Abstract
About 75% of extant sponge species use dissolved silicon (DSi) to build a siliceous skeleton. We show that silicon (Si) uptake by sublittoral Axinella demosponges follows an enzymatic kinetics. Interestingly, maximum uptake efficiency occurs at experimental DSi concentrations two orders of magnitude higher than those in the sponge habitats, being unachievable in coastal waters of modern oceans. Such uptake performance appears to be rooted in a former condition suitable to operate at the seemingly high DSi values characterizing the pre-Tertiary (>65 mya) habitats where this sponge lineage diversified. Persistence of ancestral uptake systems causes sponges to be outcompeted by the more efficient uptake of diatoms at the low ambient DSi levels characterizing Recent oceans. Yet, we show that sublittoral sponges consume substantial coastal DSi (0.01–0.90 mmol Si m−2 day−1) at the expenses of the primary-production circuit. Neglect of that consumption hampers accurate understanding of Si cycling on continental margins.
Silicic acid, a biologically assimilable dissolved form of silicon (DSi), is a key ocean nutrient1,2,3. It fuels primary production by enhancing growth of diatoms, which polymerize DSi to elaborate their skeletons of biogenic silica (BSi). Increased photosynthesis in diatom populations decreases DSi, nitrate, and phosphate levels in surface waters and facilitates transfer of atmospheric carbon dioxide to the ocean, hence connecting silicon to carbon, phosphate, and nitrogen cycles4,5,6. Therefore, there is strong interest in predicting the interplay between DSi and BSi budgets and many efforts have been made during the last decades to unravel the route of Si through the oceans. In the current global model describing the Si cycle in the ocean, diatoms are thought to biologically dominate Si cycling, with other Si-consuming organisms, such sponges, radiolarians, choanoflagellates and silicoflagellates, playing negligible roles2,3,7. Nevertheless, a concatenation of findings relative to the contribution by siliceous sponges8,9,10,11,12,13 has alerted us that the notion of a Si cycle exclusively revolving around diatoms incurs an unrealistic oversimplification that also neglects ocean history.
There have always been suspicions that the contribution of sponges to the marine Si cycle, even if never quantified in global terms owing to its complexity, could be of some importance4,14. More recent studies evaluating the contribution of sponges on continental-shelf and slope habitats have revealed that Si standing stocks in sponges may surpass the combined Si stock in living diatoms and ambient DSi of the diverse local systems investigated8,9,12,13. Moreover, upon death, sponge skeletons are far more refractory to dissolution than diatom frustules, irrespective of potential differences in skeleton surface area12,15,16. Consequently, benthic populations of siliceous sponges appear to function as BSi traps on continental margins, retarding recycling of BSi into DSi. A serious problem limiting our understanding of the magnitude of Si turnover through sponges is the current lack of data regarding uptake kinetics, with only a single available study to date10.
Fossil records and molecular clocks arguably suggest that sponges were already present in the Precambrian17,18,19, but the oldest unequivocal fossil spicules date back to the Lower Cambrian20, about 542 my ago. It means that the Si-consuming activity by sponges evolved before that of the two others major Si consumers characterizing modern oceans, namely radiolarians and diatoms. Radiolarians are also quite an old group, with arguable fossils from as early as the Lower Cambrian21 and the earliest unequivocal fossils dating from the Lower Ordovician, about 488 my ago22. Compared to sponges and radiolarians, diatoms are newcomers, with controversial remains reported from Jurassic sediments23 and unequivocal fossils from the Early Cretaceous24, about 140 my ago. Geochemical modeling25 and analysis of marine chert formations4,26 support the notion that the Precambrian and Cambrian oceans in which sponges and radiolarians thrived had average DSi concentrations ranging from 1 to 2.2 mM, that is, at least two orders of magnitude higher than the average (10 µM) in the Recent world ocean. Likewise, from the Cambrian to the Jurassic, ocean average DSi concentrations have been estimated not lower than 650 µM4,6,26. Those paleo-estimates along with an abundant fossil record of highly silicified spicules and tests27 support that the Si-uptake systems of sponges and radiolarians operated with notable success in waters with very high DSi concentrations for about 400 to 500 million years, until the evolutionary emergence and the subsequent ecological expansion of diatoms. There is a congruent body of evidence supporting that the expansion of diatoms during the Late Cretaceous and the Lower Tertiary drastically decreased DSi concentrations in surface waters, leading to the low values characterizing modern oceans, which appear to have experienced only minor variations for the last 60 my4. Such a decrease in DSi availability put a selective pressure on both sponges11,28 and radiolarians29,30,31 to evolve skeletons that required less silica. This process is well illustrated by the extant shallow-water demosponge Crambe crambe (Family Crambeidae, Order Poecilosclerida). Long-term exposure of this sponge to DSi concentrations much higher than those in its natural habitats induces secretion of not only thicker and larger spicules, but also additional types that are never produced in wild populations11. Such a response suggests that the genetic systems controlling Si-uptake and silicification are up-regulated by threshold DSi concentrations higher than those naturally available to the sponge. This opens the intriguing possibility that extant sponge species belonging to lineages that diversified before the expansion of diatoms may still silicify through uptake systems originally suitable to deal with the high DSi concentrations that seemingly characterized pre-Tertiary oceans. A better knowledge of the performance of these uptake systems could help us to understand the evolution of nutrient concentrations as well as that of the biosilicification process characterizing modern sponges . Additionally, by improving our knowledge on sponge Si uptake, we will be able to approach more realistically the role of these organisms on Si cycling in Recent marine ecosystems. To these aims, we have investigated Si-uptake kinetics in axinellid demosponges, members of a lineage with a fossil record thought to pre-date the Cretaceous-Tertiary boundary, as rhabdostyles and styles characterizing some axinellid sub-lineages have been reported from the middle Triassic (245 to 228 my ago)32.
Results
Experimental DSi uptake
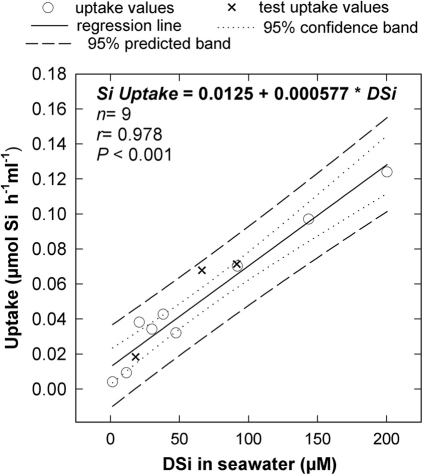
Silicon uptake was investigated in individuals of three Atlantic-Mediterranean demosponges of the genus Axinella (fam. Axinellidae; order Halichondrida) collected from sublittoral north-western Mediterranean populations (see SI 1). The bulk of uptake data was obtained from individuals of A. damicornis, a species scattered in moderated density on sublittoral rocky walls. Additional information was obtained from individuals of both A. verrucosa and A. polypoides, which are rarer, to-be-protected species. One experiment (Exp. I) examined uptake responses of 10 individuals of A. damicornis and 3 of A. verrucosa to DSi concentrations that were increased, in 48h steps, from field values (1.6 µM) to 10, 20 30, 40, 50, 100, 150, and 200 µM, using sodium hexafluorosilicate (SFS) as DSi source. In response, average Si uptake by the set of assayed sponges progressively increased with increasing DSi, significantly fitting a linear regression (Fig. 1; n = 13, r = 0.978, P< 0.001). After the 200 µM DSi step, sponges were transferred back to the natural concentration (1.6 µM) for a 5-day resting period, then exposed again to 20, 70, and 100 µM DSi experimental steps for 48h periods each. Sponges responded to these treatments after resting with readjustments in uptake rate that mirrored the marked shifts in DSi availability, showing at each concentration step an average uptake rate that fell within the 95% prediction interval of the previously calculated linear regression (Fig. 1). These results indicated that the Si uptake system of the sponges is able to react quickly and, more importantly, predictably to rapid, abrupt changes in ambient DSi.
