Abstract
Brain norepinephrine and dopamine regulate a variety of critical behaviors such as stress, learning, memory, and drug addiction. Here, we demonstrate differences in the regulation of in vivo neurotransmission for dopamine in the anterior nucleus accumbens (NAc) and norepinephrine in the ventral bed nucleus of the stria terminalis (vBNST) of the anesthetized rat. Release of the two catecholamines was measured simultaneously using fast-scan cyclic voltammetry (FSCV) at two different carbon-fiber microelectrodes, each implanted in the brain region of interest. Simultaneous dopamine and norepinephrine release was evoked by electrical stimulation of a region where the ventral noradrenergic bundle (VNB), the pathway of noradrenergic neurons, courses through the ventral tegmental area/substantia nigra (VTA/SN), the origin of dopaminergic cell bodies. The release and uptake of norepinephrine in the vBNST were both significantly slower than for dopamine in the NAc. Pharmacological manipulations in the same animal demonstrated that the two catecholamines are differently regulated. The combination of a dopamine autoreceptor antagonist and amphetamine significantly increased basal extracellular dopamine whereas a norepinephrine autoreceptor antagonist and amphetamine did not change basal norepinephrine concentration. α-Methyl-p-tyrosine, a tyrosine hydroxylase inhibitor, decreased electrically evoked dopamine release faster than norepinephrine. The dual-microelectrode FSCV technique along with anatomical and pharmacological evidence confirms that dopamine in the NAc and norepinephrine in the vBNST can be monitored selectively and simultaneously in the same animal. The high temporal and spatial resolution of the technique enabled us to examine differences in the dynamics of extracellular norepinephrine and dopamine concurrently in two different limbic structures.
Keywords: Norepinephrine, dopamine, nucleus accumbens (NAc), ventral bed nucleus of the stria terminalis (vBNST), fast-scan cyclic voltammetry (FSCV)
Introduction
Dopamine and norepinephrine, the major catecholamines in the central nervous system, are involved in a number of behaviors including learning, memory, arousal, stress, and drug addiction (Owesson-White et al. 2008, Onaka & Yagi 1998, Aston-Jones & Cohen 2005, Aston-Jones et al. 1999, Berridge & Waterhouse 2003, Forray & Gysling 2004, Weinshenker & Schroeder 2007, Aragona et al. 2008, Phillips & Wightman 2003). Noradrenergic neurons project widely throughout the brain to areas such as the thalamus, hippocampus, hypothalamus, bed nucleus of the stria terminalis (BNST), and cortex (Berridge & Waterhouse 2003, Paxinos 1995, Harley 2004). Dopaminergic neurons project heavily to the frontal cortex, striatum, and limbic areas such as the anterior nucleus accumbens (NAc) and the amygdala (Harley 2004, Moore & Bloom 1978). However, many of the rat brain regions that have appreciable amounts of catecholamines are often only a few hundred microns across. Thus, techniques with high spatial (submillimeter) as well as temporal (subsecond) resolution are required to evaluate the rapid catecholamine fluctuations in these discrete locations. The high temporal and spatial resolution achieved with fast-scan cyclic voltammetry (FSCV) at carbon-fiber microelectrodes has been successfully used to characterize dopamine dynamics in several brain regions (Stamford & Justice 1996, Robinson et al. 2008, Garris & Wightman 1995b). Our previous work showed that FSCV can also measure norepinephrine release, although it needs to be used in conjunction with select pharmacological agents and careful histological measurements of electrode placement to distinguish norepinephrine from dopamine (Park et al. 2009, Park et al. 2010). Norepinephrine was found to be the primary released catecholamine in the vBNST while dopamine was the predominant catecholamine released in the NAc.
Although the regulatory mechanisms of dopamine and norepinephrine neurotransmission have many similarities, previous studies have revealed important differences (Mitchell et al. 1994, Garris & Wightman 1995a, Park et al. 2009, Weinshenker & Schroeder 2007). Here, we used FSCV with two separate carbon-fiber microelectrodes to study regulation of norepinephrine in the vBNST and dopamine in the NAc simultaneously in the same animal. Simultaneous measurements in the same animal eliminate experimental confounds such as variable levels of anesthesia and allow direct comparison of neurotransmitter dynamics under identical conditions (Zachek et al. 2010). Here, simultaneous release of norepinephrine in the vBNST and dopamine in the NAc was evoked by electrical stimulation of the ventral tegmental area and substantia nigra (VTA/SN) region, the site of dopamine cell bodies. Because the ventral noradrenergic bundle (VNB), the pathway of noradrenergic neurons which originates mainly from nucleus of the solitary tract (NST, A2) and A1 cell group, passes directly through this region (Ungerstedt 1971, Miyahara & Oomura 1982, Saphier 1993), stimulation evokes release of both catecholamines (Park et al. 2009). In the present study, we demonstrate successfully for the first time simultaneous measurement of both electrically evoked norepinephrine and dopamine in the two brain regions for the comparison of their in vivo characteristics and the drug effects on their regulation. We observed that the regulation of the two catecholamines in the target regions were strikingly different.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing between 280–400 g were purchased from Charles Rivers (Wilmington, MA) and housed in temperature and humidity controlled rooms with a 12 h light-dark cycle. Food and water were continuously available ad libitum. All procedures for handling and caring for the laboratory animals were in accordance with the Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Surgery
Rats were anesthetized with urethane (1.5 mg/kg) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). All coordinates were obtained from the rat brain atlas (Paxinos & Watson 2007). Anteroposterior (AP), mediolateral (ML) and dorsoventral (DV) positions were referenced from bregma. The skull surface was exposed, and four small holes were drilled in the skull for insertion of electrodes. The instrumentation has been described in Takmakov et al. (Takmakov et al.). The bipolar, stainless-steel stimulating electrode (Plastics One, Roanoke, VA) was placed into the VTA/SN (AP −5.2 mm, ML 1.2 mm, DV from −8.0 to −9.0 mm). The stimulating electrode was insulated to the tip (0.2 mm diameter) and the tips were separated by ~ 1.0 mm. The Ag/AgCl reference electrode was placed into the contralateral cortex and was held with dental cement in contact with the skull to minimize the number of manipulators used. The pia mater was punctured, removed and two carbon-fiber microelectrodes were implanted vertically into vBNST (AP 0.0 mm, ML +1.2 mm, DV from −6.5 to −7.5 mm) and NAc (AP +1.7 mm, ML +1.0 mm, DV from −5.5 to −7.5 mm). Temperature was maintained at 37 °C by a heating pad (Harvard Apparatus, Holliston, MA).
Electrical stimulation
Pulses delivered to the stimulating electrode were computer-generated with a 6711 PCI card (National Instruments, Austin, TX, USA) and were optically isolated from the electrochemical system (NL 800A, Neurolog, Digitimer Ltd, Hertfordshire, UK). The electrical stimulation consists of biphasic square wave pulses (300 μA, 2 ms each phase unless otherwise noted), and stimulation frequencies between 10 to 60 Hz were applied. Normally, the number of stimulus pulses was held constant at 60. Each stimulation was applied every 4 or 5 min to allow time for releasable stores to return to their original levels (Kita et al. 2007).
Fast-Scan cyclic voltammetry (FSCV)
Glass-encased cylindrical carbon-fiber and Ag/AgCl reference electrodes were prepared as described earlier (Cahill & Wightman 1995). T-650 carbon fibers (Thornel, Amoco Corp., Greenville, SC) with an exposed length of 75 – 100 μm and 6 μm in nominal diameter were used. The experimental setup for dual measurement has been described previously (Zachek et al. 2009, Zachek et al. 2010). A locally modified version of TH1- software was used with a Quad UEI instrument that has four independent current transducers and can support four different carbon-fiber microelectrodes (University of North Carolina Department of Chemistry Electronic Shop). Both of the carbon-fiber microelectrodes were referenced to the sole reference electrode. A triangular waveform (−0.4 to +1.3 V and back to −0.4 V, 400V/s, repeated at 100 ms intervals) was simultaneously applied to both microelectrodes. The triangular waveform was low-pass filtered at 2 kHz. Data were digitized and processed using NI-6711 and NI-6251 DAC/ADC cards and TH-1 software. Background-subtracted cyclic voltammograms were obtained by digitally subtracting voltammograms collected during baseline recording from those collected during electrical stimulation event. Temporal responses were determined by monitoring the current at the peak potential for catecholamine oxidation in successive voltammograms (pH changes (Takmakov et al. 2010) caused minimal interference in the present studies). Because the carbon-fiber microelectrode was used to generate a lesion for histology, the oxidation current was converted to concentration based on the averaged dopamine or norepinephrine calibration factors 6.9 ± 0.3 pA/(μM·μm2) for dopamine, 4.5 ± 0.2 pA/(μM·μm2) for norepinephrine (Park et al. 2010). A lesion was made at the recording site by applying constant current (20 μA for 10 s) to the implanted carbon-fiber electrodes as described earlier (example in Supplementary Fig. 1) (Park et al. 2009, Park et al. 2010). Brains were removed from the skull and stored in 10 % formaldehyde for at least 3 days, and coronally sectioned into 40–50 μm thick slices with a cryostat. The sections mounted on slides were stained with 0.2 % thionin, and coverslipped before viewing under a light microscope.
