Cell polarity in epithelia depends on the PAR proteins, which interact with the machinery for exocytic and endocytic vesicular trafficking. Polarity in Drosophila neural stem cells is independent of vesicular trafficking, although it depends on the PAR proteins, revealing different mechanisms of how polarity is controlled.
Abstract
The possession of apical–basal polarity is a common feature of epithelia and neural stem cells, so-called neuroblasts (NBs). In Drosophila, an evolutionarily conserved protein complex consisting of atypical protein kinase C and the scaffolding proteins Bazooka/PAR-3 and PAR-6 controls the polarity of both cell types. The components of this complex localize to the apical junctional region of epithelial cells and form an apical crescent in NBs. In epithelia, the PAR proteins interact with the cellular machinery for polarized exocytosis and endocytosis, both of which are essential for the establishment of plasma membrane polarity. In NBs, many cortical proteins show a strongly polarized subcellular localization, but there is little evidence for the existence of distinct apical and basolateral plasma membrane domains, raising the question of whether vesicular trafficking is required for polarization of NBs. We analyzed the polarity of NBs mutant for essential regulators of the main exocytic and endocytic pathways. Surprisingly, we found that none of these mutations affected NB polarity, demonstrating that NB cortical polarity is independent of plasma membrane polarity and that the PAR proteins function in a cell type–specific manner.
INTRODUCTION
The separation of the plasma membrane into distinct apical and basolateral membrane domains is crucial for the establishment and maintenance of cell polarity in epithelia. One essential mechanism to generate membrane asymmetry is the targeted exocytosis of apical and basolateral transport vesicles to the plasma membrane, which requires recognition between the vesicles and their target membrane (Schuck and Simons, 2004; Rodriguez-Boulan et al., 2005). This process, termed vesicle tethering, is mediated by the exocyst, an evolutionarily conserved octameric protein complex consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (Hsu et al., 1996; TerBush et al., 1996; Kee et al., 1997; Matern et al., 2001; He and Guo, 2009). In Drosophila, several studies demonstrated the importance of the exocyst complex for the maintenance of epithelial apical–basal polarity. In embryos mutant for exo84, the transmembrane protein Crumbs (Crb) mislocalizes to enlarged recycling endosomes (Blankenship et al., 2007). The resulting phenotype strongly resembles the crb loss-of-function phenotype (Tepass et al., 1990), demonstrating that the proper delivery of Crb to the apical plasma membrane domain is essential for epithelial integrity. Trafficking of Drosophila E-Cadherin (DE-Cad), another transmembrane protein important for epithelial polarity, from the recycling endosome to the plasma membrane was shown to depend on the exocyst components Sec5, Sec6, and Sec15 (Langevin et al., 2005).
In addition to targeted exocytosis, the regulation of endocytosis is also crucial for the control of epithelial apical–basal polarity. Already the earliest steps of endocytosis, including the α-adaptin–dependent sorting of receptors into clathrin-coated vesicles and the dynamin-dependent scission of vesicles at the plasma membrane, are required for epithelial cell polarity (Shivas et al., 2010). In Drosophila, mutations in genes that regulate the fusion of vesicles with early endosomes, namely avalanche, encoding a syntaxin, the small GTPase Rab5, the Rab5 effector rabenosyn-5, and Vps45 (vesicular protein sorting 45) cause the loss of epithelial polarity (Lu and Bilder, 2005; Menut et al., 2007; Morrison et al., 2008). Epithelial cells lacking the function of any of these genes show loss of the zonula adherens and mislocalization of apical proteins to the basolateral membrane. Proteins of the endosomal sorting complex required for transport (ESCRT) machinery, which are required for sorting of endocytosed cargo into multivesicular bodies, are also indispensable for epithelial polarity. Loss of function of tumor susceptibility gene 101 (TSG101, named Erupted [Ept] in Drosophila), a component of the ESCRT-I complex, and Vps25, a component of the ESCRT-II complex, cause the loss of apical–basal polarity and extensive overproliferation (Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005).
Cell polarity in many different cell types is controlled by atypical protein kinase C (aPKC) and the evolutionarily conserved PDZ-domain proteins Bazooka/Par-3 (Baz) and Par-6, which form the so-called Par/aPKC complex (Suzuki and Ohno, 2006). Loss of any of the Par/aPKC proteins in epithelial cells leads to the complete loss of apical–basal polarity (Muller and Wieschaus, 1996; Petronczki and Knoblich, 2001; Harris and Peifer, 2007; Kim et al., 2009). It was shown that vesicle trafficking and the Par/aPKC complex, together with the small Rho GTPase Cdc42, control epithelial apical–basal polarity in a mutually dependent manner (Harris and Tepass, 2008).