Figure 1. Relationship between DSi uptake and DSi availability during initiation of experiment I.
The uptake-rate response by the sponges (µmol Si per h and ml of sponge) was linearly related to DSi concentration in the experimental bottles, whenever DSi availability ranged from natural values (1.6 µM) to 200 µM. Crosses are 3 further treatment steps (20, 70 and 100 µM DSi) conducted as a test after allowing sponges to rest for 5 days at natural DSi concentration. Note that all 3 test responses fell within the 95% prediction interval of the previously calculated regression equation.
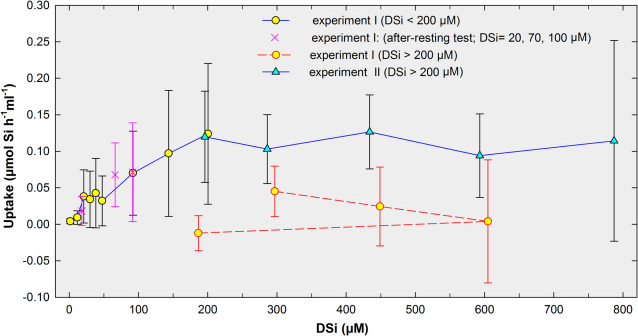
After the 48h step in the 100 µM DSi treatment, sponges were exposed to 300 µM DSi and, surprisingly, their average uptake rate decreased (Fig. 2). Furthermore, average uptake rates at subsequent 400 and 600 µM DSi treatments were even lower (Fig. 2). When sponges were transferred from 600 down to 185 µM DSi, a concentration that had elicited nearly maximum uptake during the first part of the experiment, they showed no perceptible Si uptake (Fig. 2: dashed line). Such a physiological inactivity suggested that the sponges had somehow been damaged during treatments at 300, 400 and/or 600 µM DSi. Although no sponge died either partially or entirely during those treatments, slow progressive darkening of the bright yellow-orange skin (ectosome) was noticed, supporting our concern of potential sponge damage. We suspected that the use of SFS as DSi source could proof harmful when aiming for high DSi concentrations. When using SFS to increase DSi concentration by a factor of 1 in the experimental bottles, fluorine concentration (as fluoride) was concomitantly increased by a factor of 6. For instance, in the 300 µM DSi treatment, the sponges were simultaneously exposed to 1800 µmol L−1 fluoride. Such concentration is far higher than the natural seawater average, estimated around 70 µM33. Fluoride concentrations considerably above the natural average are known to favor rapid accumulation of fluorine in many aquatic organisms, leading to internal concentrations that eventually become harmful or even lethal34. This also appeared to be the case of the assayed sponges, the external epithelia of which were sub-lethally poisoned by fluorine. For this reason, we complemented the initial experiment with a second one (Exp. II) based on the use of non-toxic sodium metasilicate (Na2SiO3 5H20) to re-assess sponge uptake, particularly at high DSi concentrations. For Exp. II, we used 10 newly collected individuals of A. damicornis, 2 of A. polypoides, and 1 of A. verrucosa. After 5 days under natural DSi concentration for acclimation to laboratory conditions, Exp. II started by exposing the sponges to 200 µM DSi for 48h, conducting subsequent 48h steps at 300, 450, 600, and 800 µM.
Figure 2. Summary of sponge uptake responses during experiments I and II.
The solid line indicates the course of average uptake rates (µmol Si per h and ml of sponge ± s.d.) by "healthy" (i.e., non-fluorine poisoned) sponge sets in response to experimental DSi concentrations during the first phase of the hexafluorosilicate-based experiment I (yellow circles) and through the metasilicate-based experiment II (blue triangles). Crosses are 3 treatment steps (20, 70 and 100 µM DSi) conducted as a test after allowing sponges to rest for 5 days at natural DSi concentration prior to initiating the second phase of experiment I. The dashed line indicates average (± s.d.) uptake rates during the second phase of experiment I (i.e., DSi >200 µM), in which sponges became poisoned by high concentrations of fluorine released from sodium hexafluorosilicate.
Interestingly, the set of new sponges exposed to the 200 µM DSi treatment during Exp. II (Fig. 2) showed an average uptake rate (0.11±0.06 µmol Si h−1 ml−1 sponge) nearly identical to that measured for the previous set of individuals during the "healthy" 200 µM DSi step of Exp. I (0.12±0.09 µmol Si h−1 ml−1), confirming repeatability in the sponge responses. Experiment II also revealed that subsequent increases in DSi to 300, 600, and 800 µM neither stimulated nor decreased uptake rates, relative to the 200 µM DSi treatment (Fig. 2). We even run a final 72 h step at 850 µM DSi to examine the possibility that the uptake system could need several days to get adapted to those high concentrations. Nevertheless, such an extended treatment—resulting in a total of five days of exposure to 800–850 µM DSi— did not stimulate any significant shift in uptake relative to values achieved at 200 µM DSi. Therefore, the results strongly suggest that the uptake system becomes saturated somewhere around 200 µM DSi.
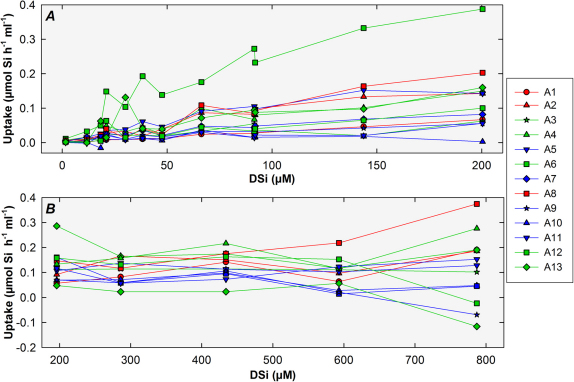
When the uptake responses of each sponge in the various DSi concentrations in both experiments were compared, considerable inter-individual variability was found (Fig. 3 a–b). It is worth noting that the few individuals of A. verrucosa and A. polypoides that we assayed for exploratory purposes did not show uptake rates substantially different from the bulk of individuals of A. damicornis, suggesting that interspecific differences in uptake kinetics, if any, should be minimal (Fig. 3 a–b). Interindividual variability showed a consistent pattern, as individuals taking up faster at a given DSi concentration were also good performers at most other concentrations (Fig. 3 a–b). Maximum uptake rate (average±sd; recorded in both experiments at 200 µM DSi) was 0.12±0.07 µmol Si h−1 ml−1 (sponges of both experiments pooled; n = 26), but again it was affected by a large inter-individual variability, ranging from 0.002 µmol Si h−1 ml−1 in individual 10 to 0.387 in individual 6 of Exp. I.
