion complexes at mature synapses could be to regulate subsynaptic receptor positioning by participating in the creation or alignment of modules within the synapse. Of particular interest is whether or how scaffold-adhesion complexes at a single synapse coordinate postsynaptic receptor positioning with presynaptic sites of glutamate release, but there is little evidence to date on this question. Recently, it was reported that AMPARs, specifically the N-termini of GluA2 subunits, enhance the synaptogenic effect of neuroligin (Ripley et al., 2011). This suggests that AMPARs themselves also have the ability to influence transsynaptic organization. Extrapolating even further, it is intriguing to speculate that such molecular bridges emanating from the receptors themselves would be ideally suited to coordinate the alignment of receptors with release sites (Fig. 1d,e).
Changes to the content or organization of oligomeric scaffold modules at the synapse could alter synaptic transmission by changing either receptor number or distribution. Indeed the induction of depression or potentiation is associated with scaffold modifications. NMDA-induced LTD triggers the loss of PSD-95 (Colledge et al., 2003b), an effect which depends on calcineurin activation (Horne and Dell’Acqua, 2007), proteolytic attack of the N-terminal region of PSD-95 (Xu et al., 2008) and its first two PDZ domains (Sturgill et al., 2009). The C-terminal tandem SH3-GK domains of PSD-95 are also important for LTD induction (Bhattacharyya et al., 2009; Xu et al., 2008). These domains can form an intramolecular complex (McGee et al., 2001; Tavares et al., 2001) that occludes binding of the SH3 domain to other ligands, and regulating this interaction may switch PSD-95 molecules from a self-clustered to an open conformation enabling recruitment of other ligands (Feng and Zhang, 2009). Such a switching mechanism could reorganize receptor-module complexes (Fig. 3B), potentially triggering changes in properties of receptors, receptor distribution or stability in the PSD, or the recruitment of other effectors such as AKAPs or calcineurin (Bhattacharyya et al., 2009; Xu et al., 2008). Intriguingly, phosphorylation of PSD-95 by CaMKII during induction of LTP also destabilizes it in the PSD, and in addition facilitates the activity-dependent loss of Shank2 (Steiner et al., 2008). Loss of Shank presumably alters cytoskeletal mechanisms within the spine that control LTP-associated spine growth (Qualmann et al., 2004). The counter-intuitive loss of scaffolds is only transient, however, as each is regained during the more stable phase of spine enlargement and functional strengthening. This suggests that some forms of protein reorganization require disruption or removal of existing complexes before establishment of the new order. In sum, subsynaptic reorganization of scaffold modules may regulate synaptic strength through diverse mechanisms involving control of receptor number and distribution, the efficiency and specificity of downstream signaling cascades, or control by the actin cytoskeleton.
3.2 Molecular confinement
3.2.1 Corrals
Aside from protein binding interactions, additional mechanisms may contribute to receptor positioning. For example, the clustering of membrane proteins can arise from formation of specialized domains within the plasma membrane by “fences” or “corrals” (Kusumi et al., 2005). These form the barriers to free diffusion that lead to accumulation and/or maintenance of receptor clusters (Saxton, 1995; Triller and Choquet, 2008). Several computational models have been developed to test whether a synaptic corral could explain observed characteristics of AMPAR dynamics, including the time receptors remain at the synapse and the size of the domain in which receptors are free to diffuse before encountering a barrier. Holcman and Triller (2006) modeled the PSD by reducing its evident complexity to two compartments: an internal disk with bound or free receptors, and a surrounding annulus where receptor movement is limited by obstacles. By positing a boundary surrounding the PSD with a small opening permitting infrequent receptor escape, their model could reproduce fluorescence recovery after photobleaching (FRAP) data measured for AMPA receptors. An extension of this framework modeled the variability in synaptic AMPA receptor number (Bressloff and Earnshaw, 2009) by considering the inclusion of a stochastic gate that allows receptor escape when opened. However, the major shortcoming of such models to date is that the interior of the PSD is treated as a homogenous compartment, contrary to observed motion of receptors within single PSDs (Triller and Choquet, 2008). Because they eliminate internal structure, these existing models cannot be used to test potential explanations of lateral organization. Consequently, models that incorporate realistic geometry of the synapse will be required.
