Abstract
The hibernator's heart functions continuously and avoids damage across the wide temperature range of winter heterothermy. To define the molecular basis of this phenotype, we quantified proteomic changes in the 13-lined ground squirrel heart among eight distinct physiological states encompassing the hibernator's year. Unsupervised clustering revealed a prominent seasonal separation between the summer homeotherms and winter heterotherms, whereas within-season state separation was limited. Further, animals torpid in the fall were intermediate to summer and winter, consistent with the transitional nature of this phase. A seasonal analysis revealed that the relative abundances of protein spots were mainly winter-increased. The winter-elevated proteins were involved in fatty acid catabolism and protein folding, whereas the winter-depleted proteins included those that degrade branched-chain amino acids. To identify further state-dependent changes, protein spots were re-evaluated with respect to specific physiological state, confirming the predominance of seasonal differences. Additionally, chaperone and heat shock proteins increased in winter, including HSPA4, HSPB6, and HSP90AB1, which have known roles in protecting against ischemia-reperfusion injury and apoptosis. The most significant and greatest fold change observed was a disappearance of phospho-cofilin 2 at low body temperature, likely a strategy to preserve ATP. The robust summer-to-winter seasonal proteomic shift implies that a winter-protected state is orchestrated before prolonged torpor ensues. Additionally, the general preservation of the proteome during winter hibernation and an increase of stress response proteins, together with dephosphorylation of cofilin 2, highlight the importance of ATP-conserving mechanisms for winter cardioprotection.
Keywords: 2D DiGE, CFL2, hibernating, Ictidomys (spermophilus) tridecemlineatus, mass spectrometry
hibernation in mammals is characterized by dramatic, but reversible, reductions in body temperature (Tb) and metabolic rate. During this torpid phase, respiration and heart rate also decline to 1–4% of their euthermic values (for review, see Ref. 11). Torpor bouts are punctuated by interbout arousals (54), when physiological rates are restored to or even surpass euthermic levels (11). For circannual hibernators such as 13-lined ground squirrels, Ictidomys tridecemlineatus, the ability to orchestrate reversible metabolic depression exists only in winter (reviewed in Ref. 38). This heterothermic winter phenotype contrasts markedly with summer, when animals remain homeotherms. A “two-switch” mechanism in which animals first switch from summer homeothermy to a state that is permissive for winter heterothermy (switch 1), and then cycle between periods of torpor and arousal in winter (switch 2) appears to model the phenomenon of circannual hibernation (31, 58).
Heterothermy poses a significant challenge to a hibernator's physiology. This may be particularly acute for the heart, because it maintains functionality across a wide Tb range. The acceleration of heart rate from 3–5 to ≥300 beats/min during arousal from torpor (44) is likely to be especially stressful. As metabolic rate peaks during this period, oxygen delivery fails to keep pace, creating transient hypoxic conditions (45) analogous to ischemia-reperfusion events. Furthermore, the rapidly increasing metabolic activity may generate a surge in oxidative stress (16, 52). Yet, remarkably, the hibernator's heart continues to function throughout all of these dramatic transitions. Understanding this cardioprotected phenotype, which is capable of low-temperature function, offers untapped potential to benefit human medicine by identifying novel strategies to improve outcomes in recovery from heart attack, thoracic heart surgery, and the treatment of epilepsy-associated arrhythmia and ischemic heart disease (14, 69).
There is evidence supporting a cardioprotected winter phenotype in hibernators. In winter-torpid animals, the upregulation of some peroxiredoxins (49) and inducible heat shock proteins (18) suggests strategies for managing oxidative stress. Winter elevations of Ca2+ ion transporter abundance and activity facilitate the maintenance of Ca2+ ion homeostasis and cardiac contraction (2, 42, 73). Depolarizing signal conduction is also promoted at the low Tb of torpor by increased levels of connexins 43 and 45 (27). These findings provide discrete mechanistic explanations for continued cardiac function under conditions pathological to nonhibernators. For example, hibernator hearts maintain contractility during torpor (Tb 0–7°C), while those of nonhibernators become arrhythmic between 30 and 16°C (35). Hibernators are also markedly resistant to ventricular fibrillation (35) and to the cardiac conduction block that generally accompanies hypothermia in mammals (26). Moreover, the hibernator's heart maintains Na+/K+ ion homeostasis during torpor, while nonhibernating mammals lose ion homeostasis over time (36).