Figure 3. Summary of individual uptake data.
Some variability was noticed in the individual uptake responses during the "healthy" phase of experiment I (a) and through experiment II (b). Green and blue lines indicate small (< 9ml) and large (> 9 ml) individuals of Axinella damicornis, respectively. Red lines indicate individuals of Axinella verrucosa or Axinella polypoides. Note that the sponge individuals used for experiments plotted in "a" and "b" graphs are different.
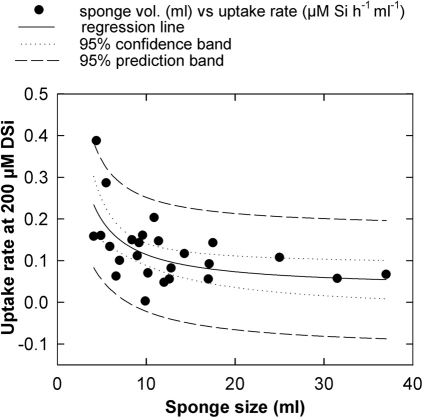
At least some of the detected inter-individual variability can be attributed to size differences. Volume of assayed sponges (ranging from 4 to 37 ml) appeared to have a moderate effect on uptake rate, with smaller individuals (<9 ml) showing slightly higher rates on average than larger individuals (>9 ml). Such relationship between size and uptake can be perceived (even if diffuse) on Fig. 3, where green lines (large sponges) often run above blue lines (smaller sponges). Likewise, the relationship between saturated uptake rate (i.e., that at 200 µM DSi) and sponge volume grossly fitted an inverted, first order polynomial regression (sponges from both experiments pooled: n = 26, r = 0.600, P = 0.001; Fig. 4), confirming a subtle negative relationship between both variables. Interestingly, in nearly all DSi concentrations assayed (Fig. 3a), the highest uptake rates were achieved by individual 6, which was the second smallest (4.4 ml) sponge out of the 26 assayed (Fig. 4). In this individual, silica spicules largely protruded from the epithelium, making a velvety body surface visible by the naked eye and indicating that the sponge was involved in a more intense process of skeletal production than the other individuals.
Figure 4. Relationship between sponge size and uptake.
A weak negative relationship between uptake rate (µmol Si h−1 ml−1) at 200 µM DSi and sponge size (ml) was detected, grossly fitting an inverted, first order, polynomial regression. The highest uptake rate corresponded to individual 6 of Exp. I.
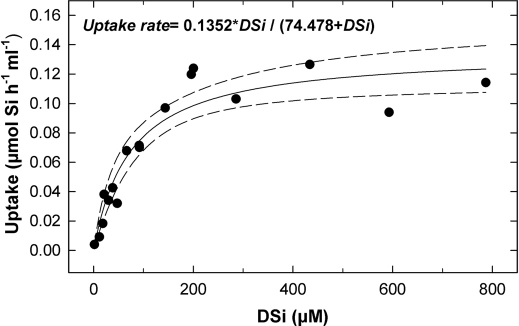
When average uptake data from the "healthy" phase of Exp. I (i.e., DSi treatments <300µM) and Exp. II were pooled together and analyzed by non-linear regression, the equation better fitting the data was a hyperbolic function (Fig. 5; n = 16, r = 0.948 , P< 0.001), similar to that of a Michaelis-Menten ligand-binding kinetic with one site saturation. Consequently, DSi uptake (V, µmol Si per hour and per sponge ml) by Axinella spp. can be regarded as an enzyme-mediated transport, with a half-saturation constant (Km) of 74.47 µM and a saturated uptake rate (Vmax) of either 0.13 µmol per hour and sponge ml or 1.74 µmol h−1 g−1, if expressed as ash-free dry weight (AFDW).
Figure 5. DSi uptake model for Axinella.
Michaelis-Menten kinetics function modeling the relationship between Axinella spp. average uptake rate and ambient DSi in the experimental bottles, obtained after pooling data of experiments I and II.
Estimates of field DSi demands
Conservative field surveys along 100 km of the relatively sponge-poor and oligotrophic Mediterranean rocky sublittoral where Axinella spp. grew (see Methods and Supplementary Information: Section 1) revealed that siliceous sponges average 0.34±0.52 L m−2 (n = 100 quadrats) and ambient DSi 0.73±0.44 µM (n = 240 water samples over a year cycle). At that ambient DSi concentration, sponge uptake rate is predicted to average 1.31±0.79 × 10−3 µmol Si per h and sponge ml (according to equation in Fig. 2). It means that the sponge fauna per m2 of rocky habitat at this Mediterranean coast use yearly about 3.9±5.9 mmol DSi. Such consumption represents yearly about 21.4±32.7% of the average DSi available in a 30m overlying sublittoral water column and about 10.7±16.3% of that in a 50 deep water column. Similarly, we have conservatively estimated (see Methods) on 21 km2 of a Mesoamerican continental shelf (Belize) that the abundance of siliceous sponges averages 2.6±14.3 L per m2 of bottom13. Mean yearly DSi concentration in the 25m-deep water column of such continental-shelf system is about 3.6±0.6 µM13, being the sponge communities predicted to consume DSi at an average rate of about 14.5±0.35 ×10−3 µmol per h and sponge ml (according to the equation in Fig. 2). It means that yearly sponge uptake is about 332±1826 mmol DSi m−2, and that it would virtually deplete that shallow Belizean shelf of DSi once every 98.9±83.7 days, if there is no DSi replenishment. The estimated Si consumption rate by the poor (in volume) sponge fauna of the oligotrophic Mediterranean sublittoral is about 0.01±0.01 mmol Si m−2 day−1, while that of the richer Caribbean sponge assemblages would be 0.90±5.00 mmol Si m−2 day−1. Both sponge figures are lower than the demand average estimated for diatoms in diverse marine systems (Table 1).