The simplest potential molecular substrate of a synaptic corral is a fence formed by perisynaptic actin. However, both EM and live-cell imaging demonstrate that actin directly at the PSD itself is sparse (Fifkova and Delay, 1982; Frost et al., 2010b), perhaps even less dense than elsewhere in the spine, and no evidence suggests an enrichment of filaments perisynaptically. Furthermore, when F-actin is pharmacologically disrupted for tens of minutes to hours some synaptic AMPARs are lost (Allison et al., 1998; Gu et al., 2010; Rust et al., 2010; Zhou et al., 2001), but the majority of synaptic AMPA receptors are retained. Thus, actin is not likely to act as a corral to retain synaptic AMPA receptors (though even sparse filaments may play a more general role in molecular crowding (see section 3.2.2). Moreover, a perisynaptic fence could only regulate total synaptic AMPAR content, and not subsynaptic receptor positioning. Thus it cannot account for the fact that single receptors are sometimes confined to areas smaller than the synapse itself (Ehlers et al., 2007; Heine et al., 2008). To date, there is no direct ultrastructural evidence for a corral at the synapse edge. Instead, there is a longstanding appreciation for a mesh or lattice-like organization of the PSD interior (Adam and Matus, 1996; Carlin et al., 1980); (Chen et al., 2011). This raises the possibility that a more relevant mechanism for AMPAR corralling could occur at the level of subsynaptic partitioning. In this case, certain synapse proteins could form small corrals to retain populations of AMPARs at particular synapse locations by retaining them within the interstices of this lattice. In some ways, this is the opposite of the scaffold module mechanism presented above which would predict receptors to be localized to the nodes of the lattice. Future experiments will be required to discriminate these possibilities.
3.2.2 Macromolecular crowding as means to control receptor positioning
The densely packed nature of the synapse suggests that PSD-resident proteins could influence the positioning of AMPARs by acting as a series of barriers to lateral diffusion within the synapse (Fig. 3C). To directly address this possibility, Santamaria et al. (2010) utilized computational modeling to simulate PSDs crowded with proteins either able to or unable to bind receptors. Remarkably, even in PSDs devoid of receptor-binding proteins, synaptic receptors were still retained for minutes to hours, simply through the extreme reduction of diffusion by molecular crowding. Consistent with a crowding mechanism, the mobility not just of receptors is reduced inside synapses compared to the extrasynaptic membrane. Even glycophosphatidylinositol-anchored probes (present only in the extracellular leaflet of the plasma membrane and unlikely to bind any synaptic proteins) as well as the endogenous membrane lipid GM1, show reduced diffusion and confinement within the synapse (Renner et al., 2009). Intriguingly, simulations of Santamaria et al. (2010) that included specific binding partners for AMPARs promoted a counterintuitive enhancement of receptor exchange, presumably because the affinity of AMPARs for their known binding partners is rather low, while the efficacy of elastic collisions to confine AMPARs in crowded portions of the synapse can be very high. Thus, during times when receptor exchange rates are modulated, e.g. during expression of LTP or LTD, specific AMPAR interactions may most likely be subject to regulation, whereas at the steady state, non-specific interactions within the crowded PSD may provide an effective mechanism to trap receptors in subregions of the synapse (Fig. 3d).