While insightful, these studies provide a partial view of cardioprotection in hibernators, because they sampled few physiological states and focused on relatively few gene products. To maximize its potential to reveal novel medical treatment strategies, however, the cardiac phenotype of hibernators must be characterized in detail. Therefore, we applied an unbiased screening method to examine the heart proteome that underlies the dynamic physiology of hibernation. To capture the protein components of the two switches in hibernation, we quantified differences in the 13-lined ground squirrel's heart among eight states defined by season or physiology using two-dimensional difference gel electrophoresis (2D DiGE). The components of the winter-protected phenotype were identified by comparing states representing summer homeothermic and winter heterothermic physiology, whereas the components of the intrawinter cycle were identified by analysis of four winter groups from both torpid and aroused states. Finally, we hypothesized that fall represents a transition between homeothermy and heterothermy that could illuminate the process underlying the summer-winter switch. Therefore, for the first time, we included two fall sampling states. This comprehensive sampling strategy and unbiased experimental approach provides new insight into elements supporting the dynamic circannual physiology of the hibernator heart.
EXPERIMENTAL PROCEDURES
Animals
Procurement and monitoring.
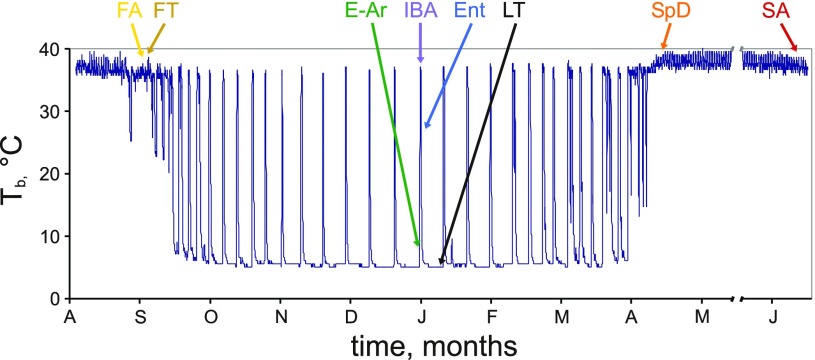
Thirteen-lined ground squirrels were purchased from the University of Wisconsin, Oshkosh captive breeding program in July-August 2006–2009. Squirrels were individually housed under standard conditions (18–21°C, 14:10 light-dark cycle, fed cat chow supplemented with sunflower seeds and water ad libitum). All animals except those in the summer active group (SA, n = 6; Fig. 1) were surgically implanted in August or early September with both an intraperitoneal datalogger (iButton, Embedded Data Systems) and a radiotelemeter (VM-FH disks; Mini Mitter, Sunriver, OR) for remote Tb monitoring until tissue collection. In early October, squirrels were moved to the hibernaculum, which was kept in constant darkness. Ambient temperature was lowered step-wise from 18 to 4°C over a 2 wk period, and food and water were withdrawn as squirrels exhibited multiday torpor bouts, assessed by Tb telemetry. Food and water were returned to the spring dark animals (SpD, n = 6; Fig. 1) at least 10 days before euthanasia. All animal protocols were approved by the University of Colorado Institutional Animal Care and Use Committee.
Fig. 1.