Table 1. Average rates of Si use by communities of planktonic diatoms and sublittoral sponge communities in various marine systems. Diatom Si demands were originally measured as BSi production rates1,2,3, while sponge demands have been derived from Si uptake rates.
| Si DEMAND | |
|---|---|
| HABITAT/ SYSTEM | (mmol Si m−2 day−1) |
| Planktonic diatoms | |
| Coastal upwelling | 90 |
| Other coastal conditions | 15 |
| Southern ocean | 15 |
| Deep ocean | 2.3 |
| World ocean average | 1.6 – 2.1 |
| Sublittoral sponges | |
| Outer shelf of Belize | 0.90 |
| Baltic sublittoral bottoms | 0.44 |
| Mediterranean rocky bottoms | 0.01 |
Discussion
Our experimental approach revealed that sponges react to ambient DSi availability with a rapid and predictable response of their uptake rate. Interestingly, the assayed sponges achieved their highest uptake rates at concentrations around 200 µM DSi, that is, nutrient values that are not available to them in shallow waters of modern oceans and that are more than 100 times the yearly DSi average experienced by these animals in their natural habitats (see Supplementary Information: Section 1). Consequently, these sponges are suffering a severe, chronic limitation by DSi (< 2µM) in their natural habitats. The idea that low DSi concentration in Recent shallow waters might limit sponges was originally disregarded35,36,37, as their Si-uptake system was expected to have adapted to the post-Cretaceous decline in the availability of this nutrient. However, a strong DSi limitation appears to be the rule in the few sponges investigated so far in this regard, i.e., H. panicea10, C. crambe11, and Axinella spp. (current results).
It is difficult to decide what accounts for the Axinella spp.'s ability to deal with high levels of ambient DSi. This ability could be the mere ancestral condition of the Si uptake system. Alternatively, such an ability could have resulted from evolvement of the original uptake system in order to maximize exploitation of the elevated DSi concentrations characterizing Jurassic and Cretaceous shallow waters, an adaption that would not have reverted during the subsequent post-Cretaceous DSi crisis and that makes these sponges little efficient Si users at the low ambient DSi concentrations characterizing modern oceans. If this is the case, investigation of DSi uptake kinetics in selected extant sponge lineages could be a potential tool to infer DSi concentrations in the particular habitats of past oceans where their ancestors thrived. There have also been recent proposals to use Si isotope analysis of fossil sponge spicules38,39 to infer past DSi concentrations. A combination of both techniques could provide an interesting biological congruence test for hypotheses on DSi concentration in ancient marine environments, which are often formulated from strictly geological approaches.
In Axinella spp., DSi uptake appears to be an enzyme-meditated process. Uptake in the only other sponge investigated to date in terms of kinetics – i.e., Halichondria panicea (family Halichondriidae, order Halichondrida) – also conformed to a Michaelis-Menten model, with saturation around 100 µM DSi10, which is nearly half the value in Axinella spp. Surprisingly, saturated uptake rate for H. panicea was 19.33 µmol Si h−1 g−1 AFDW, an order of magnitude higher than that measured for Axinella spp. Such differences in Vmax could be explained by both species having very different affinity by DSi. An alternative explanation is that H. panicea uptake measurements were not taken from complete individuals but from explants, i.e., regenerating pieces that had been obtained for the experiment by fragmenting larger sponges10. It is well known that sponges regenerate rapidly large portions of their body (including production of new silica skeleton), accomplishing regeneration at rates that are up to 2,900 times the undisturbed growth rates40. It is also worth noting that the experimental DSi concentrations to which H. panicea was exposed, which ranged from 1 to 200 µM10, were based on SFS, a compound that proved toxic for Axinella spp when used at concentrations equal or higher than 300 µM.
Although the available evidence is still scarce, it congruently supports that sponge uptake conforms to an enzymatic kinetics. Nevertheless, it remains little understood what mechanism is responsible for binding and transporting Si from seawater to the cytoplasm of the silica-secreting cells located in the internal mesenchyme (mesohyl) of the sponge body. To date the only enzymes properly identified in connection with sponge silicification belong to the silicatein family41,42, but neither this compound nor the process of direct polymerization of DSi into BSi inside the sclerocyte cells are having a demonstrated role in the internalization of DSi from ambient seawater. Suggestions have been made that a sodium-bicarbonate co-transporting system could somehow be involved in taking up silicic acid from seawater43, which would be consistent with the detected enzymatic kinetics. Although active DSi uptake in diatoms has been shown to be supplemented by some passive diffusion across the cell membrane44, which is in direct contact with the ambient water, a similar diffusion process is unlikely in sponges. Silica secreting cells of sponges (sclerocytes) occur typically at the inner mesohyl regions of the sponge body, isolated from ambient seawater by epithelial cell layers and dense intercellular deposits of collagen and other macromolecules. Furthermore, the possibility that DSi can passively diffuse into the sponges has been discarded experimentally by demonstrating that DSi uptake rates are abated following sponge starvation, what indicates that most DSi internalization results from an active process that requires energy45. Likewise, when the epithelial cells of the Axinella individuals were sub-lethally poisoned with fluorine during our experiment I, rates of DSi uptake decreased inversely to DSi concentrations, corroborating that DSi transport is not a passive diffusion process (Fig. 2). The finding that smaller Axinella individuals incorporate DSi at higher rates than larger individuals (Fig. 4) also supports active DSi uptake, because the opposite pattern should occur under a passive diffusion model, particularly in these branching sponges (Fig. S1), in which surface area increases almost exponentially with increasing individual volume. Further evidence of non-diffusive uptake is provided by investigations on the relationship between the Si isotopic composition of ambient DSi and that of sponge spicules. Sponges are known to fractionate Si isotopes during their BSi production process46, with Δ30Si (i.e., δ30Sisponge- δ30Siseawater) fractionation increasing (i.e., lower values) with increasing ambient DSi concentration38,39. More importantly, Si isotopic data in sponges show good agreement with a Michaelis-Menten function for Si uptake, what again supports uptake and δ30Si fractionation being biologically -rather than diffusively- controled38. Likewise, isotopic analyses strongly suggest that DSi transport from the ambient water to the specific silicificying sites within the sponge body is well differentiated from the process of DSi polymerizing as BSi around the organic template, because the fractionation factor is constant during uptake transport, but it appears to decrease in value (i.e., more intense fractionation) with increasing DSi concentration during strictly the polymerization process38
Ample between-individual variability in DSi uptake rate has been found in our study and it was also reported in H. panicea10,45. The physiological reasons behind such an inter-individual variability remain unclear. Nevertheless, the level of variability detected in laboratory studies is consistent with many field studies reporting that neighboring conspecific sponges subjected to nearly identical environmental conditions in terms of food supply, oxygen, DSi, and others, exhibit puzzling differences in body growth rates—and implicitly in BSi production to skeletonize the new soft tissues—over months or years40,47,48.
Si uptake by diatoms shows some fundamental differences with that of sponges. Planktonic diatoms arguably follow a Michelis-Menten kinetics, saturating at 10 µM DSi on average49 and with a half saturation constant that ranges from 0.3 to 549,50,51, except for some Antarctic species that have shown a Km from 12 to 22 µM in laboratory cultures52. Beside saturating at relatively low concentrations, planktonic diatoms are able to achieve high, diffusion-mediated uptake rates with non-saturable kinetics during short periods, what allows them to transiently store much DSi if high ambient concentrations are sporadically encountered44. Diatom uptake uses an electrogenic, sodium/silicic acid symporter53, being also able to transport the ionized form SiO (OH)3−54. Consequently, Recent planktonic diatoms are finely tuned to work with maximum efficiency under relatively low DSi values. This tuning probably resulted from their genetic uptake system being more "plastic" than that characterizing sponges, so that it was able to evolve in an Early Tertiary ocean where ambient DSi concentrations were progressively decreasing owing to their own ecological expansion. The available evidence to date indicates that the genetic systems controlling biosilicificaton in sponges, diatoms, and Si-using plants are completely unrelated, corresponding to independent evolutionary acquisitions44,55,56. Interestingly, by maintaining ambient DSi values low in the long run, planktonic diatoms favor proliferation of Si users with similar uptake kinetics (i.e., more planktonic diatoms), while limiting any other Si user characterized by a higher saturation constant, hence becoming strong competitors for sponges, radiolarians, and probably benthic diatoms (see Supplementary Information: Section 3).