Many different classes of molecules could participate in crowding: transmembrane proteins (e.g. receptors, ion channels), molecules in the cleft (e.g. adhesion molecules or even presynaptic transmembrane proteins), and intracellular proteins near the postsynaptic membrane (e.g. PSD scaffolds). NMDA receptors bind directly to PSD-95 (Niethammer et al., 1996) and would be optimally positioned to contribute to local receptor crowding, but their limited numbers and apparently central location suggest that they may not be important determinants of AMPAR movements. Still, NMDARs may act collectively with other neurotransmitter receptors and voltage-gated channels to limit the passage of AMPARs in certain synaptic regions. Because AMPARs have a very bulky extracellular domain (Sobolevsky et al., 2009) that must navigate through a potentially tortuous environment in the synaptic cleft, it might be expected that even non-specific extracellular interactions will substantially obstruct diffusion. For example, consider an intriguing candidate from the extracellular space. It was recently shown that the extracellular matrix (ECM) regulates AMPAR diffusion and that degrading the ECM increased the lateral diffusion of extrasynaptic receptors and accelerated their exchange into and out of synapses (Frischknecht et al., 2009). Interestingly, in spite of this increase in receptor exchange, the fraction of receptors immobilized at synapses did not change, nor did the diffusion properties of the synaptic receptors themselves. Thus, while the ECM is important for regulating exchange of receptors, it is unlikely to play a substantial role in subsynaptic clustering. More broadly, the synaptic cleft is certainly rich with proteins, but appears not as densely packed as the PSD (RJ Weinberg, personal communication) so the overall contribution to crowding by cleft proteins may not be more important than transmembrane or postsynaptic molecules.
Some adhesion molecules have clear means to be positioned at locations within the synapse near AMPARs and thus influence their subsynaptic positioning. For example, N-cadherin interacts directly with an extracellular domain of the AMPAR GluA2 subunit (Saglietti et al., 2007), making it a transmembrane barrier to AMPAR lateral diffusion that can also bind directly to the receptors themselves. Similarly, the transmembrane protein neuroligin directly binds to PSD-95, and may be positioned in close proximity to AMPARs. Finally, while some components of the PSD scaffold can bind AMPARs directly or via AMPAR auxiliary subunits, many PSD scaffold components do not. Given the dense packing of these proteins, they are likely to substantially crowd the intracellular portions of synaptic AMPARs. Thus, it will be important for future experiments to develop new approaches to separately test mechanisms of macromolecular crowding stemming from the extracellular, transmembrane, and intracellular portions of AMPARs and whether the local crowding within synapses can be dynamically regulated (Fig. 3d).
Finally, an interesting and largely unexplored potential avenue for generating subsynaptic protein domains is via membrane lipids. Membrane specializations such as membrane rafts are critical for the organization and compartmentalization of receptors and downstream signaling cascades in many systems. Excitatory synapses also contain markers of membrane rafts (Delint-Ramirez et al., 2010; Swanwick et al., 2009), and biochemical studies have found that many PSD components, including glutamate receptors, reside in synaptic membrane rafts (Hering et al., 2003; Hou et al., 2008), and PSD-95 (Delint-Ramirez et al., 2010; Suzuki et al., 2008) by virtue of its N-terminal palmitoylation. Further, Renner et al. (2009) found that cholesterol depletion reduced the degree of slowing of GABAA receptors and even of the lipid GM1 within inhibitory synapses. This suggests that synaptic receptor-scaffold complexes may be sequestered by a mechanism shared with rafts (Allen et al., 2007; Delint-Ramirez et al., 2010). The role of lipids may be regulated, as an important role was recently identified for the lipid-based second messenger PIP3 in regulating synaptic AMPARs (Arendt et al., 2010). PIP3-containing regions of the plasma membrane have the potential to recruit pleckstrin-homology domain containing proteins, which in turn, dock membrane-associated signaling complexes, including some which regulate the actin cytoskeleton. At hippocampal synapses, depletion of PIP3 resulted in reduced accumulation of synaptic PSD-95, decreased stability of synaptic AMPARs, and reduced synapse strength. While the role of PIP3 was investigated only in total receptor retention, it is conceivable that PIP3 or other lipid specializations help define subsynaptic areas to cluster receptors in the PSD subregions. The small size of rafts in other cells (10-200 nm; (Pike, 2006) is consistent with this notion. Future experiments to unravel the spatial and temporal regulation of membrane lipids at synapses will be essential to elucidate their potential roles in facilitating the organization of PSD scaffold modules or the assembly of subsynaptic domains of macromolecular crowding. As discussed below, single-molecule tracking appears to be ideal for these tasks.