Body temperature trace of a 13-lined ground squirrel from August through July of the following year. Seasonal and physiological groups are labeled; these colors are used throughout. Groups (n = 6) are fall active [FA: late September–mid-October, Tb = 32–37°C, 3 of these animals had not yet exhibited torpor (56), the other 3 had been torpid for short periods, no more than 7 h and with a minimum Tb of no less than 19°C]; fall torpor [FT: same dates as FA, except all had exhibited previous multiday torpor bouts of at least 2.5 days with Tb <10°C; 4 of these animals were torpid (Tb = 6–7°C) and the other 2 had Tb of 19 or 36°C]; early arousing (E-Ar: Tb = 6–12.8°C); interbout aroused (IBA: Tb ∼37°C, 3 h after inflection point demarking the end of rapid rewarming), entrance (Ent: Tb = 27–23°C); late torpor (LT: Tb = 5–6°C, time torpid = 80–95% of previous bout); spring dark (SpD: Tb = 37°C for >10 days after spontaneous termination of hibernation); summer active (SA: animals in July-August). Tb, body temperature.
Tissue collection.
Hearts were collected from animals representing the eight different seasonal and physiological groups (n = 6 per group) defined in Fig. 1. Animals were euthanized by exsanguination under isoflurane anesthesia. They were perfused with ice-cold isotonic saline and decapitated before dissection.
2D DiGE
Protein sample preparation.
Each heart was frozen in N2(l) immediately after dissection and stored at −80°C. Protein extracts were prepared as described previously (20) in ice-cold homogenization buffer (0.5 M sucrose, 100 mM phosphate, 5 mM MgCl2, 1 mM PMSF, 10 μg/ml protease inhibitors). The homogenate was centrifuged and the nuclear-free supernatant was snap frozen in aliquots using N2(l). Aliquots were stored at −80°C, and each aliquot was used only once. Protein concentration was determined using a BCA assay (Pierce, Rockford, IL).
2D DiGE labeling.
DiGE labeling was completed as previously described (46). A pooled reference standard containing equal amounts (μg) of n = 3 heart protein extracts from each sampling group (24 samples total) was stored in single-use aliquots. For labeling, 90 μg of each experimental sample and reference standard were denatured overnight at room temperature in lysis buffer (8 M urea, 2 M thiourea, 4% CHAPS, and 25 mM Tris, pH 8.8). Denatured protein samples were labeled with Cy2, Cy3, or Cy5 (CyDye DiGE Fluors; GE Healthcare, Piscataway, NJ). Cy2 always labeled the pooled reference sample. Cy3 and Cy5 labels were alternated among the experimental samples to control for bias in the dye labeling: in each group of six, three samples were labeled with Cy3 and the remaining three samples were labeled with Cy5.
2D DiGE and pick gels.
2D electrophoresis was completed as described previously (22). For each gel, two experimental samples (one Cy3 and the other Cy5-labeled) were mixed with a Cy2-labeled reference sample. The experimental gels were always prepared with two group combinations (e.g., FT-Cy3 and IBA-Cy5 or IBA-Cy3 and FT-Cy5) so that no two samples of the same group were used in a single gel. The labeled protein mixture for each gel was precipitated with methanol-chloroform and then resuspended as previously described (22). For first dimension protein separation, resuspended samples were absorbed overnight onto Immobiline DryStrips (pH 3–10 NL, 18 cm; GE Healthcare) and focused (Multiphor II isoelectric focusing apparatus, GE Healthcare). The strips were incubated in reducing (50 mM Tris pH 6.8, 2% SDS, 15% glycerol, 6 M urea, and 1% DTT) and alkylating (50 mM Tris pH 6.8, 2% SDS, 15% glycerol, 6 M urea, 1.25% iodoacetamide, and 0.05% bromphenol blue) buffers, and then proteins were size separated by SDS-PAGE using 9–16% polyacrylamide gels. Each gel was scanned with three lasers (Typhoon 9400, GE Healthcare) to collect the Cy2, Cy3, and Cy5 images within 4 h of completion of electrophoresis.
Gels used for spot picking were prepared as described above, except that an unlabeled reference sample was used, and the SDS-PAGE gels were poured onto a bind-silane (PlusOne, GE Healthcare)-treated plate. After electrophoresis, these “pick” gels were fixed in 10% methanol and 7.5% acetic acid, stained in SYPRO Ruby gel stain (Bio-Rad, Hercules, CA) overnight, and fully destained in 10% methanol and 7.5% acetic acid before scanning.