As in epithelial cells, the Par/aPKC complex has an essential function in the control of apical–basal polarity in neuronal stem cells of Drosophila, so-called neuroblasts (NBs; Wodarz, 2005; Knoblich, 2008). The Par/aPKC complex localizes to the apical cortex in mitotic NBs and is responsible for the basal localization of the cell fate determinants Prospero (Pros), Brain tumor (Brat), and Numb, as well as their adaptor proteins Miranda (Mira) and Partner of Numb (Pon) (Schober et al., 1999; Wodarz et al., 1999; Petronczki and Knoblich, 2001; Bello et al., 2006; Betschinger et al., 2006; Lee et al., 2006; Kim et al., 2009).
In contrast to the clear evidence for the function of targeted vesicle trafficking in apical–basal polarity in epithelial cells, data addressing the involvement of vesicle trafficking in the establishment and maintenance of polarity in Drosophila NBs are lacking. We therefore analyzed the polarity of NBs in several mutant conditions affecting different steps of vesicle trafficking. Surprisingly, we did not detect any defect of apical–basal NB polarity in any of the mutants analyzed, leading to the conclusion that targeted vesicle trafficking is dispensable for proper polarization of NBs. Moreover, our data indicate that the PAR/aPKC complex may control polarity of NBs in a manner fundamentally different from its function in epithelial cells.
RESULTS
Loss of exocyst function does not affect polarity of embryonic NBs
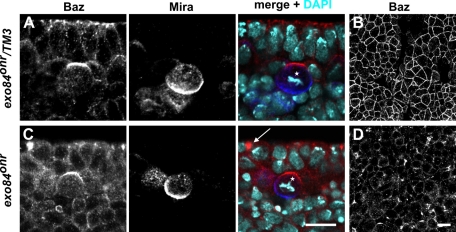
The function of the exocyst complex is crucial for apical–basal epithelial polarity (Blankenship et al., 2007). We therefore checked whether NB polarity is affected in embryos mutant for the exocyst component Exo84. exo84onr represents a hypomorphic allele of exo84, and exo84onr mutant embryos display epithelial defects caused by impaired apical targeting of the transmembrane protein Crumbs. This in turn results in mislocalization of proteins normally localized to the apical junctional region, including Baz, aPKC, DE-Cad, and Armadillo, to aggregates along the basolateral membrane of epithelial cells (Blankenship et al., 2007). Consistent with this report, we observed that Baz was lost from the apical junctional region in the embryonic neuroectoderm and localized to intracellular aggregates in exo84onr mutant embryos (Figure 1, C and D; compare to control, Figure 1, A and B). Despite of these severe epithelial polarity defects, Baz and Mira localized as in wild-type embryos in mitotic NBs of exo84onr mutant embryos (Figure 1C). Although we cannot exclude that the Exo84 protein encoded by the hypomorphic exo84onr allele possesses residual Exo84 activity sufficient to control polarity in NBs, this result indicates that the polarization mechanisms in epithelial cells and NBs are different.
FIGURE 1:
NB polarity is normal in exo84onr mutant embryos. (A and B) Baz (red) and Mira (blue) localization in an embryo heterozygous for exo84onr. Baz localizes to the apical margin of the lateral membrane in epithelial cells and to an apical crescent in NBs (A, star), whereas Mira is not expressed in the epithelium and forms a basal crescent in NBs. (B) An optical section of the same embryo as shown in A at the plane of the zonula adherens. (C and D) Baz and Mira localization in an embryo homozygous mutant for exo84onr. Baz is mislocalized to scattered aggregates (arrow) in the neuroectoderm of exo84onr mutant embryos, whereas Baz and Mira localization in NBs is indistinguishable from wild type (C, star). (D) An optical section of the same embryo as shown in C at the plane of the zonula adherens. In A and C apical is up. Scale bars, 10 μm. DNA is stained with 4′,6-diamidino-2-phenylindole (DAPI) (turquoise).