4. Future directions: Understanding the role of PSD interior organization in disease
Overall, accumulating evidence clearly suggests that even subtle disruptions of receptor positioning may lead to functional disturbance. Consistent with this possibility, mutations in several components of the PSD scaffold (Bayes et al., 2011), spine actin cytoskeleton (Hayashi-Takagi et al., 2010; Woolfrey et al., 2009), and synaptic adhesion (Betancur et al., 2009) that would be expected to influence PSD lateral organization have been linked to human disease. While these diseases may sometimes involve synapse loss or altered connectivity, the convergence upon proteins responsible for synaptic regulation suggests that mechanisms of synaptic AMPARs positioning are altered in the pathology. In this way, many diseases may initially involve dysregulation of synaptic transmission resulting from defects in synaptic organization. In the case of the PSD, Shank3 genetic mutations and deletions are linked to autism spectrum and other neurodevelopmental disorders (Bonaglia et al., 2001; Durand et al., 2007) as well as schizophrenia (Gauthier et al., 2010). Mutations in GKAP family member SAPAP3 have been linked to pathological grooming and obsessive-compulsive disorders (OCD) in human patients (Bienvenu et al., 2009) and leads to both a synaptic and OCD-like behavioral phenotype when deleted in mice (Welch et al., 2007).
The complex roles of actin governing synaptic transmission and plasticity are particularly important to decipher. If actin participates in mechanisms regulating lateral PSD organization and receptor distribution, as outlined above (Fig. 2), then cytoskeletal dysfunction may represent an important route to synaptic pathophysiology. In fact, dysregulation of the spine cytoskeleton has emerged as a strong candidate mechanism in many diseases (Penzes et al., 2011) in part because disruptions to spine structure are readily observed in diseases as diverse as addiction (Shen et al., 2009), schizophrenia (Glantz and Lewis, 2000), and Alzheimer’s disease (Walsh and Selkoe, 2004). However, the dramatic spine morphology changes in these diseases may be only the most obvious of multiple consequences of cytoskeletal dysregulation near the synapse. Because the PSD itself may be an important location for regulating actin (Frost et al., 2010a), more subtle changes to subsynaptic structure seem likely to occur even if overall spine morphology or number is not grossly disrupted. In one intriguing example, the activity of the Rho-GEF Kalirin-7, which activates the potent cytoskeletal modulators Rac1 and Rap1, is regulated by its binding to PSD-95. This binding is altered by disease-associated mutations of the schizophrenia susceptibility gene DISC1 (Hayashi-Takagi et al., 2010), raising the possibility of highly localized disruption of actin at the PSD potentially capable of generating such synapse-specific changes in actin regulation. It will be important to expand our analysis of synaptic dysfunction in disease models beyond simply measurements of spine density and morphology.
The subsynaptic dimensions over which these various mechanisms organize receptors are extremely small, and the events of interest are in many cases transient. What technical advances might facilitate understanding these processes? Identifying defects of subsynaptic structure associated with disease processes and understanding their functional impact will come from convergence of many fields of work, but will likely rely on aggressive development in three areas: EM tomography, super-resolution microscopy, and biophysical modeling.
EM tomography offers clear visualization of subcellular structures due to virtual sectioning at 2nm resolution (Chen et al., 2008c). It is ideal for discriminating PSD internal organization, where protein density is so high that it obstructs traditional EM resolution even within ultrathin EM sections. However, the incompatibility of EM with live-cell analysis means that an exciting wave of developments in fluorescence imaging with sub-diffraction resolution (Lippincott-Schwartz and Manley, 2009) will be critical for measuring mobility of receptors and other PSD constituents within the synapse. For live-cell approaches, stimulated-emission-depletion (STED) (Hell and Wichmann, 1994; Klar and Hell, 1999) and structured-illumination microscopy (SIM) (Gustafsson, 2005), though technically demanding, appear poised to at least double the lateral and axial resolution of confocal microscopy. STED has recently been used to reveal unprecedented morphological detail of spine head and neck dynamics (Ding et al., 2009; Nagerl et al., 2008), as well as to resolve real-time movements of presynaptic vesicles (Kamin et al., 2010; Wilhelm et al., 2010).