Quantitative analysis of 2D gels.
A total of 24 analytical gels containing data from 48 individuals, plus 5 SYPRO Ruby stained “pick” gels were evaluated with DeCyder 2D 7.0 software (GE Healthcare). The Cy2 gel containing the most protein spots was designated the master gel, and spots in the remaining 23 Cy2 images and 5 pick gels were matched to it using DeCyder's Biological Variation Analysis (BVA) Module. We first manually landmarked ∼275 spots then applied the automated spot matching feature. Each Cy3 or Cy5 spot intensity value was normalized to its respective Cy2 internal reference spot value.
Random Forests (9), an unsupervised machine learning classification method, was applied to cluster individual samples based solely upon protein spot abundance variation and to identify the protein spots that best discriminated the clusters. The initial analyses considered all spots present in every sample, i.e., 6/6 samples per group. Subsequent Random Forests were run with the top 6–10 classifiers from the top 20 identified by the initial analyses. The Random Forests that used the fewest spots to give the greatest biological separation of individual samples into clusters were considered the best. Random Forests were generated in R (64), n = 50,000 classification trees.
To identify the proteins that changed significantly between the summer homeothermic (SpD and SA) and winter heterothermic [interbout arousal (IBA), entrance (Ent), late torpor (LT), and early arousing (E-Ar)] seasonal groups, a student's two-tailed t-test followed by a Benjamini-Hochberg false discovery rate (FDR) correction (6) was performed on spots present in 5/6 individuals per group across all groups. These same spots were evaluated by a one-way ANOVA analysis followed by FDR correction. Post hoc Tukey pairwise comparisons were performed for all spots significant by one-way ANOVA. All tests were performed in R (64) with α set to 0.05.
Spot picking and mass spectral analysis.
Protein spots comprising the 20 most important classifiers from the Random Forests were identified by tandem mass spectrometry, as were those significant by either t-test or ANOVA. Spots were robotically picked (1.4 mm diameter, 1.5 mm deep head) and digested from three pick gels using an Ettan system (v 1.10, GE Healthcare) at the University of Colorado School of Medicine Mass Spectrometry and Proteomics Core Facility. After digestion, they were stored at −20°C in 0.1% formic acid. Each digest was identified by LC MS/MS as previously described (22), except that the peptides were separated using an HPLC-Chip Cube (Chip #G4240-62001 Zorbax 300SB-C18; Agilent Technologies, Santa Clara, CA) along a 3–50% acetonitrile hydrophobicity gradient for 7 min followed by a 3 min wash in 90% acetonitrile. Peptides were analyzed by MS/MS in an ESI ion trap (LC/MSD XCT Plus, Agilent Technologies) in positive ion mode with an initial 300–2,200 m/z scan followed by tandem mass spectral scans of the four highest peaks, with a dynamic exclusion of 30 s. Spectral data were collected with 6300 Series TrapControl software (V6.1, Build 83, Agilent Technologies) and analyzed with Spectrum Mill MS Proteomics Workbench (revision A.03.02.075) using an in-house database comprising all National Center for Biotechnology Information (NCBI) nr mammalian sequences from June 2009, which contained 724,584 entries. Settings for this program, such as precursor ions and number of missed cleavages, were the same as previously described (22), except that oxidized methionine was included as a variable modification. If a picked spot returned a unique protein identification (ID), with ≥2 peptides and a score ≥30, no further attempt was made at identification. However, over the course of this analysis, an updated in-house database became available, which included all NCBI mammalian sequences from April 2010 in addition to 13-lined ground squirrel sequences from Ensembl and Arctic ground squirrel (Urocitellus paryii) sequences published by Shao et al. (59). This database contained 792,655 entries. Proteins that were not uniquely identified with the initial June 2009 database were reanalyzed (previous Spectrum Mill data were