Dynamin function is not required for NB polarity
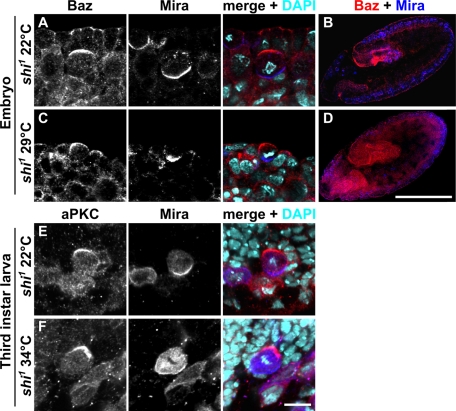
Epithelial apical–basal polarity depends on endocytosis to maintain the proper balance between apical and basolateral transmembrane proteins and lipids in the plasma membrane (Shivas et al., 2010). To test whether dynamin dependent endocytosis is required for regulating cortical polarity of Drosophila NBs we used a temperature-sensitive allele of dynamin (shibire1, shi1) and blocked endocytosis during embryonic neurogenesis. We observed severe disruption of epithelial organization (Figure 2, C and D), probably as a result of disturbed Notch signaling (Poodry, 1990) in shi1 embryos reared at the restrictive temperature of 29°C but not at the permissive temperature of 22°C (Figure 2, A and B). Although epithelial organization was severely disturbed in shi1 embryos reared at 29°C and NBs frequently failed to ingress and remained positioned in the cell layer facing the outside of the embryo (Figure 2, C and D), the apical localization of Baz and the basal localization of Mira in NBs was unaffected (Figure 2C). The same observation was made for shi1 mutant NBs in third-instar larvae shifted to 34°C (Figure 2F; Chabu and Doe, 2008), indicating that NB polarity is not dependent on dynamin function.
FIGURE 2:
Blocking dynamin-dependent endocytosis does not affect NB polarity. (A–D) Baz (red) and Mira (blue) localization in shi1 embryos reared at the permissive temperature (22°C) (A and B) and at the restrictive temperature (29°C) (C and D). Embryos are at stage 10. (E and F) Baz and Mira localization in NBs of shi1 wandering third-instar larvae reared at 22°C (E) and at 34°C (F). Except for B and D, apical is up. In B and D, dorsal is up, anterior is to the left. Scale bar except for B and D, 10 μm. Scale bar for B and D, 200 μm. DNA is stained with DAPI (turquoise).
Larval NBs mutant for different regulators of vesicle trafficking exhibit normal polarity
Recently it was shown that different components of the vesicle trafficking machinery are crucial for the maintenance of apical–basal cell polarity in epithelial cells of Drosophila (Langevin et al., 2005; Lu and Bilder, 2005; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Blankenship et al., 2007). Null mutations in most of the genes coding for the components of the vesicle-trafficking machinery either cause arrest during oogenesis if the gene product is missing in germline cells or cause the death of the animal at the end of embryogenesis or during early larval development when the zygotic gene product is missing (Murthy et al., 2003; Murthy and Schwarz, 2004; Wucherpfennig et al., 2003; Beronja et al., 2005). This complicates the analysis of NB polarity in these mutants because embryonic phenotypes are at least partially rescued by residual maternal gene product, and zygotic mutant animals do not develop far enough to analyze the phenotype of NBs at third larval instar.
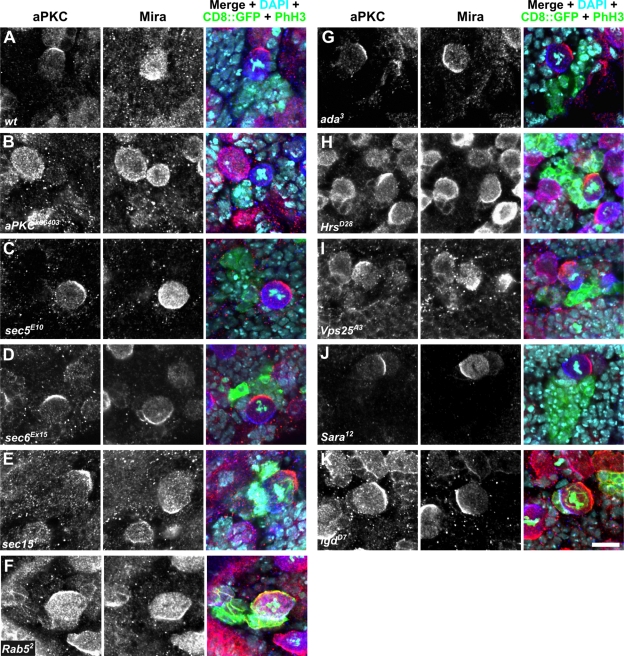
To circumvent these problems, we generated positively labeled homozygous mutant clones containing NBs in the brains of heterozygous larvae using mosaic analysis with a repressible cell marker (MARCM; Lee and Luo, 1999). As a control, clones were generated with a wild-type chromosome harboring the same FRT site as the gene under investigation (Figure 3A). In dividing NBs of these control clones, Mira and aPKC formed crescents at opposing poles of the NB cortex (Figure 3A). To check whether the MARCM technique is suitable for causing polarity defects in larval NBs, we generated positively labeled clones of the amorphic aPKC allele aPKCk06403 (Wodarz et al., 2000; Rolls et al., 2003). Loss of aPKC in embryonic and larval NBs leads to mislocalization of Mira to the whole NB cortex (Rolls et al., 2003; Kim et al., 2009). NBs in aPKCk06403 clones lacked any detectable aPKC staining, consistent with the fact that aPKCk06403 is a null allele (Figure 3B). Concomitantly, Mira was distributed all around the cell cortex, demonstrating that the MARCM technique is a suitable method to study the effect of mutations on NB polarity.