Despite chronic DSi limitation of siliceous sponges owing to diatom overcompetence, measured uptake rates (which ranged from V1µM = 0.001 to V200µM = 0.098 µmol Si per h and sponge mL) suggest that DSi consumption by sponges on coastal systems is of some relevance. We conservatively estimated average Si consumption rate by the poor (in volume) sponge fauna of the oligotrophic Mediterranean sublittoral in about 0.01±0.01 mmol Si m−2 day−1, while that of the richer Caribbean sponge assemblages is about 0.90±5.00 mmol Si m−2 day−1 (Table 1). Note that these calculations are not maxima, but conservative averages. They come from estimates of sponge biomass with enforced mechanisms to prevent overestimation (see Methods) and are based on highly-replicated field measurements scattered over large continental shelf areas, realistically including large extensions of habitats that are not favorable to sponges, as indicated by the large standard deviation values associated to mean sponge volumes per bottom area unit (i.e., 0.34±0.52 and 2.6±14.3 L m−2 for the investigated Mediterranean and Mesoamerican continental shelf, respectively). Similarly, by using available uptake data for the seasonal Baltic populations of the sponge H. panicea10 and the mean biomass (20 ml m−2) in the less favorable sponge habitat57, a conservative average consumption of 0.44 mmol Si m−2 day−1 during summer months may be arrived at. These Si consumption rates by sponges (Table 1) are somewhat smaller than the average BSi production by diatoms in the global ocean, estimated at 1.6–2.1 mmol Si m−2 day−1 1,2.
At present, it is impossible to estimate with any accuracy Si consumption by sponges in the world oceans—as will be the case for decades to come owing to the extremely variable distribution of sponge biomass on the continental margins at depths that prohibit extensive measurements of individual Si contents per bottom area. However, if the Si consumption rates herein estimated for sponges per unit area of bottom at the Mediterranean, the Baltic, and the Caribbean sea are extrapolated over the entire continental shelf of the earth (22 × 106 Km2), a first, very tentative estimate of the global Si consumption by sponges could be suggested, falling somewhere between 8.6 × 1010 and 7.3 × 1012 mol Si year−1. This figure would still be about two to four orders of magnitude smaller than the 2.0 to 2.8 × 1014 mol Si year−1 estimated for diatoms1. By incorporating into the calculations the large sponge populations that are being discovered at bathyal depths by the advent of ROVs and manned oceanographic submersibles12,58,59,60,61,62, a generous -but unlikely to be ever globally quantified- increase of the yearly sponge Si demands herein estimated for only continental shelves might be arrived at (see Supplementary Information: Section 4 and Figs. S5).
Nevertheless, the ecological importance of sponge DSi demands does not derive only from the magnitude of its uptake rate, but also from the fact that it largely concentrates on continental margins, where substantial amounts of coastal DSi are progressively accumulated in long-lived (often centennial) sponges under the form of BSi skeletal pieces that are extremely reluctant to dissolution following sponge death12,13,15,63. Therefore, even when sponge DSi uptake rates are clearly lower on average that those of diatoms, populations of siliceous sponges may still operate as relevant biological traps, slowing down Si cycling and favoring Si sinking on continental margins. To improve the current knowledge of Si fluxes on continental margins is one of the most urgent needs in order to refine modeling of both the Si cycle and its connections to the Carbon cycle64. Unfortunately, most on-going research efforts towards these aims are guided by the extended notion attributing the preponderance of DSi removal on continental margins to formation and burial of BSi primarily by diatoms and radiolarians, disregarding the contribution by the large sponge populations characterizing most continental margins (see also Figs. S5). For instance, after the recent realization that early estimates of BSi accumulation in the Southern Ocean and Antarctic deep sea were about 35% overestimated65, the widely accepted model of steady-state balance for the marine Si cycle became unbalanced, because a Si sink equivalent to approximately one quarter of the global BSi burial is now missing. Because in order to bring back the cycle into its assumed balance an additional BSi sink should be identified, it has been proposed that BSi accumulation by diatoms on continental margins should account for most of the "missing" BSi burial64,65. Admittedly, reliable direct estimates of diatom Si retention on continental margins are still lacking64. Our current data on sponge DSi demands, along with those already available on BSi standing stocks in sponge populations12,13, suggest that much of the "missing" BSi could correspond to that in the sponge populations of continental margins. Therefore, if we are to refine realistically our current understanding of continental margins as transitory and permanent Si sinks, the role played by sponges has to be incorporated into regional budget calculations. Additionally, the fact that all DSi used by sponges on continental shelves is at the expense of the stock available for diatoms deserves careful consideration, as the DSi slowly –but progressively– accumulated into sponge BSi on continental shelves (and slopes) is taken away from the primary-production circuit for a long time, given the longevity of most sponges and the low levels of dissolution characterizing sponge BSi. By disregarding this sponge DSi-sequestering process while considering exclusively diatom-related DSi and BSi stocks, we are currently overestimating the real levels of connection between Si and C cycles on continental shelves.
Methods
Uptake laboratory experiments
Silicon uptake was investigated in the laboratory using erect, branching demosponges belonging to the genus Axinella (Family Axinellidae, Order Halichondrida; see Fig. S1). Sponges for the uptake experiment were collected from the rocky sublittoral bottoms of the Spanish Mediterranean, between the sites 41° 42' 21" N –2° 48' 17" E and 41° 42' 25" N –2° 54' 51" E, at depths ranging from 12 to 25 m.
There are three major reasons why species of Axinella were selected as a laboratory model: 1) Unlike encrusting species, they can be detached from natural bottoms with minor damage and their volume can easily be calculated with minor error owing to tissue retraction; 2) Unlike most other sponges, they cope well with laboratory manipulation and can even be pulled out of the water periodically for a few seconds with no further negative consequence; 3) They have an intermediate skeleton/organic-tissue weight ratio (54.9±11.5 %) suggesting that they may appropriately represent a standard shallow-water sponge regarding Si use; and, 4) There is fossil evidence indicating that the axinellid lineage was already diversified prior to the Cretaceous-Tertiary boundary32. Indeed, the ancestry of the axinellid lineage can be traced back -although no without controversy- to the Middle Ordovician (471-461 my ago)66,67.