Drugs and reagents
All chemicals and drugs were reagent-quality and were used without additional purification. Drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA). In vitro postcalibration of carbon-fiber microelectrodes was performed in a Tris buffer solution at pH 7.4 containing 15 mM Tris, 140 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2, and 2.0 mM Na2SO4 in double distilled water (Mega Pure System, Corning Glasswork, Corning, NY). The following drugs, desipramine-HCl, raclopride-HCl, yohimbine-HCl, idazoxan-HCl, d-amphetamine sulfate, α-methyl-DL-p-tyrosine methyl ester-HCl and L-3,4-dihydroxyphenylalanine methyl ester-HCl were dissolved in saline. GBR 12909-HCl was dissolved in double distilled water and then diluted with saline. All drugs were injected intraperitoneally (i.p.).
Data analysis
Voltammetric data are presented in a form of color plots where abscissa represents time, ordinate represents potential and current is encoded in false color (Michael et al. 1999). Clampfit 8.1 as part of pCLAMP 8.1 software package (Axon Instruments, Foster City, CA) was used to analyze all data as described earlier (Park et al. 2006). tr is the time to reach the maximum concentration and t1/2, was taken as the time to descend from its maximum value to half of that value. [CA]max is the maximal evoked catecholamine concentration. Catecholamine transients were identified by a principal component regression algorithm as descried earlier (Heien et al. 2004, Keithley et al. 2009). Catecholamine transients were defined as signals that were greater than five times the root-mean-square noise level and were analyzed for frequency, amplitude, and t1/2 using Mini Analysis Software (Synaptosoft, Decatur, GA, USA) (Park et al. 2010). Data are represented as mean ± S.E.M. and ‘n’ values indicate the number of rats. Mean values were compared by using the Student’s t test (GraphPad Software, San Diego, CA, USA) and P < 0.05 was regarded as statistically significant.
Results
Simultaneous monitoring of dopamine in the NAc and norepinephrine in the vBNST
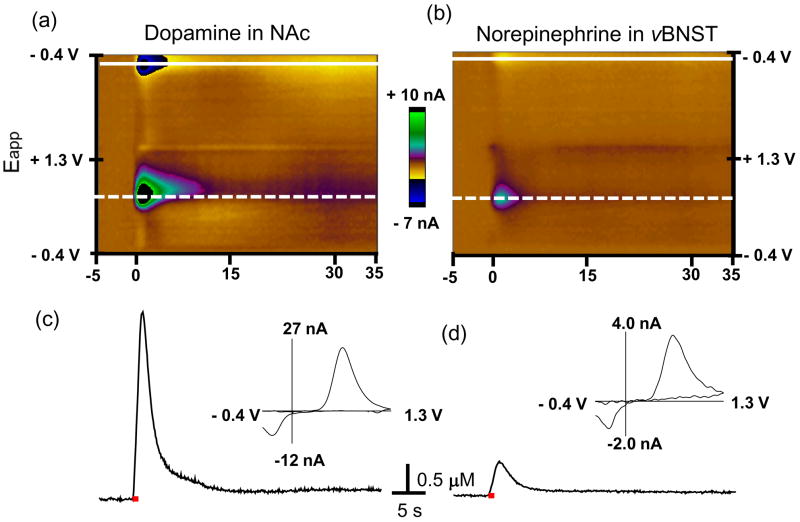
Electrical stimulation (60 Hz) of the VTA/SN causes simultaneous catecholamine release that can be monitored in the NAc and vBNST in a single animal (Fig. 1). Both the color plots and the individual background subtracted voltammograms establish the signals are due to catecholamines. Both catecholamines are oxidized at ~ +0.65 V (dashed line) and their catecholamine-o-quinone forms are reduced at ~ −0.23 V (solid line). The current at ~ +0.65 V in both regions increased rapidly during electrical stimulation and afterwards decreased back to the prestimulation basal level (Fig. 1c and d). This time-dependent concentration during the stimulation increase is due to the altered balance between release and uptake of catecholamines and diffusion from multiple varicosities near the microelectrodes. After the stimulation catecholamine concentrations decrease due to uptake coupled to diffusion (Garris & Wightman 1995b). Our prior pharmacological and histological characterization of these regions established that dopamine is the predominant catecholamine released in the NAc and norepinephrine is the predominant catecholamine in the vBNST (Park et al. 2009, Park et al. 2010). The average maximum dopamine concentration evoked in the NAc was ~ 4 fold higher than the maximal norepinephrine concentration in the vBNST evoked by the same stimulation (Table 1). The rise time (tr), the time from the signal onset to the point the maximum is reached of the dopamine concentration and its half-decay time (t1/2), the time to descend from its maximum value to half of that value, were significantly faster than those of norepinephrine in the vBNST (P < 0.05 for both tr and t1/2) (Table 1).
Figure 1. Signal identification of voltammetric data recorded in the NAc and vBNST.
Upper panels: color plots for the voltammetric data, with current changes encoded in false color for dopamine in NAc (a) and norepinephrine in the vBNST (b). The sets composed of all background-subtracted cyclic voltammograms recorded for 40 s before and after electrical stimulation of the VTA/SN and VNB (60 Hz, 60 pulses; delivered at 0 s). Catecholamine concentration changes are apparent in the color plots at the potential for their oxidation (~ 0.65 V, dotted line) and reduction (~−0.23 V, solid line). The traces of dopamine in the NAc (c) and norepinephrine in the vBNST (d) evoked by the electrical stimulation measured. The traces (dotted line) were shown at the potential at which catecholamine is oxidized. Electrical stimulation is indicated with the solid red bars under the traces. Insets: background-subtracted cyclic voltammograms recorded at the maximum of the evoked release.
Table 1.
Numerical parameters measured from the dopamine in the NAc and norepinephrine in the vBNST evoked by the electrical stimulation (60 Hz, 60 pulses).
| Rats (n=6) | Dopamine in the NAc | Norepinephrine in the vBNST |
|---|---|---|
| [CA]max (μM) | 1.35 ± 0.33 | 0.31 ± 0.05* |
| tr (s) | 1.04 ± 0.14 | 1.70 ± 0.17* |
| t1/2 (s) | 1.19 ± 0.38 | 2.59 ± 0.47* |
[CA]max is the maximal evoked catecholamine concentration; tr is the time to reach to [CA]max from the start of the stimulation; t1/2 is the time required for catecholamine overflow to decay to 50 % of the maximum. Values represent the mean ± S.E.M. The values were compared by unpaired t-tests.
(P < 0.05) indicates significantly different from the evoked dopamine in the NAc.