FIGURE 3:
Dividing larval neuroblasts mutant for different vesicle-trafficking genes do not exhibit polarity defects. Larval neuroblasts in wild-type (A) and mutant (B–K) MARCM clones were stained for aPKC (red), Mira (blue), GFP, and phospho-histone H3 (both in green). The genotype of the mutant clones is indicated. CD8::GFP (green) marks the homozygous mutant cells. Scale bar, 10 μm. DNA is stained with DAPI (turquoise).
To investigate exocyst function in third-instar larval NBs, we generated positively labeled clones homozygous mutant for the loss-of-function alleles sec5E10 (Figure 3C; Murthy et al., 2003), sec6Ex15 (Figure 3D; Murthy et al., 2005), and sec151 (Figure 3E; Mehta et al., 2005). NBs in these clones displayed normal localization of aPKC and Mira, indistinguishable from control clones (Figure 3A). The efficiency of the MARCM technique in the reduction of the protein levels for the protein under investigation could only be checked for Sec15. Here, Sec15 levels were strongly reduced in the clones as compared to adjacent cells expressing a wild-type allele of sec15 (Supplemental Figure S1, A and B). For Sec5 and Sec6, no antibodies suitable for immunohistochemical stainings were available.
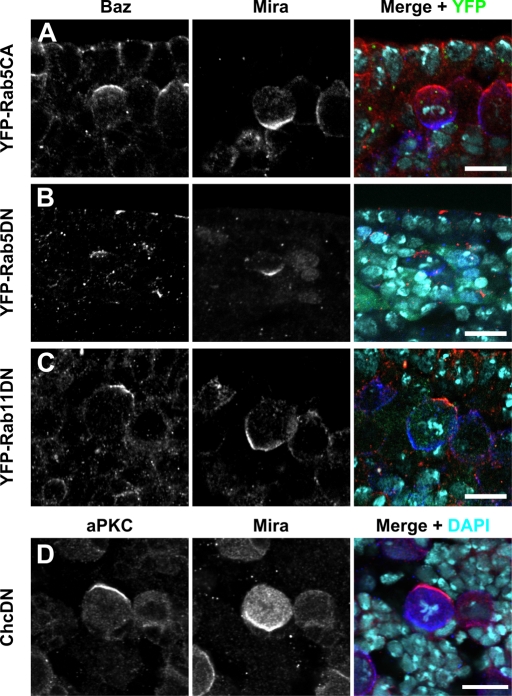
Rab5 has been implicated in the control of apical–basal polarity in epithelial cells of Drosophila (Lu and Bilder, 2005). Therefore we checked whether loss of Rab5 in larval NBs affects cell polarity. NBs of MARCM clones homozygous mutant for Rab52, a Rab5-null mutation (Wucherpfennig et al., 2003), had a normal distribution of aPKC and Mira (Figure 3F). In a complementary approach, we interfered with Rab5 function by overexpression of a constitutively active and a dominant-negative version of Rab5 in embryos (Zhang et al., 2007). In both cases, NB polarity was unaffected (Figure 4, A and B), consistent with our loss-of-function data (Figure 3F). We also overexpressed a dominant-negative version of Rab11 (Zhang et al., 2007), which has been demonstrated to affect the function of recycling endosomes. Again, NB polarity was normal under these conditions (Figure 4C), further corroborating our hypothesis that cortical NB polarity is established by a mechanism independent of endosomal trafficking.
FIGURE 4:
NB polarity is independent of Rab5, Rab11, and clathrin function. (A–C) Embryos overexpressing the yellow fluorescent protein–tagged, constitutively active (CA) or dominant-negative (DN) versions of the Rab proteins indicated on the left were stained for Baz (red) and Mira (blue). Overexpression was driven by tubulin::GAL4 (A) or worniu::GAL4 (B and C). (D) A larval brain overexpressing dominant-negative clathrin heavy chain under control of worniu::GAL4 was stained for aPKC (red) and Mira (blue). DNA was stained with DAPI (turquoise). Apical is up. Scale bar, 10 μm.