By using hammer and chisel, sponges were collected along with a small piece of the rocky substratum on which they naturally grew (Fig. S3), preventing tissue damage at their attachment zone. For 5 to 15 days, sponges acclimated to laboratory conditions in a 40L food-grade polyethylene tank filled with unfiltered seawater that was re-circulated and oxygenated using a pump, with water replacement every 3 days. Not one sponge became sick or died during acclimating periods. Immediately prior to the onset of the Si-uptake experiments, sponges were placed for a few minutes into a 3L container filled with filtered seawater, a precautionary transitory step taken to minimizing the chance of diatom contamination when transferring specimens to their experimental bottles. Uptake experiments were conducted using 13 individuals in each run. Each sponge was placed into a translucent polycarbonate bottle filled with 3L of filtered seawater (Figs. S3–S4). We used 3µm-pore polycarbonate membranes, so that bacteria (which serve as food) passed through the filter, but diatoms were excluded. Further microscopic inspection of water subsamples revealed that diatom contamination occurred at no step of the experiments. Each experimental bottle also contained a Micra pump, the outlet of which lengthened into a 10 cm upright tube ending 2 cm below the water surface (Fig. S4). Pumps automatically activated every hour for 30 minutes, re-circulating seawater to prevent gradients of food, excretes, and dissolved nutrients within bottles. Additionally, the outflow through the outlet tube broke the air-water interface, oxygenating the seawater. As controls, we used an additional set of 13 bottles (N = 13). They were filled with 3L of the same filtered seawater as the sponge-treatment bottles and included a pump but no sponge. During the experiment, bottles were exposed to a 16∶8 h light-dark cycle and a thermal regime ranging from 20.0 to 21.7 °C within a cold room.
The experiments consisted of a stepwise process. At each step, sponge-containing bottles (n = 13) and control bottles (n = 13) were filled with experimental seawater at an initially known DSi concentration and changes in concentration assessed after 48h (Supplementary Information: Section 2). Because at least 40h are needed to built a 200 µm-long sponge spicule68, we opted for 48h monitoring periods to ensure that the taken DSi was already being used by the sponges to built their spicules, which range from about 300 to 1200 µm in length. Initial DSi concentration in each bottle was determined by collecting a 20 ml water sample once the pump mixed the water for 10 minutes. Final DSi concentrations after 48h were determined by collecting another 20ml sample while the pump was operating. All water samples were analyzed immediately upon collection following a standardized colorimetric method69 run through an Integral Futura Autoanalyzer (Alliance Instruments). Briefly, DSi concentrations (µM) were estimated as silicic acid converted to molybdate-reactive silicate and the colorimetric reactions compared with that of a calibration equation obtained from a 1000±2 mg/l Silicon standard solution (Merck 1.2310.0500). For DSi concentrations higher than 40µM DSi, water samples were processed through the appropriated dilution steps (determination precision about 4 to 6%); otherwise, samples were processed undiluted (determination precision about 2%). Individual uptake at each concentration step was estimated by subtracting the final DSi concentration from the initial one in each bottle and correcting by the average concentration change in the set of control bottles, caused typically by Si adsorption to container walls at high DSi concentrations (Supplementary Information: Section 2).
Using the above-described laboratory set-up, we performed two consecutive, but complementary, experimental runs. In experiment I, we assessed uptake by 13 sponges, which were exposed to DSi concentrations ranging from natural values (1.6 µM) to 600 µM DSi, using sodium hexafluorosilicate (Na2SiF6) as the source of silicic acid. In experiment II, 13 different sponges were exposed to DSi concentrations ranging from 200 to 850 µM DSi, using sodium metasilicate (Na2SiO3 5H20) as the source of silicic acid. In both experiments, seawater Si concentrations were increased by adding 500 ml of a previously prepared DSi stock solution to each of two 50L acid-cleaned, food-grade polyethylene barrels provided with a tap to further delivering Si-enriched seawater to the experimental 3L bottles. Both the DSi stock solution and the 100 L of Si-enriched seawater were prepared 12 hours before starting each treatment step, a period required to take the large volume of seawater to the selected temperature (21–21. °C) and allow the 500 ml of stock solution to homogeneously diffuse into the 49.5L of filtered seawater by pump mixing. While DSi stock solutions prepared from sodium hexafluorosilicate did not need any special treatment once the powder had slowly dissolved in distill water, sodium metasilicate solutions required further buffering with 37% HCl to adjust their initial extremely basic pH (> 10) to values typical for seawater in the natural sponge habitat (pH = 8.1–8.3).
After each experiment, we measured the size of the tested individuals by determining their displacement volume (ml). Subsequently, we weighed the sponges wet (g), dried them at 60° to constant dry weight (g), and finally combusted them at 540°C for 10 hours and weighed the ashes to estimate ash-free dry weight (AFDW). Hourly uptake rates measured for each sponge individual were then normalized by volume (ml) or AFDW weight (g). Finally, experimental uptake data over the assayed range of DSi concentrations were fitted using linear and a non-linear regression approaches.
Field data on DSi availability and sponge abundance
To estimate the annual average of DSi available to the Mediterranean sublittoral sponges in the study area, we sampled seawater from 1 cm above the rocky substrata where sponges grew using syringes during scuba dives; we also sampled the open water column above the continental shelf (about 0.5 km off the coastline) using Niskin bottles (see Supplementary Information: Section 1). These nutrient analyses (n = 240 water samples) extended over a year cycle. To estimate field DSi demands by sponges, we measured average volume of siliceous sponges per m2 of rocky bottom, using scuba and 50 × 50 cm random quadrats (n = 100) scattered along 100 Km of Mediterranean coastline (see Supplementary Information: Section 1), following the methodology detailed elsewhere12,13. We finally extrapolated DSi average uptake rates determined for Axinella spp. in the laboratory experiments to infer potential DSi demand for field sponges. Additionally, we used recent field measurements published by our team13 on sponge abundance (n = 409, 1×1m quadrats) and DSi availability (n = 48 water samples) obtained from 21.7 km2 of Belizean continental shelf (Caribbean Sea), including reefs, mangroves, seagrass beds, and sandy non-vegetated bottoms, to comparatively infer the magnitude of the annual DSi demand by the sponge populations in that ecosystem. It is worth noting that to prevent overestimation, we applied a one-fourth reduction to volume values calculated for each individual in both the Mediterranean and the Caribbean field studies.
Author Contributions
M.M. designed research, analyzed data and wrote the paper. M.M., L.A. A.Gr., A.Go, and I.V. performed research.
Supplementary Material
Supplementary Information
Acknowledgments
The authors thanks Roser Ventosa for help with DSi analyses. María Bardají, Ferrán Crespo, Angel Luque, Cèlia Sitga, María López, Eugenia Martí and Miquel Ribot are specially thanked for their varied help during either preparation or daily maintenance of the complex logistics of the laboratory experiments. Arianna Bucci is thanked for help with collection of field Mediterranean DSi data and Ana Riesgo for help with field data on sponge abundances. Craig M. Young, Sally Leys and Ricardo Aguilar are thanked for contributing pictures to the Supplementary Information, and Klaus Rützler for comments on the manuscript. This research was supported by a MINYC grant (BFU2008- 00227/BMC) and benefited from CSIC funds addressed to implement the CEAB Wet Laboratory.