Photoactivated localization microscopy (PALM) (Betzig et al., 2006; Hess et al., 2006) offers important advantages for live-cell visualization. PALM makes use of genetically expressed fluorophores that can be individually photoactivated and localized with nm-scale precision, similar in principle to STORM, but compatible with the study of structural and molecular dynamics in live cells (Manley et al., 2008; Shroff et al., 2008). Importantly, though live-cell PALM is currently limited to rather thin structures such as cultured neurons, its power lies in the essentially unique ability to localize and track intracellular proteins (Frost et al., 2010b; Izeddin et al., 2011) instead of only those membrane proteins accessible to an extracellular antibody. Aside from its high resolution, the utility of PALM to measure diffusion and trajectories of individual molecules gives access to otherwise inaccessible information of direct, biochemical interpretability (Frost et al., 2010b), and a superior ability to resolve behavior of small kinetic or spatial subpopulations (Saxton and Jacobson, 1997). These advantages make PALM a uniquely powerful approach to study the dynamics of synaptic molecules.
Using this experimental information to assess the roles of protein lateral organization within the PSD will require structurally accurate computational models of synapse function. To understand the PSD interior, the best models will expand on current, state-of-the-art models (Bressloff and Earnshaw, 2009; 2006; Santamaria et al., 2010) that simplify PSD complexity for the sake of computational ease. A next generation of models will include accurate geometric representation of synapse and spine structure determined from EM, will take advantage of growing structural and biochemical information on PSD constituents, and will incorporate realistic estimates of protein mobility from live-cell and single-molecule dynamic assays (Alber et al., 2007). Such models can be used to predict physiological properties of synapses to be tested experimentally, helping us characterize important features of synaptic interior organization that control synapse function. These features will be important clues to guide our searches in human tissue and within model systems for aberrations of subsynaptic structure associated with disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam G, Matus A. Role of actin in the organisation of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;43:246–250. doi: 10.1016/s0169-328x(96)00177-5. [DOI] [PubMed] [Google Scholar]
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA. PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci. 2010;13:36–44. doi: 10.1038/nn.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash MA, Park JM, Melnikova T, Wang D, Jeon SK, Lee D, Syeda S, Kim J, Kouser M, Schwartz J, et al. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. doi: 10.1016/j.cell.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E, Patterson G, Sougrat R, Lindwasser O, Olenych S, Bonifacino J, Davidson M, Lippincott-Schwartz J, Hess H. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12:172–181. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Kerr JM, Ehlers MD. Structural plasticity with preserved topology in the postsynaptic protein network. Proc Natl Acad Sci U S A. 2008;105:12587–12592. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001;69:261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressloff PC, Earnshaw BA. A dynamic corral model of receptor trafficking at a synapse. Biophys J. 2009;96:1786–1802. doi: 10.1016/j.bpj.2008.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capani F, Martone ME, Deerinck TJ, Ellisman MH. Selective localization of high concentrations of F-actin in subpopulations of dendritic spines in rat central nervous system: a three-dimensional electron microscopic study. J Comp Neurol. 2001;435:156–170. doi: 10.1002/cne.1199. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Holderith NB, Nusser Z, DiGregorio DA, Cull-Candy SG. Changes in synaptic structure underlie the developmental speeding of AMPA receptor-mediated EPSCs. Nat Neurosci. 2005;8:1310–1318. doi: 10.1038/nn1534. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 Is Required to Sustain the Molecular Organization of the Postsynaptic Density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith J, Leapman R, Reese T. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008a;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008b;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters CA, Reese TS. Life inside a thin section: tomography. J Neurosci. 2008c;28:9321–9327. doi: 10.1523/JNEUROSCI.2992-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder E, Crozier R, Soderling J, Jin Y, Langeberg L, Lu H, Bear M, Scott J. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003a;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003b;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan M, Otto S, Tiglio K, Savas J, Yates C, D T, P G. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgis J, Galbraith J, Dosemeci A, Chen X, Reese T. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biology. 2008 doi: 10.1007/s11068-007-9017-0. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Fernandez E, Bayes A, Kicsi E, Komiyama NH, Grant SG. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci. 2010;30:8162–8170. doi: 10.1523/JNEUROSCI.1792-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Takasaki KT, Sabatini BL. Supraresolution imaging in brain slices using stimulated-emission depletion two-photon laser scanning microscopy. Neuron. 2009;63:429–437. doi: 10.1016/j.neuron.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll R, Bredt D. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- El-Husseini A, Craven S, Chetkovich D, Firestein B, Schnell E, Aoki C, Bredt D. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000a;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A, Schnell E, Chetkovich D, Nicoll R, Bredt D. PSD-95 involvement in maturation of excitatory synapses. Science. 2000b;290:1364–1368. [PubMed] [Google Scholar]
- Elias G, Funke L, Stein V, Grant S, Bredt D, Nicoll R. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Kreitzer AC, Regehr WG. Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron. 2002;36:1115–1126. doi: 10.1016/s0896-6273(02)01106-6. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Wilson M. Saturation of postsynaptic receptors at central synapses? Curr Opin Neurobiol. 1996;6:395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Frost NA, Kerr JM, Lu HE, Blanpied TA. A network of networks: cytoskeletal control of compartmentalized function within dendritic spines. Curr Opin Neurobiol. 2010a;20:578–587. doi: 10.1016/j.conb.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010b;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Champagne N, Lafreniere RG, Xiong L, Spiegelman D, Brustein E, Lapointe M, Peng H, Cote M, Noreau A, et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci U S A. 2010;107:7863–7868. doi: 10.1073/pnas.0906232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MG. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc Natl Acad Sci U S A. 2005;102:13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Kim E. Synaptic adhesion molecules and PSD-95. Prog Neurobiol. 2008;84:263–283. doi: 10.1016/j.pneurobio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S, Girirajan T, Mason M. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J. 2006;91:4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne EA, Dell’Acqua ML. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J Neurosci. 2007;27:3523–3534. doi: 10.1523/JNEUROSCI.4340-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Regulation of AMPA receptor localization in lipid rafts. Mol Cell Neurosci. 2008;38:213–223. doi: 10.1016/j.mcn.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang W, Bates M, Zhuang X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 2008;319:810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T, Südhof T. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Izeddin I, Specht CG, Lelek M, Darzacq X, Triller A, Zimmer C, Dahan M. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS One. 2011;6:e15611. doi: 10.1371/journal.pone.0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin D, Lauterbach MA, Westphal V, Keller J, Schonle A, Hell SW, Rizzoli SO. High- and low-mobility stages in the synaptic vesicle cycle. Biophys J. 2010;99:675–684. doi: 10.1016/j.bpj.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annual Review Of Neuroscience. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Klar TA, Hell SW. Subdiffraction resolution in far-field fluorescence microscopy. Opt Lett. 1999;24:954–956. doi: 10.1364/ol.24.000954. [DOI] [PubMed] [Google Scholar]
- Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J Neurosci. 2006;26:7693–7706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Manley S. Putting super-resolution fluorescence microscopy to work. Nat Methods. 2009;6:21–23. doi: 10.1038/nmeth.f.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. doi: 10.1038/nrn2191. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Dakoji SR, Olsen O, Bredt DS, Lim WA, Prehoda KE. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell. 2001;8:1291–1301. doi: 10.1016/s1097-2765(01)00411-7. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Neher E, Schneggenburger R. Estimation of quantal size and number of functional active zones at the calyx of held synapse by nonstationary EPSC variance analysis. J Neurosci. 2001;21:7889–7900. doi: 10.1523/JNEUROSCI.21-20-07889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Willig KI, Hein B, Hell SW, Bonhoeffer T. Live-cell imaging of dendritic spines by STED microscopy. Proc Natl Acad Sci U S A. 2008;105:18982–18987. doi: 10.1073/pnas.0810028105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TA, DiGregorio DA, Silver RA. Modulation of glutamate mobility reveals the mechanism underlying slow-rising AMPAR EPSCs and the diffusion coefficient in the synaptic cleft. Neuron. 