Next we generated MARCM clones homozygous for the ada3-null allele of the vesicle coat protein α-adaptin (Gonzalez-Gaitan and Jackle, 1997). NBs in ada3 clones displayed normal distribution of aPKC and Mira (Figure 3G). The same result was obtained in MARCM clones for the adaear4 allele (data not shown). adaear4 is a hypomorphic mutation in α-adaptin that specifically affects asymmetric cell divisions in sensory organ precursor cells (Berdnik et al., 2002). We also tested the function in NB polarization of another vesicle coat protein, clathrin heavy chain, by overexpression of a dominant-negative version in larval NBs. Again, apical–basal NB polarity was unaffected under these conditions (Figure 4D).
To analyze the role of ESCRT proteins for polarity of larval NBs, we generated MARCM clones homozygous for null alleles of Hrs (HrsD28), Vps25 (Vps25A3), and erupted/TSG101 (ept2) (Lloyd et al., 2002; Moberg et al., 2005; Vaccari and Bilder, 2005). MARCM clones for HrsD28 and Vps25A3 showed normal NB polarity (Figure 3, H and I). We rarely observed small clones of cells homozygous for ept2, but we never observed NBs in these clones (data not shown). It is possible that these clones result from inappropriate NB differentiation, but it is more likely that NB loss is caused by cell lethality of the mutation.
We also tested two additional mutations in genes involved in vesicular trafficking that had been shown to affect asymmetric division of larval sense organ precursor cells for defects in apical–basal NB polarity. Neither larval NBs mutant for the Sara12-null allele of Smad anchor for receptor activation (Sara) (Bokel et al., 2006) nor NBs mutant for the lgdD7-null allele of lethal (2) giant discs (lgd) (Jaekel and Klein, 2006) showed polarity defects (Figure 3, J and K).
Table 1 summarizes the genes and alleles that were used to generate MARCM clones and the effects on Mira and aPKC localization in NBs homozygous for the indicated alleles.
TABLE 1:
Summary of MARCM experiments to study the function of vesicular trafficking during asymmetric division of larval NBs.
| Miranda localization | aPKC localization | ||||
|---|---|---|---|---|---|
| Gene | Allele | Normal | Mislocalized | Normal | Mislocalized |
| sec5 | sec5E10 | 18 | 0 | 18 | 0 |
| sec6 | sec6Ex15 | 23 | 0 | 23 | 0 |
| sec15 | sec151 | 14 | 0 | 14 | 0 |
| Rab5 | Rab52 | 18 | 0 | 18 | 0 |
| alpha-adaptin | ada3 | 20 | 0 | 20 | 0 |
| Hrs | HrsD28 | 21 | 0 | 21 | 0 |
| Vps25 | Vps25A3 | 14 | 0 | 14 | 0 |
| Sara | Sara12 | 15 | 0 | 15 | 0 |
| erupted (TSG101) | ept2 | No clones obtained | |||
| lgd | lgdd7 | 15 | 0 | 15 | 0 |
| aPKC | aPKCk06403 | 0 | 10 | Not detectable | Not detectable |
Numbers indicate in how many dividing NBs in MARCM clones of the indicated genotype Mira and aPKC were normal or mislocalized. All analyzed NBs were in late prophase to anaphase. “No clones obtained” indicates that under the chosen experimental conditions no clones containing NBs homozygous for the indicated null alleles were recovered.
DISCUSSION
Our data strongly indicate that vesicle trafficking is not involved in polarization of NBs, in contrast to epithelia, where it is essential for polarity. In epithelial cells vesicle trafficking controls cell polarity mainly by regulating the levels of the transmembrane proteins Crumbs and DE-Cad at the membrane (Langevin et al., 2005; Lu and Bilder, 2005; Blankenship et al., 2007). In NBs no asymmetrically localized transmembrane protein has been described so far, except for one: Numb-interacting protein (NIP) is a multipass transmembrane protein that colocalizes with Numb at the basal cortex of dividing NBs. In Drosophila Schneider cells, Numb and NIP colocalize at the plasma membrane, and RNA interference–mediated knockdown of NIP results in a release of Numb from the plasma membrane (Qin et al., 2004). Whether NIP is required for proper localization of Numb in dividing NBs is not known since no null mutation in moladietz, the gene encoding NIP, is available. It has also not been studied whether Numb may be required for the asymmetric localization of NIP in NBs.