Effects of the stimulation conditions on dopamine release in the NAc and norepinephrine release in the vBNST
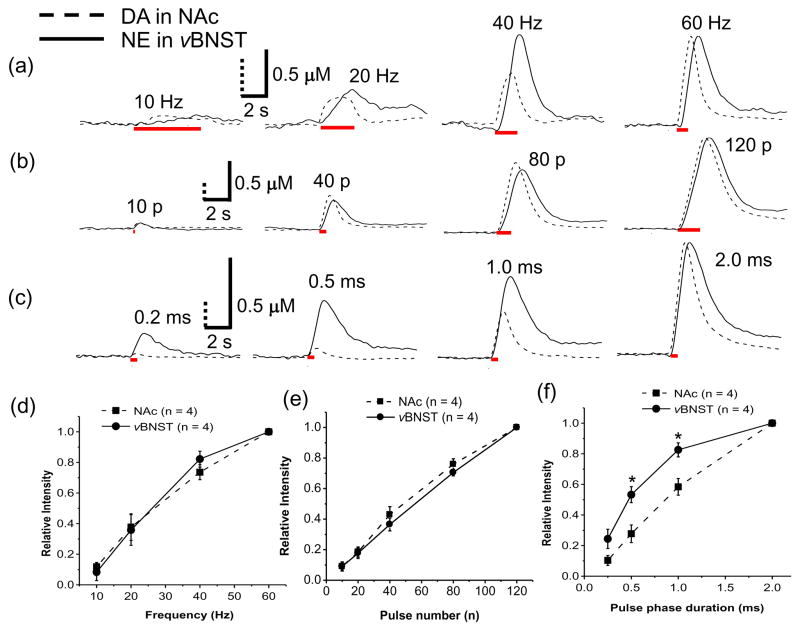
Subsecond changes of dopamine and norepinephrine concentration simultaneously elicited by various electrical stimulation parameters (stimulation frequency, pulse number and pulse width) were investigated and compared (Fig. 2). Increasing the stimulation frequency from 10 to 60 Hz (60 pulses with 2 ms pulse width) caused an increase in both evoked dopamine in the NAc and norepinephrine in the vBNST (representative examples in Fig. 2a). While the evoked dopamine concentration in the NAc during low frequency stimulations (≤20 Hz) reached a plateau, the norepinephrine concentration in the vBNST continued to increase during the stimulation. At higher frequencies (> 20 Hz), dopamine and norepinephrine concentrations increased continuously during the stimulation. The maximal responses observed in all animals are shown in Figure 2d.
Figure 2. Comparison of dopamine response in the NAc to norepinephrine response in the vBNST.
Individual dopamine (dotted line) trace in the NAc and norepinephrine (solid line) trace in the vBNST as a function of stimulation frequency (10, 20, 40, and 60 Hz) at 60 pulses (a), pulse number (10, 40, 80, and 120) at 60 Hz (b) and pulse width (0.2, 0.5, 1.0, and 2.0 ms) (c) are shown. Electrical stimulation is indicated with the solid red bars under the traces. Maximal catecholamine responses in the NAc and the vBNST as a function of stimulation frequency (d), pulse number (e) and pulse width (f). Relative responses in (d), (e) and (f) are the monitored response ([CA]x) divided by the maximum response ([CA]max). *indicates significantly different from the evoked dopamine in the NAc (P < 0.05).
Both evoked dopamine and norepinephrine concentrations increased with pulse numbers from 10 to 120 pulses (at 60 Hz with 2 ms pulse width, representative examples in Fig. 2b, average responses in Fig. 2e). Measurable responses (signal to noise (S/N) ≥ 5) in this study were normally observed with greater than 10 pulses for norepinephrine and greater than 5 pulses for dopamine at 60 Hz. The effect of stimulus pulse width was also evaluated. Evoked norepinephrine concentrations in the vBNST were less sensitive to pulse width than was evoked dopamine in the NAc (representative example in Fig. 2c, average responses in Fig. 2f).
Spatial distribution of VTA/SN sites for evoked catecholamine release
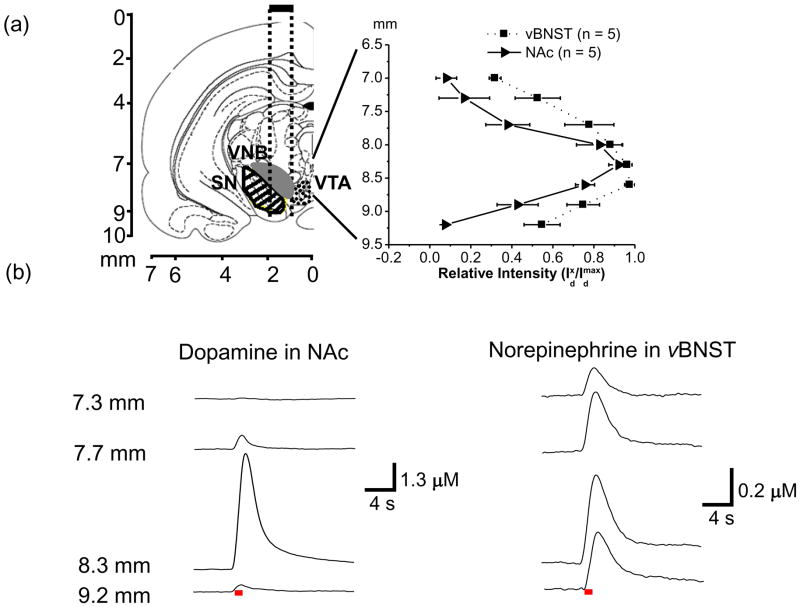
The VNB, the pathway of noradrenergic neurons, passes within the VTA/SN. We examined the distribution of sites of the VNB that project to the vBNST and of the VTA/SN that project to the NAc by mapping the maximal stimulated release at different depths of the tips of the bipolar stimulating electrode. The carbon-fiber microelectrodes were fixed at locations in the NAc and the vBNST that supported robust catecholamine release. The stimulating electrode was lowered through the VTA/SN region in ~ 100 to 200μm increments from 6.7 to 9.2 mm below the skull surface as electrically evoked (60 Hz, 60 pulses) release was recorded in the two terminal regions. Figure 3a (left) shows the coronal plane (AP −5.2 mm) and track (dotted lines, centered on 1.2 mm ML) of the tips (spaced ~ 1.0 mm apart) of the bipolar stimulating electrode for this experiment (Park et al. 2009).
Figure 3. Maps of electrically evoked catecholamine responses measured in the NAc and vBNST as a function of depth of the stimulating electrode.
Coronal section (AP −5.2 mm from bregma) illustrates the approximate path of the stimulating electrode tips (dotted lines) aimed at the VNB (filled region SN (striped) and VTA (dotted)) (a, left). Relative intensity of dopamine in NAc (—with triangles) and norepinephrine in vBNST (··· with squares) evoked at different positions of the stimulating electrode (60 Hz, 60 pulses) (a, right). The coronal sections were taken from the atlas of Paxinos and Watson (2007). Approximate placements of the VTA/SN and VNB in the diagram were based on the previous studies (Ungerstedt 1971, Paxinos & Watson 2007, Park et al. 2009). Representative concentration versus time traces for dopamine (b, left) and norepinephrine (b, right) release at different positions of the stimulating microelectrode in the same animal. Distance (mm) on the left is depth from the skull for stimulating electrode. Electrical stimulation is indicated with the solid red bars under the traces. Abbreviations used: VTA, ventral tegmental area; SN, substantia nigra; VNB, ventral tegmental area.
When averaged from multiple animals, evoked release of dopamine in the NAc was immeasurable with the stimulating electrode above 7.0 mm or below 9.2 mm. The maximal evoked dopamine concentration was observed when the stimulating electrode was ~ 8.3 mm from the skull surface (solid line, Fig. 3a, right). In the individual example shown in Figure 3b, dopamine in the NAc was not observed until the stimulating electrode was below a depth of ~ 7.5 mm. The maximal concentration evoked concentration was observed when the stimulating electrode was at ~ 8.3 mm and then it decreased dramatically when the stimulating electrode was lowered to 9.2 mm. In contrast, the average evoked norepinephrine concentration in the vBNST showed less dependence on the depth of the stimulating electrode (dotted line, Fig. 3a right). The individual traces for evoked norepinephrine in the vBNST show release with the stimulating electrode above 7.3 mm (Fig. 3b). When the stimulating electrode was lowered to 9.2 mm, norepinephrine release only decreased 20 % from the maximal response that was observed at 8.3 mm. In general, maximum dopamine and norepinephrine concentrations were evoked when the stimulating electrodes was at a depth of ~ 8.3 mm.