discarded) using this updated database. If protein spots were still not identified, they were repicked in two additional gels. Faint spots were submitted for analysis by LC MS/MS on an LTQ-XL Linear Ion Trap Mass Spectrometer (Thermo/Finnigan, Waltham, MA) at the University of Colorado School of Medicine Mass Spectrometry and Proteomics Core Facility. For this mass spectrometer, 2 μl of each sample were injected onto a reversed-phase column using a cooled (9°C) autosampler (AS-1; Eksigent, Dublin, CA) connected to an HPLC system run at 0.12 μl/min before the split and ∼400 nl/min postsplit (1100 Series, Agilent Technologies). The column was made from an in-house pulled 360/100 nm (outer/inner diameter) fused silica capillary packed with 80 Å Synergi C18 resin (Phenomenex, Torrance, CA) kept at a constant 40°C using an in-house built column heater. Peptides were separated using a 12–30% acetonitrile gradient over 60 min. The column effluent was coupled directly to an LTQ XL Linear Ion Trap mass spectrometer with an in-house built nanospray ion source. Data acquisition was performed using the instrument-supplied Xcalibur (version 2.0.6) software. The LC runs were monitored by sequentially recording the precursor scan (MS) followed by three collision-induced dissociation (CID) acquisitions (MS/MS). Singly charged ions were excluded from CID selection. Normalized collision energies were employed using helium as the collision gas. An in-house script was used to create deisotoped centroided peak lists from the raw spectra (.mgf format). The spectral data were then analyzed with Spectrum Mill as described above. All of the resulting peptide data were combined to account for multiple MS/MS runs and orthologous identifications using an in-house program, ExtracTags (23). Spots were considered unambiguously identified if they returned an overall score ≥30 and contained ≥2 peptides for only one protein. If multiple proteins were identified in a spot but one (with ≥2 peptides) had 4× greater spectral intensity than any other, this was also accepted as the unique ID, as previously described (46). The resulting peptide data are available in Supplemental Table S1.1 To eliminate ambiguity inherent with protein nomenclature, we generally refer to proteins by their unambiguous gene ID throughout the text and figures; both protein name and gene symbol appear in Supplemental Table S2.
Biological pathway classification.
To identify meaningful biological components underlying the cycles of hibernator heart physiology, we used the functional annotation clustering tool in DAVID (Database for Annotation, Visualization and Integrated Discovery; 34). Official gene symbols of identified proteins were submitted to the DAVID database; functional annotation clusters (FACs) were generated using a Homo sapiens background and clustering stringency set to “high,” with default settings for all other parameters. The Entrez Gene database at NCBI was used to assign functions to individual genes.
2D Gel Analysis of Phosphoproteins
Phosphoprotein abundance in heart extracts from IBA, Ent, LT, and E-Ar samples (n = 3 per state) were evaluated on 2D gels as above, except that 90 μg (unlabeled) were separated on a single gel. After electrophoresis, the free-floating gels (12 total) were fixed overnight (50% methanol, 10% acetic acid), then stained in 1/3 diluted Pro-Q Diamond phosphoprotein gel stain (Molecular Probes, Eugene, OR) for 2 h, destained 4× (50 mM sodium acetate, pH 4.0, 20% acetonitrile) and scanned (1). The gels were then stained in SYPRO Ruby for >24 h, destained in 10% methanol and 7.5% acetic acid, and rescanned for total protein.
The Pro-Q Diamond phosphoprotein and SYPRO Ruby total protein 2D gel images were uploaded into DeCyder's BVA module for spot matching to the Cy2 experimental gel images. Because the phosphoprotein-stained gels did not reveal every protein spot seen in the SYPRO or Cy2 gels, each Pro-Q Diamond 2D gel and its corresponding SYPRO Ruby gel image were overlaid in Adobe Photoshop, which served as a visual guide during matching in DeCyder. Overlay images were generated in Adobe Photoshop (CS3 Extended; Adobe Systems, San Jose, CA).