So, how could cell polarity be established and maintained in NBs? Baz, PAR-6, and aPKC are all localized to the apical junctional region of the neuroectodermal epithelium at the time when NBs ingress from the epithelium. Thus the components of the PAR/aPKC complex are already apically enriched in NBs prior to their first division. We furthermore know that Baz can associate with the plasma membrane by direct binding to phosphoinositide lipids (Krahn et al., 2010). However, there is no evidence for an asymmetric distribution of phosphoinositides in NBs, which might cause the asymmetric localization of Baz. In analogy to the mechanism that operates in the Caenorhabditis elegans zygote (Gonczy, 2008), we favor the hypothesis that the apical localization of Baz is stabilized by a mutual repression mechanism involving phosphorylation of Baz by the basally localized kinase PAR-1 (Benton and St Johnston, 2003; Krahn et al., 2009) and phosphorylation of PAR-1 by aPKC (Hurov et al., 2004; Kusakabe and Nishida, 2004). Although this mechanism may be sufficient to stably polarize an NB, extrinsic cues from adjacent neuroectodermal cells contribute to the positioning of the Baz crescent to the apical cortex (Siegrist and Doe, 2006).
In conclusion, our work shows for the first time that cortical polarity in NBs can be established even when intracellular vesicular trafficking is blocked, in striking contrast to the situation in epithelia. Although we cannot completely rule out the possibility that the lack of polarity phenotypes in NBs homozygous for the mutations that we analyzed may be due to the perdurance of the respective wild-type protein in the clones, we consider this possibility unlikely. It has been shown that the same mutations that we analyzed in NBs cause strong polarity phenotypes when clones are induced in epithelia. Furthermore, in some of our experiments we can rule out perdurance, for example in the experiments using the shi1 allele, and these also showed no polarity defects in NBs.
Our findings imply that the PAR/aPKC complex can function in different ways, to polarize only the cortex, as in NBs or the C. elegans zygote, or the cortex and the plasma membrane, as in epithelia and probably also in neurons. In the future it will be important to dissect these different mechanisms at the molecular level in order to understand the function of the PAR proteins in a specific cellular context.
MATERIAL AND METHODS
Fly stocks and genetics
The following stocks and alleles were used in this study: w1118 shi1 (#7068), Df(3R)Espl3/TM6C cu1 Sb1 Tb1 ca1 (#5601), elav::Gal4 UAS-mCD8::GFP hsFLP (#5146), y1 w*; FRT42D GAL80/CyO y+ (#9917), y1 w*; GAL80 FRT40A/CyO (#5192), y1 w*;; GAL80 FRT80B (#5191), y1 w*;; FRT82B GAL80 (#5135), y1 w*; P{w[+mC] = tubP-GAL4}LL7/TM3 Sb1 (#5138), w*;; UAS-Chc.DN (#26874) (Bloomington Drosophila Stock Center, Indiana University, Bloomington, IN; stock number given in parenthesis), and onr142-5/TM3 hb-lacZ (Giansanti et al., 2004; Blankenship et al., 2007), w; adaear4 FRT40A (Berdnik et al., 2002), w; ada3 FRT40A (Gonzalez-Gaitan and Jackle, 1997), w; HrsD28 FRT40A (Lloyd et al., 2002), w; Rab52 FRT40A (Wucherpfennig et al., 2003), w; lgdd7 FRT40A ( Jaekel and Klein, 2006), w; FRT42D Sara12 (Bokel et al., 2006), w; FRT42D aPKCk06403 (Wodarz et al., 2000; Rolls et al., 2003), w; FRT42D Vps25A3 (Vaccari and Bilder, 2005), w;; FRT80B ept2 (Moberg et al., 2005), y w; sec5E10 FRT40/CyO y+ (Murthy et al., 2003), w; FRT42D sec6Ex15 (Murthy et al., 2005), w;; FRT82B sec151/TM3 (Mehta et al., 2005), y1 w*; P{UASp-YFP.Rab5.Q88L}24, y1 w*; P{UASp-YFP.Rab5.S43N}01, y1 w*; P{UASp-YFP.Rab11.S25N}06 (Zhang et al., 2007), and w; worniu::GAL4 (Siegrist and Doe, 2005).
Embryos maternally and zygotically mutant for onion rings142-5 (Exo84onr) were obtained as described in Blankenship et al. (2007), with the exception that Exo84onr was balanced over TM3 ftz::lacZ to facilitate genotyping by the absence of lacZ expression.