Selective dopamine and norepinephrine drug effects on dopamine in the NAc and norepinephrine in the vBNST
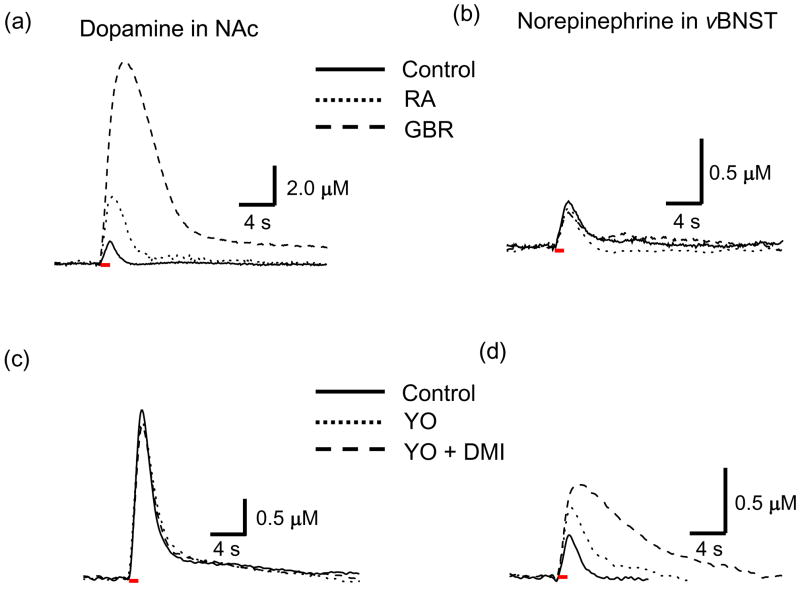
Dopamine and norepinephrine cannot be distinguished based on their voltammograms though the cyclic voltammograms for the catecholamines are distinct from those for all other substances establishing a unique identifier for catecholamines (Heien et al. 2003, Park et al. 2009). Although pharmacological evidence previously confirmed that the main catecholamines released in the vBNST and NAc were norepinephrine and dopamine, respectively (Park et al. 2009, Park et al. 2010), here we examined the effects of selective dopamine or norepinephrine drugs simultaneously in the different brain regions of the same animal. Presynaptic D2 and α2-adrenergic receptors are activated by dopamine and norepinephrine, respectively, which regulate their release while the norepinephrine transporter (NET) and dopamine transporter (DAT) regulate their clearance. We studied simultaneously the influence of the α2-adrenergic receptor antagonist, yohimbine, and D2 receptor antagonist, raclopride, on both electrically evoked norepinephrine and dopamine release. In addition, the effects of the NET inhibitor, desipramine, and DAT inhibitor, GBR 12909, on the evoked catecholamine responses were simultaneously investigated in both brain regions.
Twenty minutes after administration of raclopride (2 mg/kg, i.p.), electrically evoked dopamine concentration ([DA]) and the t1/2 value for dopamine disappearance both increased in the NAc. Subsequent administration of GBR 12909 (15 mg/kg, i.p.) further increased [DA] and t1/2 (Fig. 4a). Table 2 presents a summary of the average catecholamine responses evoked at 60 Hz both before (control) and 20 min after drug administration. In the presence of raclopride and GBR 12909, only the [DA] and the t1/2 valuein the NAc significantly increased. In contrast, the dopamine drugs had little effect on evoked norepinephrine concentration ([NE]) and the t1/2 value in the vBNST within the same animal (Fig. 4b, Table 2). The norepinephrine drugs, yohimbine (5 mg/kg, i.p.) and desipramine (15 mg/kg, i.p.), were ineffective in altering dopamine overflow in the NAc (Fig. 4c, Table 2). In contrast, yohimbine increased electrically evoked [NE] and the t1/2 value in the vBNST and subsequent administration of desipramine (15 mg/kg) following yohimbine, further increased both [NE] and t1/2 (Fig. 4d, Table 2).
Figure 4.
Selective dopamine and norepinephrine drug effect on the release and uptake of catecholamines in the NAc and vBNST. Raclopride (RA, ···, 2 mg/kg) increased both [DA] and t1/2 in the NAc and administration of GBR 12909 (GBR, ---, 15 mg/kg) after RA further increased both [DA] and t1/2 in the NAc (a) but these dopamine drugs have no effect on [NE] and t1/2 in vBNST (b). Yohimbine (YO, ···, 5 mg/kg) and yohimbine with desipramine (DMI, ---, 15 mg/kg) have no effect on [DA] and t1/2 in the NAc (c) but increased [NE] and t1/2 of the signal in the vBNST (d). Electrical stimulation is indicated with the solid red bars under the traces.
Table 2.
Effects of yohimbine (YO, 5 mg/kg), YO + desipramine (DMI, 15 mg/kg), raclopride (RA, 2 mg/kg) and RA + GBR 12909 (GBR,15 mg/kg) on evoked catecholamine release.
| Drug (n = 4) | (%) of control | ||
|---|---|---|---|
| Region | NAc | vBNST | |
| YO | [CA] | 102 ± 3 | 153 ± 7* |
| t1/2 | 105 ± 10 | 171 ± 18* | |
|
| |||
| Yo + DMI | [CA] | 94.0 ± 3.8 | 203 ± 15*# |
| t1/2 | 107 ± 9.5 | 368 ± 49*# | |
|
| |||
| RA | [CA] | 169 ± 6* | 102 ± 3 |
| t1/2 | 200 ± 21* | 89.8 ± 6.0 | |
|
| |||
| RA + GBR | [CA] | 299 ± 38*# | 95.1 ± 3.5 |
| t1/2 | 305 ± 48*# | 96.9 ± 5.0 | |
Data are mean ± S.E.M. and were obtained during 60 Hz stimulations (60 pulses). The evoked catecholamine concentration, [CA], and the time required for catecholamine overflow decay to 50 % of the maximum, t1/2, are shown.
Indicates significantly different from control values (P < 0.05).
Significantly different versus a single drug (yohimbine or raclopride) (P < 0.05). Values represent the mean ± S.E.M.
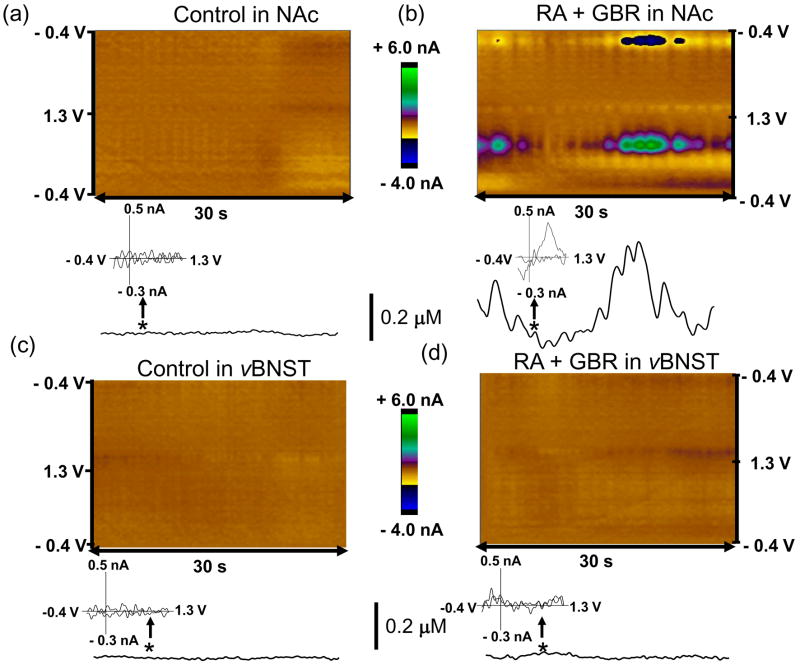
Spontaneous dopamine transients in the NAc were observed after the two dopamine drugs (Fig. 5). These transients were not observed after the two norepinephrine drugs, and neither were transients observed under any conditions in the vBNST. The average concentration amplitude of the dopamine transients in the NAc was 0.18 ± 0.04 μM following GBR 12909 and raclopride, and these occurred with a frequency of ~ 0.41 Hz and t1/2 (0.86 ± 0.06 s, n = 3 rats).
Figure 5.
Dopamine drugs induced dopamine concentration transients in the NAc. Two-dimensional color plot representation of the background-subtracted cyclic voltammograms collected over 30 sec (a, c) before and (b, d) following administration of the dopamine drugs (raclopride (2 mg/kg, i.p.) and GBR 12909 (15 mg/kg, i.p.)) in the NAc and the vBNST. Dopamine concentration changes are apparent in the color plot (b) at the potential for its oxidation (~ 0.65 V) and its reduction (−0.2 V). Principal component regression was used to extract the time course of the catecholamine concentration transients (lower traces in a-d). Times are indicated by the vertical bars. Inset: The cyclic voltammogram recorded at the time indicated by the arrow (b) was identical to that for dopamine.