Western Blotting
Heart protein samples (30 μg) from all eight groups (Fig. 1, n = 3 per group) were denatured in 1× Laemmli sample buffer and separated by electrophoresis through 15% polyacrylamide gels. Proteins were transferred to PVDF membranes (Immobilon-FL; Millipore, Bedford, MA), stained with Memcode protein blot stain (Pierce, Rockford, IL) and then scanned to quantify total protein loading. Blots were blocked for 1 h (Odyssey blocking buffer; LI-COR, Lincoln, NE), and incubated overnight at 4°C in both anti-cofilin 2 (CFL2, 1:5,000, goat polyclonal; Abcam, Cambridge, MA) and anti-phospho-cofilin 2 (P-CFL2, 1:500, rabbit polyclonal; Upstate, Lake Placid, NY) primary antibodies. This was followed by 50 min incubation in secondary antibodies: anti-rabbit IgG (1:20,000, IRDye 800CW conjugated; LI-COR) and anti-goat IgG (1:30,000, IRDye 680LT conjugated; LI-COR). Protein bands were visualized using the Odyssey near-infrared imaging system (LI-COR) and analyzed with ImageQuant TL software (GE Healthcare). To correct for inconsistencies in protein loading, band intensities were normalized to the lane containing the greatest protein pixel volume. Bands were further normalized by setting the most intense band for each antibody in each blot to 100. A one-way ANOVA followed by a Tukey post hoc test was used to assess both total CFL2 and P-CFL2 for significant abundance differences among the groups.
RESULTS
Protein Spot Separation and Matching
Proteomic changes in the heart of 13-lined ground squirrels from eight stages across their circannual hibernation cycle were assessed by 2D DiGE. A representative 2D pick gel showing the separation of heart protein spots is presented in Fig. 2. Of the analytical gels (24 total), the “master” gel contained 2,080 Cy2 spots and was used to match spots in all of the other Cy2 gel images. A total of 278 spots were matched in all 24 analytical gels and used for Random Forests. For t-tests and ANOVA statistical analyses, 432 spots were used that matched in at least 5/6 samples per group for all sampling groups.
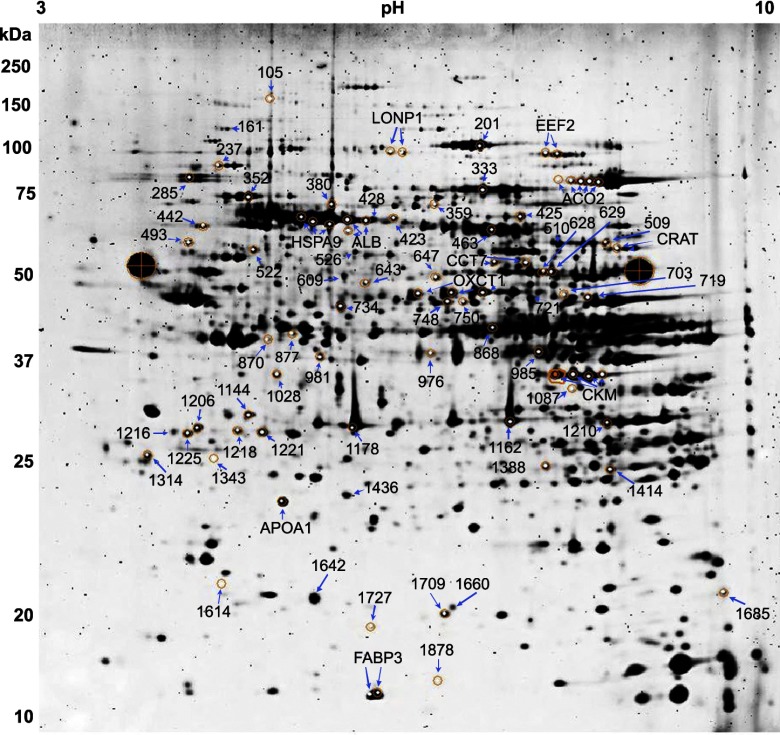
Fig. 2.