To generate embryos lacking shibire (shi) function, w1118 and shi1 flies were allowed to lay eggs for 1 h at 22°C; embryos were kept at 22°C for 5 h and were then shifted to 29°C for 2 h. As a control w1118 and shi1 embryos were kept at 22°C for 2 h instead.
w1118 shi1 wandering third-instar larvae were shifted to 34°C at 96 h after larval hatching for 6 h and directly dissected and fixed.
To generate positively labeled MARCM clones, the following crosses were conducted (females listed first):
elav::Gal4 UAS-mCD8::GFP hsFLP; GAL80 FRT40A/CyO × w; ada3 FRT40A/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; GAL80 FRT40A/CyO × y w; sec5E10 FRT40/CyO y+
elav::Gal4 UAS-mCD8::GFP hsFLP; GAL80 FRT40A/CyO × w; rab52 FRT40A/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; GAL80 FRT40A/CyO × w; HrsD28 FRT40A/CyO
elav::Gal4 UAS-mCD8::GFP, hsFLP; GAL80 FRT40A/CyO × w; lgdd7 FRT40A/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; GAL80 FRT40A/CyO × w; FRT40A
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT42D GAL80/CyO × w; FRT42D vps25A3/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT42D GAL80/CyO × w; FRT42D Sara12/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT42D GAL80/CyO × w; FRT42D sec6Ex15/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT42D GAL80/CyO × w; FRT42D aPKCk06403/CyO
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT42D GAL80/CyO × w; FRT42D
elav::Gal4 UAS-mCD8::GFP hsFLP;; GAL80 FRT80B/CyO × w;; ept2 FRT80B/TM6B
elav::Gal4 UAS-mCD8::GFP hsFLP;; GAL80 FRT80B/CyO × w;; FRT80B
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT82B GAL80 × w;; FRT82B sec151/TM6B
elav::Gal4 UAS-mCD8::GFP hsFLP; FRT82B GAL80 × w;; FRT82B
For induction of MARCM clones, eggs were collected for a period of 24 h. After incubation at 25°C for another 24 h, larvae were heat shocked during L1 once for 2 h in a 37°C water bath to induce mitotic recombination. Brains of wandering third-instar larvae were dissected and stained.
Immunohistochemistry
Embryos and brains of wandering third-instar larvae were fixed in 4% formaldehyde, phosphate-buffered saline (PBS), pH 7.4. The primary antibodies used were rabbit anti-PKCζ C20 (Santa Cruz Biotechnology, Santa Cruz, CA) 1:1000, rabbit anti-Baz (Wodarz et al., 1999) 1:1000, guinea pig anti-Mira (Kim et al., 2009) 1:1000, rabbit anti-Sara (Bokel et al., 2006) 1:200, guinea pig anti-Hrs (Lloyd et al., 2002) 1:1000, mouse anti–phospho-histone H3 (6G3; Cell Signaling Technology, Danvers, MA) 1:1000, mouse anti–green fluorescent protein (GFP; Invitrogen, Carlsbad, CA) 1:1000, mouse anti–β-galactosidase JIE7 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) 1:50, and guinea pig anti-Sec15 (Mehta et al., 2005) 1:500. Secondary antibodies conjugated to Cy2, Cy3, Cy5 (Jackson Laboratories, West Grove, PA), Alexa Fluor 647, and Alexa Fluor 488 (Invitrogen) were used at 1:400. Images were taken on a Zeiss (Wetzlar, Germany) LSM 510 Meta confocal microscope and processed using Gimp and Inkscape.
Supplementary Material
Acknowledgments
We thank Hugo Bellen, Todd Blankenship, David Bilder, Dave Featherstone, Marcos Gonzalez-Gaitan, Thomas Klein, Jürgen Knoblich, Kenneth Moberg, Thomas Schwarz, Ulrich Tepass, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks and antibodies. We also thank Mona Honemann-Capito and Katja Brechtel-Curth for technical assistance and Ernst A. Wimmer, Marcos Gonzalez-Gaitan, and members of the Wodarz laboratory for discussion. This work was supported by grants of the Deutsche Forschungsgemeinschaft to A.W. (Sonderforschungsbereich 523, Teilprojekt B15, Research Center for Molecular Physiology of the Brain).