Effects of amphetamine and autoreceptor antagonists on stimulated release of dopamine in the NAc and norepinephrine in the vBNST
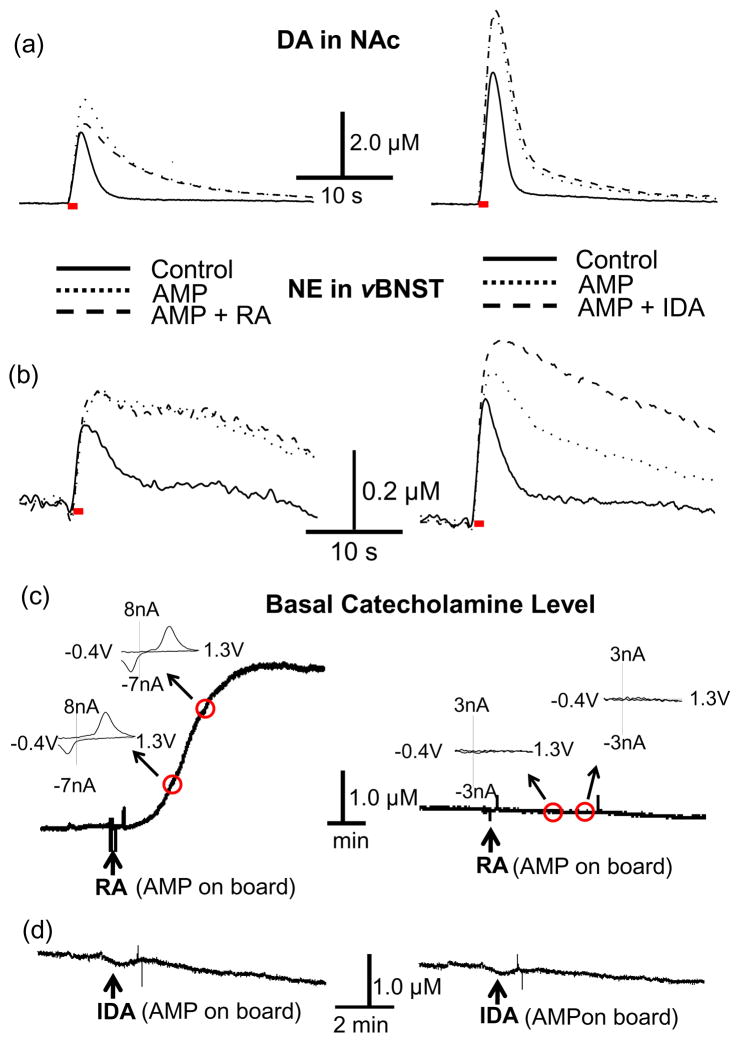
Amphetamine releases newly synthesized catecholamines from a readily releasable pool and blocks their uptake (Sulzer et al. 2005), but the effects seem to be different at norepinephrine and dopamine terminals (Sanghera et al. 1979). In the present study, amphetamine (3.0 mg/kg) caused electrically evoked dopamine and norepinephrine concentration to increase ([DA] = 147 ± 6.0 % of predrug, P < 0.001 and [NE]=163 ± 7.8 % of predrug, P < 0.001, n = 5 animals) and it also increased half decay times (t1/2, AMP = 215 ± 18 % for dopamine, P < 0.001 and t1/2, AMP = 220 ± 16 % for norepinephrine, P < 0.0001, n = 5) (Fig. 6a and b). Subsequent administration of raclopride (2.0 mg/kg) following amphetamine suppressed electrically evoked dopamine significantly ([DA] AMP+RA = 94.5 ± 11.5 % of predrug, P < 0.01, n = 4) but did not alter evoked norepinephrine from its post-amphetamine level ([NE] AMP+RA =153.8 ± 7.3, P > 0.05, n = 4 animals)(Fig. 6b, left). The suppressed dopamine release following amphetamine and raclopride was accompanied by a dramatic increase in the extracellular basal dopamine concentration (to 2.52 ± 0.76 μM, n = 5) that occurred within 4 min after administration of raclopride (Fig. 6c, left). The combination of amphetamine and raclopride did not alter the basal norepinephrine level significantly (Fig. 6c, right). Normally measurement of basal concentrations with FSCV is complicated by electrode drift (Hermans et al. 2008), but, with these conditions, the cyclic voltammograms clearly revealed the large increase in basal dopamine concentration (Fig. 6c inset).
Figure 6.
Amphetamine, D2 and α2-adrenergic receptor inhibitor effect on the release and uptake of catecholamines in the NAc and vBNST. Amphetamine (AMP, ···, 3 mg/kg) increased both [CA] and t1/2 (a and b) but administration of raclopride (RA, ---, 2 mg/kg) following AMP decreased electrically evoked [DA] in the NAc (a, left). The combined drug (AMP + RA) has no effect on [NE] in vBNST (b, left) but idazoxan (IDA, ---, 3 mg/kg) following AMP further increased [NE] in the vBNST (b, right). The combined drug (AMP + IDA) has no effect on [DA] in the NAc (a, right). The combined drug (AMP + RA) increased basal dopamine concentration but not basal norepinephrine concentration (c). The combined drug (AMP + IDA) has no effect on both basal catecholamine concentrations (d). Electrical stimulation is indicated with the solid red bars under the traces.
In contrast, idazoxan administration following amphetamine changed neither basal dopamine levels in the NAc (Fig. 6d, left) nor basal norepinephrine levels in the vBNST (Fig. 6d, right). The administration of idazoxan (3.0 mg/kg), an α2-adrenergic receptor inhibitor, following amphetamine further increased electrically evoked norepinephrine concentration ([NE]AMP+IDA = 208 ± 18 % of predrug value, P < 0.05, n = 4) while it was ineffective in altering dopamine overflow in the NAc ([DA]AMP+IDA = 134 ± 4.7 %, P > 0.05, n = 4)(Fig. 6a and b, right). Idazoxan was used in this study because its effects are manifested more rapidly than those of yohimbine. The time to reach maximum effect of yohimbine is ~ 5 to 10 min longer than idazoxan while the effects of yohimbine on dopamine and norepinephrine are not significantly different from those of idazoxan.
Effects of synthesis inhibition on stimulated dopamine and norepinephrine release
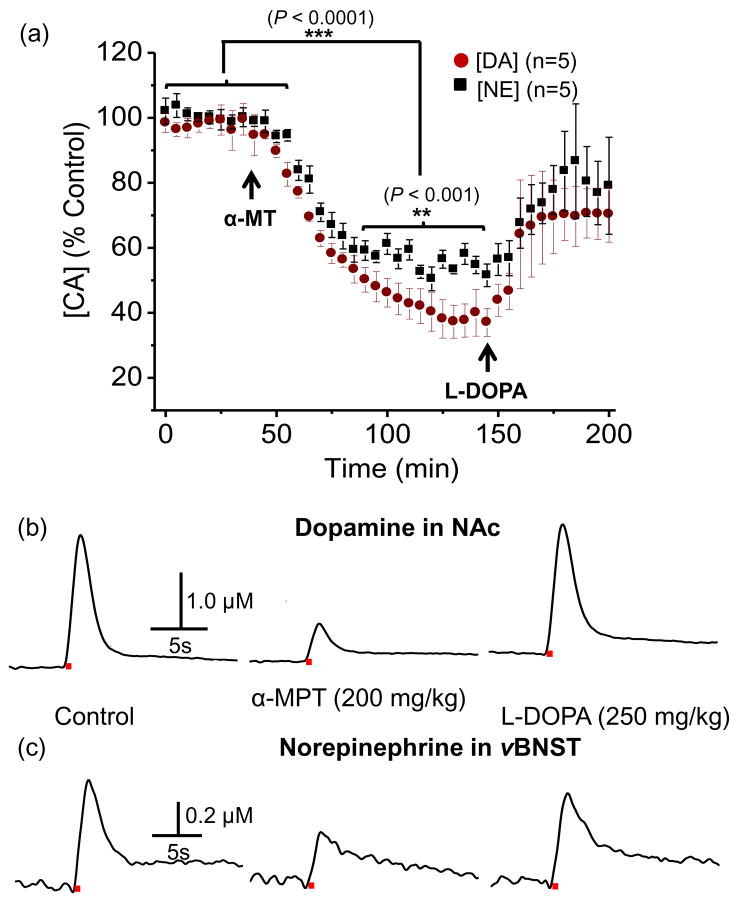
α-Methyl-p-tyrosine (α-MT), a tyrosine hydroxylase inhibitor, has been used to estimate the turnover of catecholamines (Kizer et al. 1975). Here, we monitored the release of both catecholamines evoked by electrical stimulations (60 Hz, 60 pulses) repeated at 5 min intervals. Evoked catecholamine release following a saline injection (0.5 mL) was stable over a 2-h period. However, ~ 15 min after α-MT (200 mg/kg) administration, the electrically evoked dopamine and norepinephrine release began to decrease (Fig. 7a). Norepinephrine release in the vBNST approached a new base line ~ 60 min after α-MT administration, where the amplitude was 56.6 ± 3.6 % of the predrug value. Electrically evoked dopamine decreased continuously and reached a level that was 38.2 ± 5.3 % of the predrug release at 95 min after administration. Statistical analysis revealed that the two groups were significantly different 60 min after α-MT administration (P < 0.001) (Fig. 7a). Both dopamine and norepinephrine release attenuated by α-MT was rapidly restored following administration of L-DOPA (250 mg/kg), the biochemical precursor of dopamine (Fig. 7a). Individual representative dopamine and norepinephrine traces recorded in the NAc and vBNST, simultaneously, before and after the drugs in a same rat are shown in Figure 7b and c, respectively.