2004;42:757–771. doi: 10.1016/j.neuron.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Molecular and Cellular Neuroscience. 2007;34:503. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24:2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Renner M, Choquet D, Triller A. Control of the postsynaptic membrane viscosity. J Neurosci. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B, Otto S, Tiglio K, Williams ME, Ghosh A. Regulation of synaptic stability by AMPA receptor reverse signaling. Proc Natl Acad Sci U S A. 2011;108:367–372. doi: 10.1073/pnas.1015163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur J Neurosci. 2006;24:3463–3474. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Gorlich A, Sassoe-Pognetto M, Banchaabouchi MA, Giustetto M, Triller A, et al. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Santamaria F, Gonzalez J, Augustine GJ, Raghavachari S. Quantifying the effects of elastic collisions and non-covalent binding on glutamate receptor trafficking in the post-synaptic density. PLoS Comput Biol. 2010;6:e1000780. doi: 10.1371/journal.pcbi.1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB, Saviane C, Nielsen TA, DiGregorio DA, Silver RA. Rapid vesicular release, quantal variability, and spillover contribute to the precision and reliability of transmission at a glomerular synapse. J Neurosci. 2005;25:8173–8187. doi: 10.1523/JNEUROSCI.2051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Utvik JK, Camoletto P, Watanabe M, Stephenson FA, Bredt DS, Ottersen OP. Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 2003;463:237–248. doi: 10.1002/cne.10745. [DOI] [PubMed] [Google Scholar]
- Saxton MJ. Single-particle tracking: effects of corrals. Biophys J. 1995;69:389–398. doi: 10.1016/S0006-3495(95)79911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton MJ, Jacobson K. Single-particle tracking: Applications to membrane dynamics. Annual Review of Biophysics and Biomolecular Structure. 1997;26:373–399. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt D, Nicoll R. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: Long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shroff H, Galbraith CG, Galbraith JA, Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat Methods. 2008;5:417–423. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill JF, Steiner P, Czervionke BL, Sabatini BL. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J Neurosci. 2009;29:12845–12854. doi: 10.1523/JNEUROSCI.1841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Du F, Tian QB, Zhang J, Endo S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J Neurochem. 2008;104:596–610. doi: 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Shapiro ME, Yi Z, Chang K, Wenthold RJ. NMDA receptors interact with flotillin-1 and -2, lipid raft-associated proteins. FEBS Lett. 2009;583:1226–1230. doi: 10.1016/j.febslet.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Swulius MT, Kubota Y, Forest A, Waxham MN. Structure and composition of the postsynaptic density during development. J Comp Neurol. 2010;518:4243–4260. doi: 10.1002/cne.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J-i, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and Density of AMPA Receptors in Single Synapses in Immature Cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. Embo J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares GA, Panepucci EH, Brunger AT. Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol Cell. 2001;8:1313–1325. doi: 10.1016/s1097-2765(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994;12:51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BG, Groemer TW, Rizzoli SO. The same synaptic vesicles drive active and spontaneous release. Nat Neurosci. 2010;13:1454–1456. doi: 10.1038/nn.2690. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, Penzes P. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xie X, Liaw J-S, Baudry M, Berger TW. Novel expression mechanism for synaptic potentiation: Alignment of presynaptic release site and postsynaptic receptor. PNAS. 1997;94:6983–6988. doi: 10.1073/pnas.94.13.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Schluter OM, Steiner P, Czervionke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Kanda T, Eguchi K, Takahashi T. Vesicular glutamate filling and AMPA receptor occupancy at the calyx of Held synapse of immature rats. J Physiol. 2009;587:2327–2339. doi: 10.1113/jphysiol.2008.167759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Subunit- and pathway-specific localization of NMDA receptors and scaffolding proteins at ganglion cell synapses in rat retina. J Neurosci. 2009;29:4274–4286. doi: 10.1523/JNEUROSCI.5602-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]