References
- Nelson D. M., Tréguer P., Brzezinski M. A., Leynaert A. & Quéguiner B. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycles 9, 359–372 (1995). [Google Scholar]
- Ragueneau O. et al. A review of the Si cycle in the modern ocean: recent progress and missing gaps in the application of biogenic opal as paleoproductivity proxy. Global Planet. Change 26, 317–365 (2000). [Google Scholar]
- Sarmiento J. & Gruber N. Ocean biogeochemical dynamics. (Princeton University Press, 2006). [Google Scholar]
- Siever R. in Scientists on Gaia eds S. H. Scheneider & P. J. Boston) 287–295 (MIT Press, 1991). [Google Scholar]
- Harrison K. G. The role of increased marine silica input on paleo-pCO2 levels. Paleoceanography 15, 292–298 (2000). [Google Scholar]
- Planavsky N. J. et al. The evolution of the marine phosphate reservoir. Nature 467, 1088–1090, http://www.nature.com/nature/journal/v467/n7319/abs/nature09485.html#supplementary-information (2010). [DOI] [PubMed] [Google Scholar]
- Tréguer P. et al. The silica balance in the world ocean: a reestimate. Science 268, 375–379 (1995). [DOI] [PubMed] [Google Scholar]
- Conley D. J. & Schelske C. L. Potencial role of sponge spicules in influencing the silicon biogeochemistry of Florida lakes. Can. J. Fish. Aquat. Sci. 50, 296–302 (1993). [Google Scholar]
- Rützler K. & Macintyre I. G. Siliceous sponge spicules in coral reefs sediments. Mar. Biol. 49, 147–159 (1978). [Google Scholar]
- Reincke T. & Barthel D. Silica uptake kinetics of Halichondria panicea in Kiel Bight. Mar. Biol. 129, 591–593 (1997). [Google Scholar]
- Maldonado M., Carmona M. C., Uriz M. J. & Cruzado A. Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature 401, 785–788 (1999). [Google Scholar]
- Maldonado M. et al. Siliceous sponges as a silicon sink: An overlooked aspect of the benthopelagic coupling in the marine silicon cycle. Limnol. Oceanogr. 50, 799–809 (2005). [Google Scholar]
- Maldonado M., Riesgo A., Bucci A. & Rützler K. Revisiting silicon budgets at a tropical continental shelf: Silica standing stocks in sponges surpass those in diatoms. Limnol. Oceanogr. 55, 2001–2010, doi:10.4319/lo.2010.55.5.2001 (2010). [Google Scholar]
- Harriss R. C. Biological buffering of oceanic silica. Nature 212, 275–276 (1966). [Google Scholar]
- Hurd D. C. in Silicon geochemistry and biochemistry (ed S. R. Aston) 187–244 (Academic Press, 1983). [Google Scholar]
- Hurd D. C. & Birdwhistell S. On producing a more general model for biogenic silica dissolution. Am. J. Sci. 283, 1–28 (1983). [Google Scholar]
- Li C.-W., Chen J.-Y. & Hua T.-E. Precambrian sponges with cellular structures. Science 279, 879–882, doi:10.1126/science.279.5352.879 (1998). [DOI] [PubMed] [Google Scholar]
- Love G. D. et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721, http://www.nature.com/nature/journal/v457/n7230/suppinfo/nature07673_S1.html (2009). [DOI] [PubMed] [Google Scholar]
- Sperling E. A., Robinson J. M., Pisani D. & Peterson K. J. Where's the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8, 24–36, doi:10.1111/j.1472-4669.2009.00225.x (2010). [DOI] [PubMed] [Google Scholar]
- Bengtson S., Conway-Morris S., Cooper B. J., Jell P. A. & Runnergar B. N. Earlier Cambrian fossils from South Australia. Mem. Assoc. Australs. Paleo. 9, 1–364 (1990). [Google Scholar]
- Braun A., Chen J., Waloszek D. & Maas A. First Early Cambrian Radiolaria. Geol. Soc. London, Special Pub. 286, 143–149, doi:10.1144/sp286.10 (2007). [Google Scholar]
- Fortey R. A. & Holdsworth B. K. The oldest known well-preserved Radiolaria. Boll. Soc. Paleontol. Ital. 10, 35–41 (1971). [Google Scholar]
- Rothpletz A. Uber die Flysch Fucoiden und einige andere fosile Algen, soure uber liassische diatomeenfuhrende Hornschwamme. Z. Dsch. Geol. Ges. 48, 854–914 (1896). [Google Scholar]
- Harwood D. M. & Nikolaev V. A. in Siliceous microfossils Vol. 8 Short Courses in Paleontology eds C. D. Blome, P. M. Whalen, & R. Katherine) 81–106 (Paleontological Society, Short Courses in Paleontology 1995). [Google Scholar]
- Siever R. The silica cycle in the Precambrian. Geochim. Cosmochim. Acta 56, 3265–3272 (1992). [Google Scholar]
- Maliva R. G., Knoll A. H. & Simonson B. M. Secular change in the Precambrian silica cycle: Insights from chert petrology. Geol. Soc. Am. Bull. 117, 835–845, doi:10.1130/b25555.1 (2005). [Google Scholar]
- Lowenstam H. A. & Weiner S. On biomineralization. (Oxford University Press, 1989). [Google Scholar]
- Maldonado M. Embryonic development of verongid demosponges supports independent acquisition of spongin skeletons as alternative to the siliceous skeleton of sponges. Biol. J. Linn. Soc. 97, 427–447 (2009). [Google Scholar]
- Lazarus D. B., Kotrc B., Wulf G. & Schmidt D. N. Radiolarians decreased silicification as an evolutionary response to reduced Cenozoic ocean silica availability. Proc. Natl. Acad. Sci. USA 106, 9333–9338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. C. Radiolaria: change in skeletal weight and resistance to solution. Geol. Soc. Am. Bull. 80, 2103–2108 (1969). [Google Scholar]
- Harper H. E. & Knoll A. H. Silica, diatoms and Cenozoic radiolarian evolution. Geology 3, 175–177 (1975). [Google Scholar]
- Wiedenmayer F. Contributions to the knowledge of post-Paleozoic neritic and archibental sponges (Porifera). Schweiz. Paläontol. Abhand. 116, 1–147 (1994). [Google Scholar]
- Greenhalgh R. & Riley J. P. The determination of fluoride in natural waters, with particular reference to seawater. Anal. Chim. Acta, 25:179–188. Anal. Chim. Acta 25, 179–188, doi:10.1016/S0003-2670(01)81541-5 (1961). [Google Scholar]
- Camargo J. A. Fluoride toxicity to aquatic organisms: a review. Chemosphere 50, 251–264 (2003). [DOI] [PubMed] [Google Scholar]
- Hartman W. D. in Silicon and Siliceous Structures in Biological Systems eds T. L. Simpson & B. E. Volcani) 453–493 (Springer Verlag, 1981). [Google Scholar]
- Vacelet J. Indications de profondeur donnés par les Spongiaires dans les milieux benthiques actuels. Geol. Med. 15, 13–26 (1988). [Google Scholar]
- Pisera A. Upper Jurasic siliceous sponges from Swabian Alb: taxonomy and paleoecology. Palaeontol. Polon. 57, 1–216 (1997). [Google Scholar]