Figure 7.
Effects of α-methyl-p-tyrosine (α-MT) and L-DOPA on the release of dopamine in the NAc and norepinephrine in the vBNST. (a) The time course of the change in evoked dopamine concentration in the NAc and norepinephrine in the vBNST and (b) dopamine and (c) norepinephrine concentration changes in a same animal after intraperitoneal injection of α-MT and L-DOPA. Each datum point is shown as mean ± S.E.M. α-MT decreased both electrically evoked dopamine and norepinephrine release and L-DOPA restored both attenuated dopamine and norepinephrine release. Electrical stimulation is indicated with the solid red bars under the traces.
Discussion
In vivo voltammetry has been demonstrated to be a useful tool to monitor the dynamics of extracellular neurotransmitters. The use of multiple electrodes allows several experiments that are not possible with single probes. For example, electrodes in arrays have been used to minimize chemical and electrical interference for enzyme-based electrodes (Parikh et al. 2004, Burmeister et al. 2002, Garguilo & Michael 1996). Electrode arrays have been used to monitor stimulated dopamine release within the striatum (Lu et al. 1998, Zachek et al. 2010) and to compare stimulated release in the caudate nucleus and basal lateral amygdala (Garris & Wightman 1994). Here, we show that in vivo dual-microelectrodes can be used with FSCV to monitor evoked release of dopamine and norepinephrine simultaneously. The simultaneous measurements minimize the number of animals required for comparison of the two different neurotransmitters while allowing direct comparison of their dynamic processes and drug effects on them in the same preparation. Here we show that the dynamics of norepinephrine in the vBNST are slower than for dopamine in the NAc. Furthermore, the two neurotransmitter systems respond quite differently to pharmacological agents that are known to act on these systems.
We have taken advantage of the fact that noradrenergic fibers traverse through the VTA/SN region. Thus, stimulation of this region promotes simultaneous release in the terminal regions of both catecholamines (Park et al. 2009). Movement of the stimulating electrode reveals that the region of excitation of noradrenergic fibers is broader than for dopaminergic processes (Fig. 3). Dopamine content in the NAc is similar to that of norepinephrine in the vBNST (Park et al. 2009, Park et al. 2010, Kilts & Anderson 1986). However, with the conditions employed here, it appears that the broad spatial distribution of norepinephrine fibers limits electrically evoked release in the vBNST to approximately one quarter of that for dopamine release evoked in the NAc even when the stimulating electrode is placed in a site that evokes maximal release (Table 1). An alternate interpretation, previously described in a review, is that the tissue-content normalized release rate of norepinephrine is half that of dopamine (Garris & Wightman 1995b).
Both dopamine and norepinephrine fibers are unmyelinated, have processes with diameters of less than 0.5 μm, and have slow conduction velocities of ~0.5 m/s (Aston-Jones et al. 1980, Connor 1975, Grace & Bunney 1983, Phelix et al. 1992). Despite these similarities, the dynamics of the evoked release profiles of the two catecholamines are quite different. The rise time for dopamine release is faster than for norepinephrine (Fig. 1 and Table 1). While the origin of these differences is not understood, it is not due to the difference in stimulation sites in the present experiments (fibers of passage for norepinephrine, cell body region for dopamine) because stimulation of dopamine fibers in the medial forebrain bundle also results in rapid release (Garris & Wightman 1994). Furthermore, norepinephrine release is less dependent on pulse width than dopamine, with short pulse widths (duration ≤1.0 ms) being more effective for the activation of noradrenergic release (Fig. 2). Dopamine release with medial forebrain bundle stimulation also diminishes with short stimulation pulses (Millar et al. 1985). While the dependence of release on pulse width can be profound (Albert et al. 2009, Ranck 1975), in this case the difference observed may be due to the different mechanisms responsible for release at catecholamine terminals. Indeed, release from dopaminergic terminals has been shown to be more sensitive to the availability of Ca2+ both in synaptosomes (Okada et al. 1990) and in vivo (Mitchell & Adams 1993). However, the difference cannot be explained solely in the catecholamine terminal fields. It may be also due to different biophysical properties of the catecholaminergic fibers.
The slow disappearance of evoked norepinephrine in the vBNST, as measured by t1/2, indicates that the rate of norepinephrine uptake is approximately half that for dopamine uptake in the NAc. Consistent with this, norepinephrine uptake in the vBNST was reported to be significantly slower than dopamine uptake in the striatum and even compared to norepinephrine uptake in other brain regions (Dugast et al. 2002, Dugast et al. 1994, Capella et al. 1993, Park et al. 2009). Slow uptake has several consequences. First, slower uptake allows released norepinephrine in the vBNST to diffuse further from its release sites than dopamine in the NAc, and allows norepinephrine to participate to a greater degree in “volume transmission” (Cragg & Rice 2004). Second, because extracellular catecholamines during a stimulus train are the balance between uptake and release (Wightman et al. 1988), slow uptake allows protracted increases in norepinephrine concentrations during low frequency stimuli as illustrated in the 20 Hz stimulations in Figure 2a. Extracellular dopamine reaches a new steady-state level during the stimulation while norepinephrine shows a continuous increase. At higher frequencies, there is less time between impulses in a train for uptake to operate, so both dopamine and norepinephrine concentrations increase during the stimulation.
The cyclic voltammograms for dopamine and norepinephrine are virtually identical (Heien et al. 2004, Park et al. 2009), but they are distinct from metabolites and other interferences in the extracellular fluid (Baur et al. 1988, Heien et al. 2003). Thus, along with the voltammetric, anatomical, and neurochemical evidence, the use of select pharmacological agents is necessary to distinguish the two catecholamines. The simultaneous measurements shown here clearly confirm the selectivity of the drugs employed. The dopamine drugs (raclopride, GBR 12909) did not affect electrically evoked norepinephrine release in the vBNST (Fig. 4). Likewise, the selective norepinephrine drugs (yohimbine, idazoxan, desipramine) did not affect dopamine release or its spontaneous release in the NAc (Fig. 4). The results are consistent with our previous studies (Park et al. 2009, Park et al. 2010) and support the concept that dopamine in the NAc and norepinephrine in the vBNST can be selectively monitored simultaneously within the same animal using dual-microelectrode FSCV.
A major difference of the two catecholamine systems is that norepinephrine transients in the vBNST did not occur following the norepinephrine drugs but, following dopamine drugs, robust dopamine transients were observed in the NAc (Fig. 5). This difference may be explained by the different types of phasic (burst) firing that have been documented for dopaminergic and noradrenergic neurons. Dopaminergic neurons display tonic and phasic modes of firing that occur over longer time periods than for noradrenergic neurons that display only brief phasic discharge events (Berridge & Waterhouse 2003). If phasic noradrenergic excitatory responses in anesthetized animals occur with a shorter duration (< 100 ms) and lower frequency ( 10 Hz) than the phasic dopaminergic responses, the small concentration changes of norepinephrine transmission may not be observed. Alternatively, the firing rate of noradrenergic neurons may be affected with anesthesia more than that of dopaminergic neurons. Anesthesia or sleep causes a dramatic depression in firing of noradrenergic cells (< 1 Hz) in the locus coeruleus (Foote et al. 1980, Berridge & Waterhouse 2003). In contrast, the average firing rate of dopaminergic neurons does not significantly vary between awakened and deeply anesthetized rats, although there is a reduction of their burst firing (Fa et al. 2003).