Abbreviations used:
- ada
α-adaptin
- aPKC
atypical protein kinase C
- Baz
Bazooka
- Brat
Brain tumor
- Cdc42
cell division control protein 42
- Chc
clathrin heavy chain
- Crb
Crumbs
- DAPI
4′,6-diamidino-2-phenylindole
- DE-Cad
Drosophila Epithelial Cadherin
- elav
embryonic lethal abnormal vision
- Ept
Erupted
- ESCRT
Endosomal sorting complex required for transport
- Exo
Exocyst
- FRT
flipase recombinase target
- Hrs
hepatocyte growth factor regulated tyrosine kinase substrate
- Lgd
lethal (2) giant discs
- MARCM
mosaic analysis with a repressible cell marker
- Mira
Miranda
- NBs
neuroblasts
- NIP
Numb-interacting protein
- Onr
onion rings
- Par
partitioning-defective
- PDZ
postsynaptic density 95, Discs-large, Zonula occludens-1
- Pon
Partner of Numb
- Pros
Prospero
- Rab
Ras related GTP binding protein
- Sara
smad anchor for receptor activation
- Sec
secretory
- Shi
Shibire
- TSG101
tumor susceptibility gene 101
- Vps
vesicular protein sorting
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0219) on September 21, 2011.
REFERENCES
- Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 2006;133:2639–2648. doi: 10.1242/dev.02429. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA. The endocytic protein alpha-adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Fuller MT, Zallen JA. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci. 2007;120:3099–3110. doi: 10.1242/jcs.004770. [DOI] [PubMed] [Google Scholar]
- Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science. 2006;314:1135–1139. doi: 10.1126/science.1132524. [DOI] [PubMed] [Google Scholar]
- Chabu C, Doe CQ. Dap160/intersectin binds and activates aPKC to regulate cell polarity and cell cycle progression. Development. 2008;135:2739–2746. doi: 10.1242/dev.024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Farkas RM, Bonaccorsi S, Lindsley DL, Wakimoto BT, Fuller MT, Gatti M. Genetic dissection of meiotic cytokinesis in Drosophila males. Mol Biol Cell. 2004;15:2509–2522. doi: 10.1091/mbc.E03-08-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M, Jackle H. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Jaekel R, Klein T. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev Cell. 2006;11:655–669. doi: 10.1016/j.devcel.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Kee Y, Yoo JS, Hazuka CD, Peterson KE, Hsu SC, Scheller RH. Subunit structure of the mammalian exocyst complex. Proc Natl Acad Sci USA. 1997;94:14438–14443. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Gailite I, Moussian B, Luschnig S, Goette M, Fricke K, Honemann-Capito M, Grubmuller H, Wodarz A. Kinase-activity-independent functions of atypical protein kinase C in Drosophila. J Cell Sci. 2009;122:3759–3771. doi: 10.1242/jcs.052514. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Egger-Adam D, Wodarz A. PP2A antagonizes phosphorylation of Bazooka by PAR-1 to control apical-basal polarity in dividing embryonic neuroblasts. Dev Cell. 2009;16:901–908. doi: 10.1016/j.devcel.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Klopfenstein DR, Fischer N, Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol. 2010;20:636–642. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Kusakabe M, Nishida E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 2004;23:4190–4201. doi: 10.1038/sj.emboj.7600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lee CY, Wilkinson BD, Siegrist SE, Wharton RP, Doe CQ. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev Cell. 2006;10:441–449. doi: 10.1016/j.devcel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Matern HT, Yeaman C, Nelson WJ, Scheller RH. The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc Natl Acad Sci USA. 2001;98:9648–9653. doi: 10.1073/pnas.171317898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SQ, et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–232. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Menut L, Vaccari T, Dionne H, Hill J, Wu G, Bilder D. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;19:4167–4176. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. Sec6 mutations and the Drosophila exocyst complex. J Cell Sci. 2005;118:1139–1150. doi: 10.1242/jcs.01644. [DOI] [PubMed] [Google Scholar]
- Murthy M, Schwarz TL. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development. 2004;131:377–388. doi: 10.1242/dev.00931. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Poodry CA. shibire, a neurogenic mutant of Drosophila. Dev Biol. 1990;138:464–472. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- Qin H, Percival-Smith A, Li C, Jia CY, Gloor G, Li SS. A novel transmembrane protein recruits numb to the plasma membrane during asymmetric cell division. J Biol Chem. 2004;279:11304–11312. doi: 10.1074/jbc.M311733200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Schaefer M, Knoblich JA. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature. 1999;402:548–551. doi: 10.1038/990135. [DOI] [PubMed] [Google Scholar]
- Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development. 2006;133:529–536. doi: 10.1242/dev.02211. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Wodarz A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol. 2005;17:475–481. doi: 10.1016/j.ceb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.