- Wille M. et al. Silicon isotopic fractionation in marine sponges: A new model for understanding silicon isotopic variations in sponges. Earth Planet. Sci. Lett. 292, 281–289 (2010). [Google Scholar]
- Hendry K. R. et al. Silicon isotopes in Antarctic sponges: an interlaboratory comparison. Antarct. Sci. 23, 34–42, doi:10.1017/S0954102010000593 (2011). [Google Scholar]
- Ayling A. L. Growth and regeneration rates in thinly encrusting demospongiae from temperate waters. Biol. Bull. Mar. Biol. Lab. Woods Hole 165, 343–352 (1983). [DOI] [PubMed] [Google Scholar]
- Shimizu K., Cha J. N., Stucky G. D. & Morse D. E. Silicatein alpha: cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 95, 6234–6238 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. N. et al. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 96, 361–365 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H.-C. et al. Silica transport in the demosponge Suberites domuncula: fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem. J. 381, 665–673, doi:10.1042/bj20040463 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamatrakoln K. & Hildebrand M. Silicon uptake in diatoms revisited: A model for saturable and nonsaturable uptake kinetics and the role of silicon transporters. Plant Physiol. 146, 1397–1407, doi:10.1104/pp.107.107094 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøhlich H. & Barthel D. Silica uptake on the marine sponge Halichondria panicea in Kiel Bight. Mar. Biol. 128, 115–125 (1997). [Google Scholar]
- De La Rocha C. L. Silicon isotope fractionation by marine sponges and the reconstruction of the silicon isotope composition of ancient deep water. Geology 31, 423–426, doi:10.1130/0091-7613(2003)0310423:sifbms>2.0.co;2 (2003). [Google Scholar]
- Turon X., Tarjuelo I. & Uriz M. J. Growth dynamics and mortality of the encrusting sponge Crambe crambe (Poecilosclerida) in two contrasting habitats: correlation with population structure and investment in defence. Funct. Ecol. 12, 631–639 (1998). [Google Scholar]
- Pansini M. & Pronzato R. in New Perspectives in Sponge Biology (ed K. Rützler) 404–415 (Smithsonian Institution Press, 1990). [Google Scholar]
- Paasche E. Silicon and the ecology of marine plankton diatoms. II. Silicate-uptake kinetics in five diatom species. Mar. Biol. 19, 262–269 (1973). [Google Scholar]
- Conway H. L. & Harrison P. L. Marine diatoms grown in chemostats under silicate or ammonium limitation. IV Transient response of Chaetoceros debilis, Skeletonema costatum and Thalassiosira gravida to a single addition of the limiting nutrient. Mar. Biol. 43, 33–43 (1977). [Google Scholar]
- Nelson D. M. & Brzenzinski M. A. Kinetics of silicic acid uptake by natural diatom assemblages in two Gulf Stream warm-core rings. Mar. Ecol. Prog. Ser. 62, 283–292 (1990). [Google Scholar]
- Jacques G. Some ecophysiological aspects of Antarctic phytoplankton. Polar Biol. 2, 27–33 (1983). [Google Scholar]
- Bhattacharyya P. & Volcani B. E. Sodium-dependent silicate transport in the apochlorotic marine diatom Nitzschia alba. Proc. Natl. Acad. Sci. USA 77, 6386–6390 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo Y. D. & Brzezinski M. A. The chemical form of dissolved Si taken up by marine diatoms. J. Phycol. 35, 1162–1170, doi:10.1046/j.1529-8817.1999.3561162.x (1999). [Google Scholar]
- Ma J. F. et al. A silicon transporter in rice. Nature 440, 688–691, http://www.nature.com/nature/journal/v440/n7084/suppinfo/nature04590_S1.html (2006). [DOI] [PubMed] [Google Scholar]
- Mock T. et al. Whole-genome expression profiling of the marine diatom Thalassiosira pseudonana identifies genes involved in silicon bioprocesses. Proc. Natl. Acad. Sci. USA 105, 1579–1584, doi:10.1073/pnas.0707946105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel D. On the ecophysiology of the sponge Halichondria panicea in Kiel Bight. II. Biomass, production, energy budget and integration in environmental processes. Mar. Ecol. Prog. Ser. 43, 87–93 (1988). [Google Scholar]
- Richardson M. D. & Young D. K. Abyssal benthos of the Venezuela Basin, Caribbean Sea: standing stock considerations. Deep Sea Res. I 34, 145–164 (1987). [Google Scholar]
- Rice A. L., Thurston M. H. & New A. L. Dense aggregations of a hexactinellid sponge, Pheronema carpenteri, in the Porcupine Seabight (northeast Atlantic Ocean), and possible causes. Prog. Oceanogr. 24, 179–196 (1990). [Google Scholar]
- Genin A., Paull C. A. & Dillon W. P. Anomalous abundances of deep-sea fauna on a rocky bottom exposed to strong currents. Deep-Sea Res. I 39 (2), 293–302 (1992). [Google Scholar]
- Maldonado M. & Young C. M. Bathymetric patterns of sponge distribution on the Bahamian slope. Deep-Sea Res. I 43, 897–915 (1996). [Google Scholar]
- Leys S. P. et al. Patterns of glass sponge (Porifera, Hexactinellida) distribution in coastal waters of British Columbia, Canada. Mar. Ecol. Prog. Ser. 283, 133–149 (2004). [Google Scholar]
- Katamani A. Physical and chemical characteristics of biogenous silica. Mar. Biol. 8, 89–95 (1971). [Google Scholar]
- Ragueneau O., Conley D. J., DeMaster D. J., Dürr H. H. & Dittert N. in Carbon and Nutrient Fluxes in Continental Margins Global Change - The IGBP Series eds Kon-Kee Liu, Larry Atkinson, Renato Quiñones, & Liana Talaue-McManus) 515–527 (Springer Berlin Heidelberg, 2010). [Google Scholar]
- DeMaster D. J. The accumulation and cycling of biogenic silica in the Southern Ocean: revisiting the marine silica budget. Deep Sea Research Part II: Topical Studies in Oceanography 49, 3155–3167 (2002). [Google Scholar]
- Finks R. M. The structure of Saccospongia laxata Bassler (Ordovician) and the phylogeny of the Demospongea. J. Paleontol. 41, 1137–1149 (1967). [Google Scholar]
- Bergquist P. R. Sponges. (University of California Press, 1978). [Google Scholar]
- Weissenfels N. & Landschoff H. W. Bau und Funktion des Süsswasserschwamms Ephydatia fluviatilis L. (Porifera). IV. Die Entwicklung der monaxialen SiO2-Nadeln in Sandwich-Kulturen. Zool. Jarhrb. 98, 355–371 (1977). [Google Scholar]
- Grasshoff K., Ehrhardt M. & Kremling K. Methods of seawater analysis. (Verlag Chemie, 1983). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information