The dual microelectrode approach is particularly useful to contrast the actions of drugs that are nonselective. For example, amphetamine can inhibit NET and DAT and also causes reverse transport of catecholamines due to its inhibition of the vesicular monoamine transporter (Sulzer et al. 2005). Here, amphetamine increased the half-life of both extracellular catecholamines after stimulation and elevated the maximal concentration, consistent with its actions as a transport inhibitor (Fig. 6)(Ramsson et al. 2011). However, when amphetamine was followed by the selective D2 antagonist, raclopride, release of dopamine returned to pre-amphetamine levels whereas evoked norepinephrine release was unaffected (Fig. 6). Inspection of the baseline level in amphetamine treated animals after administration of the autoreceptor antagonists provides evidence of a massive spontaneous dopamine efflux following raclopride, a phenomenon not seen under most other conditions. The basal level of catecholamines following amphetamine alone does not change sufficiently to be detected by in vivo voltammetry (Ramsson et al. 2011, Wiedemann et al. 1991), although microdialysis studies have reported basal level increases in catecholamine concentrations following amphetamine (Kuczenski et al. 1997, L’Heureux et al. 1986). Raclopride can reverse the decrease in firing rates of dopaminergic neurons caused by amphetamine (Shi et al. 2007, Shi et al. 2000), and this should lead to this spontaneous release. Similar spontaneous release has been observed following the combination of the dopamine uptake inhibitors such as nomifensine, GBR 12909, and cocaine with D2 antagonists (Venton & Wightman 2007, Park et al. 2010). However, only amphetamine coupled with a D2 antagonist decreased subsequent stimulated dopamine release. For example, release following GBR 12909 and raclopride increased evoked dopamine concentration (Fig. 4). We attribute the decreased stimulated release following amphetamine and a D2 antagonist to a dual-mechanism: the displacement of vesicular dopamine by amphetamine and the depletion of releasable stores caused by release following increased neuronal firing after raclopride administration.
Following amphetamine, evoked norepinephrine release was further increased by the subsequent administration of the α2-adrenoceptorinhibitor, idazoxan, whereas dopamine release was unaffected. The different effects on noradrenergic neurons of the combination of amphetamine and an autoreceptor blocker are consistent with the idea that releasable stores of norepinephrine are more able to exchange with reserve stores (Sanghera et al. 1979). The lack of spontaneous release on noradrenergic neurons occurs despite the fact that amphetamine causes activation of the terminal norepinephrine autoreceptor (Nakamura et al. 1982), a process reversed by a norepinephrine autoreceptor antagonist (Curet et al. 1992). It has been previously noted that the degree of amphetamine’s actions on dopaminergic and noradrenergic neurotransmission are different (Ryan et al. 1985). This may be due to the potency of amphetamine for inducing release is greater for dopaminergic than noradrenergic neurons (Raiteri et al. 1974, Ryan et al. 1985), although the opposite conclusion was reached for release in isolated brain slices (Rothman & Baumann 2003).
α-MT, a central tyrosine hydroxylase inhibitor, decreased both dopamine release in the NAc and norepinephrine release in the vBNST, which is in accordance with earlier studies with in vivo microdialysis (Shimizu et al. 1990) and FSCV (Millar et al. 1985) although the degree of inhibition of brain catecholamine synthesis is dependent on dose and stimulating pulse numbers. α-MT levels in the brain reach a maximum 1–2 h after i.p. injection (Widerlov & Lewander 1978). Our result shows that norepinephrine is depleted less than dopamine by α-MT administration. Consistent with this, the duration of synthesis inhibition and storage depletion after α-MT administration was shorter lasting for norepinephrine than for dopamine from endogenous catecholamine measurements by biochemical methods (Widerlov & Lewander 1978). In addition, the 50% effective dose (ED50) for synthesis inhibition of dopamine was half of the ED50 for that of norepinephrine (Widerlov & Lewander 1978). The faster depletion of dopamine than norepinephrine is again consistent with a marked difference between dopamine and norepinephrine containing neurons in the functional relationship between their releasable and storage pools (Sanghera et al. 1979). In dopamine neurons, the rate of transfer between stored and readily-releasable amine pools is quite slow. In contrast, in norepinephrine neurons, there appears to be a more rapid mobilization of the stored amine to readily releasable sites.
It is interesting that two neuronal systems, which superficially seem anatomically and biochemically similar, are so significantly different in their storage and control mechanisms for regulating release and clearance (McMillen et al. 1980, Mitchell & Adams 1993). The results of this study suggest that neural regulation of dopamine and norepinephrine in the brain differ and the ability to simultaneously study dopamine and norepinephrine neurotransmission in different brain regions will facilitate research on the distinct roles and functions of these catecholamines in animal behaviors and disease states for future study.
Supplementary Material
Histological verification of recording site.
Acknowledgments
This research was generously supported by National Institutes of Health (NS 15841).
Footnotes
Disclosure/conflict of interest
The authors have no conflict of interest.
Additional supporting information may be found in the online version of this article:
References
- Albert GC, Cook CM, Prato FS, Thomas AW. Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: An overview of stimulation parameters and neurotransmitter release. Neurosci Biobehav Rev. 2009;33:1042–1060. doi: 10.1016/j.neubiorev.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis - A target site for noradrenergic actions in opiate withdrawal. Advancing from the Ventral Striatum to the Extended Amygdala. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Segal M, Bloom FE. Brain aminergic axons exhibit marked variability in conduction velocity. Brain Res. 1980;195:215–222. doi: 10.1016/0006-8993(80)90880-x. [DOI] [PubMed] [Google Scholar]
- Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Fast-Scan Voltammetry of Biogenic-Amines. Anal Chem. 1988;60:1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Cahill PS, Wightman RM. Simultaneous Amperometric Measurement of Ascorbate and Catecholamine Secretion from Individual Bovine Adrenal-Medullary Cells. Anal Chem. 1995;67:2599–2605. doi: 10.1021/ac00111a017. [DOI] [PubMed] [Google Scholar]
- Capella P, Ghasemzadeh MB, Adams RN, Wiedemann DJ, Wightman RM. Real-Time Monitoring of Electrically Stimulated Norepinephrine Release in Rat Thalamus. 2 Modeling of Release and Reuptake Characteristics of Stimulated Norepinephrine Overflow. J Neurochem. 1993;60:449–453. doi: 10.1111/j.1471-4159.1993.tb03171.x. [DOI] [PubMed] [Google Scholar]
- Connor JD. Electrophysiology of the nigro-caudate dopamine pathway. Pharmacol Ther B. 1975;1:357–370. doi: 10.1016/0306-039x(75)90042-2. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Curet O, De Montigny C, Blier P. Effect of desipramine and amphetamine on noradrenergic neurotransmission: electrophysiological studies in the rat brain. Eur J Pharmacol. 1992;221:59–70. doi: 10.1016/0014-2999(92)90772-v. [DOI] [PubMed] [Google Scholar]
- Dugast C, Cespuglio R, Suaud-Chagny MF. In vivo monitoring of evoked noradrenaline release in the rat anteroventral thalamic nucleus by continuous amperometry. J Neurochem. 2002;82:529–537. doi: 10.1046/j.1471-4159.2002.00991.x. [DOI] [PubMed] [Google Scholar]
- Dugast C, Suaudchagny MF, Gonon F. Continuous in-Vivo Monitoring of Evoked Dopamine Release in the Rat Nucleus-Accumbens by Amperometry. Neuroscience. 1994;62:647–654. doi: 10.1016/0306-4522(94)90466-9. [DOI] [PubMed] [Google Scholar]
- Fa M, Mereu G, Ghiglieri V, Meloni A, Salis P, Gessa GL. Electrophysiological and pharmacological characteristics of nigral dopaminergic neurons in the conscious, head-restrained rat. Synapse. 2003;48:1–9. doi: 10.1002/syn.10177. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Garguilo MG, Michael AC. Amperometric microsensors for monitoring choline in the extracellular fluid of brain. J Neurosci Methods. 1996;70:73–82. doi: 10.1016/S0165-0270(96)00105-7. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. In vivo voltammetric measurement of evoked extracellular dopamine in the rat basolateral amygdaloid nucleus. J Physiol. 1994;478 (Pt 2):239–249. doi: 10.1113/jphysiol.1994.sp020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Distinct Pharmacological Regulation of Evoked Dopamine Efflux in the Amygdala and Striatum of the Rat in-Vivo. Synapse. 1995a;20:269–279. doi: 10.1002/syn.890200311. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Regional differences in dopamine release, uptake, and diffusion measured by fast-scan cyclic voltammetry. In: Boulton A, Baker G, Adams RN, editors. Voltammetric Methods in Brain Systems. Humana; Totowa, NJ: 1995b. pp. 179–220. [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- Hermans A, Keithley RB, Kita JM, Sombers LA, Wightman RM. Dopamine detection with fast-scan cyclic voltammetry used with analog background subtraction. Anal Chem. 2008;80:4040–4048. doi: 10.1021/ac800108j. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trac-Trends in Anal Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Anderson CM. The simultaneous quantification of dopamine, norepinephrine and epinephrine in micropunched rat brain nuclei by on-line trace enrichment HPLC with electrochemical detection: Distribution of catecholamines in the limbic system. Neurochem Int. 1986;9:437–445. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- Kita JM, Parker LE, Phillips PE, Garris PA, Wightman RM. Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem. 2007;102:1115–1124. doi: 10.1111/j.1471-4159.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- Kizer JS, Kopin IJ, Zivin JA. Proceedings: Estimates of catecholamine turnover rates in individual hypothalamic nuclei of the rat by use of alpha-methyl-para-tyrosine. Br J Pharmacol. 1975;54:243P. [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Melega WP, Cho AK, Segal DS. Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther. 1997;282:591–596. [PubMed] [Google Scholar]
- L’Heureux R, Dennis T, Curet O, Scatton B. Measurement of endogenous noradrenaline release in the rat cerebral cortex in vivo by transcortical dialysis: effects of drugs affecting noradrenergic transmission. J Neurochem. 1986;46:1794–1801. doi: 10.1111/j.1471-4159.1986.tb08498.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, Michael AC. Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. J Neuroche. 1998;70:584–593. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- McMillen BA, German DC, Shore PA. Functional and Pharmacological Significance of Brain Dopamine and Norepinephrine Storage Pools. Biochem Pharmacol. 1980;29:3045–3050. doi: 10.1016/0006-2952(80)90444-x. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Joseph JD, Kilpatrick MR, Travis ER, Wightman RM. Improving data acquisition for fast scan cyclic voltammetry. Anal Chem. 1999;71:3941–3947. doi: 10.1021/ac990491+. [DOI] [PubMed] [Google Scholar]
- Millar J, Stamford JA, Kruk ZL, Wightman RM. Electrochemical, Pharmacological and Electrophysiological Evidence of Rapid Dopamine Release and Removal in the Rat Caudate-Nucleus Following Electrical-Stimulation of the Median Forebrain-Bundle. Euro J Pharmacol. 1985;109:341–348. doi: 10.1016/0014-2999(85)90394-2. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Adams RN. Comparison of the Effects of Voltage-Sensitive Calcium-Channel Antagonism on the Electrically Stimulated Release of Dopamine and Norepinephrine Invivo. Brain Res. 1993;604:349–353. doi: 10.1016/0006-8993(93)90390-9. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Oke AF, Adams RN. In-Vivo Dynamics of Norepinephrine Release Reuptake in Multiple Terminal Field Regions of Rat-Brain. J Neurochem. 1994;63:917–926. doi: 10.1046/j.1471-4159.1994.63030917.x. [DOI] [PubMed] [Google Scholar]
- Miyahara S, Oomura Y. Inhibitory-Action of the Ventral Noradrenergic Bundle on the Lateral Hypothalamic Neurons through Alpha-Noradrenergic Mechanisms in the Rat. Brain Res. 1982;234:459–463. doi: 10.1016/0006-8993(82)90887-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tepper JM, Young SJ, Groves PM. Changes in noradrenergic terminal excitability induced by amphetamine and their relation to impulse traffic. Neuroscience. 1982;7:2217–2224. doi: 10.1016/0306-4522(82)90132-4. [DOI] [PubMed] [Google Scholar]
- Okada M, Mine K, Fujiwara M. Differential calcium dependence between the release of endogenous dopamine and noradrenaline from rat brain synaptosomes. J Neurochem. 1990;54:1947–1952. doi: 10.1111/j.1471-4159.1990.tb04896.x. [DOI] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Research. 1998;788:287–293. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natil Acad Sci US A. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169:132–142. doi: 10.1016/j.neuroscience.2010.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Galligan JJ, Fink GD, Swain GM. In vitro continuous amperometry with a diamond microelectrode coupled with video microscopy for simultaneously monitoring endogenous norepinephrine and its effect on the contractile response of a rat mesenteric artery. Anal Chem. 2006;78:6756–6764. doi: 10.1021/ac060440u. [DOI] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Euro J Neuro. 2009;30:2121–2133. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Academic Press; San Diego: 1995. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Wightman RM. Critical guidelines for validation of the selectivity of in-vivo chemical microsensors. Trac-Trends in Anal Chem. 2003;22:509–514. [Google Scholar]
- Raiteri M, Levi G, Federico R. d-amphetamine and the release of 3H-norepinephrine from synaptosomes. Eur J Pharmacol. 1974;28:237–240. doi: 10.1016/0014-2999(74)90140-x. [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. J Neurochem. 2011;117:937–48. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB. Which Elements Are Excited in Electrical-Stimulation of Mammalian Central Nervous-System - Review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Tepper JM, Young SJ, Groves PM. Amphetamine’s effects on terminal excitability of noradrenergic locus coeruleus neurons are impulse-dependent at low but not high doses. Brain Res. 1985;341:155–163. doi: 10.1016/0006-8993(85)91483-0. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, German DC, Kiser RS, Shore PA. Differences in norepinephrine and dopamine neurotransmitter storage systems. Brain Res Bull. 1979;4:217–221. doi: 10.1016/0361-9230(79)90285-5. [DOI] [PubMed] [Google Scholar]
- Saphier D. Electrophysiology and neuropharmacology of noradrenergic projections to rat PVN magnocellular neurons. Am J Physiol. 1993;264:R891–902. doi: 10.1152/ajpregu.1993.264.5.R891. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Zhang XY, Pun CL, Bunney BS. Clozapine blocks D-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2007;32:1922–1928. doi: 10.1038/sj.npp.1301334. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Duan SM, Hori T, Oomura Y. Glutamate modulates dopamine release in the striatum as measured by brain microdialysis. Brain Res Bull. 1990;25:99–102. doi: 10.1016/0361-9230(90)90258-2. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Justice JB., Jr Probing brain chemistry. Anal Chem. 1996;68:359A–363A. [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Takmakov P, McKinney CJ, Carelli RM, Wightman RM. Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev Sci Instrum. 2011;82 doi: 10.1063/1061.3610651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takmakov P, Zachek MK, Keithley RB, Bucher ES, McCarty GS, Wightman RM. Characterization of local pH changes in brain using fast-scan cyclic voltammetry with carbon microelectrodes. Anal Chem. 2010;82:9892–9900. doi: 10.1021/ac102399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic Mapping of Monoamine Pathways in Rat Brain. Acta Physiologica Scand Supple. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Wightman RM. Pharmacologically induced, subsecond dopamine transients in the caudate-putamen of the anesthetized rat. Synapse. 2007;61:37–39. doi: 10.1002/syn.20343. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Widerlov E, Lewander T. Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after alpha-methyl-p-tyrosine; time- and dose-response relationships. Naunyn Schmiedebergs Arch Pharmacol. 1978;304:111–123. doi: 10.1007/BF00495547. [DOI] [PubMed] [Google Scholar]
- Wiedemann DJ, Kawagoe KT, Kennedy RT, Ciolkowski EL, Wightman RM. Strategies for low detection limit measurements with cyclic voltammetry. Anal Chem. 1991;63:2965–2970. doi: 10.1021/ac00024a030. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Zachek MK, Takmakov P, Moody B, Wightman RM, McCarty GS. Simultaneous Decoupled Detection of Dopamine and Oxygen Using Pyrolyzed Carbon Microarrays and Fast-Scan Cyclic Voltammetry. Anal Chem. 2009;81:6258–6265. doi: 10.1021/ac900790m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS. Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosens Bioelectron. 2010;25:1179–1185. doi: 10.1016/j.bios.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological verification of recording site.