Representative 2-dimensional gel showing the separation and location of picked heart proteins. Numbers to the left of the gel denote the approximate molecular weight (kDa) of the proteins, while the numbers at top represent the pH gradient of the 1st dimension. Orange circles represent the area targeted by the robotic spot picker. The 2 large black circles with cross-hairs are reference markers for the robotic spot picker. Protein spots are labeled by either their DeCyder assigned spot number or gene symbol in cases where multiple isoforms were identified.
Top Discriminating Proteins for Unsupervised Group Classification
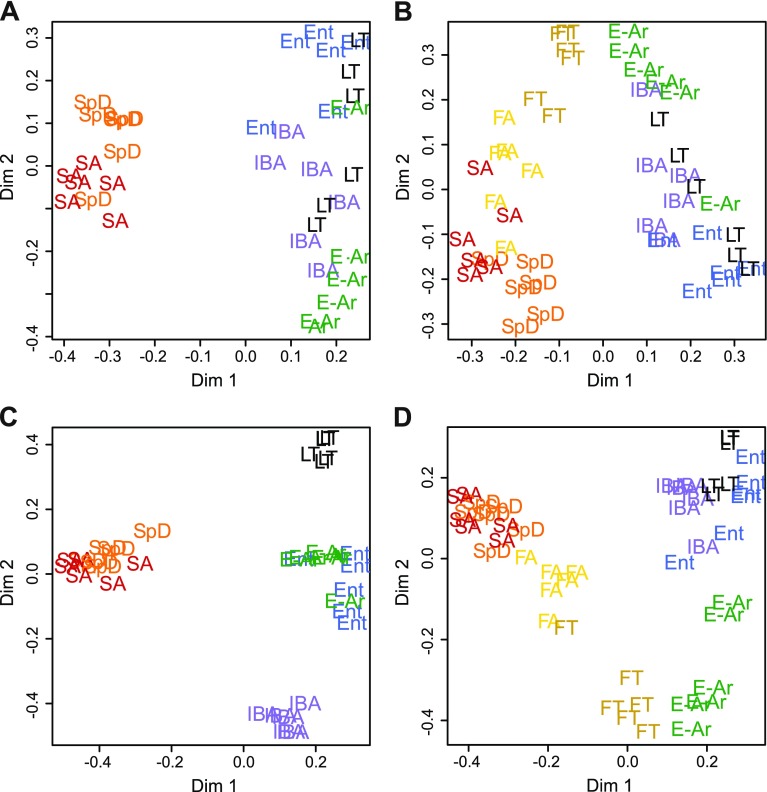
A striking seasonal separation between the summer homeothermic (SpD and SA) and winter heterothermic (IBA, Ent, LT and E-Ar) groups was revealed by Random Forests classification (Fig. 3A). However, our a priori defined groups within each season (Fig. 1) were not clearly distinguished. Thus, these data define a seasonal heart proteome with distinct summer and winter components. When the two fall groups, fall torpor (FT) and fall active (FA), were added to the analysis, a separation between the summer and fall active (SpD, SA, FA) versus the winter groups (IBA, Ent, LT, E-Ar) was apparent (Fig. 3B). It is noteworthy, however, that 4/6 FT individuals clustered between summer and winter (Fig. 3B); these four were torpid with Tb at 4–6°C when the hearts were collected. The remaining two FT samples, taken from squirrels with Tb at 37 or 19°C, clustered adjacent to FA and closer to the homeothermic individuals, indicating that Tb as well as seasonal components define the fall cardiac proteome in 13-lined ground squirrels. The proteome of the four FT individuals that fell between the summer and winter clusters may be truly distinct or comprise proteins characteristic of both.
Fig. 3.
Unsupervised clustering of individual ground squirrels using heart proteins separates summer homeotherms from winter heterotherms and highlights the fall transition. 2-dimensional scaling plots from Random Forests (RF) analyses. Individual samples are plotted and labeled by group symbol. Results of analysis using animals from: A: SpD, SA, IBA, Ent, LT, and E-Ar; B: same as in A, except that FA and FT were included. The top discriminators from these analyses were reanalyzed by RF using: C: top 6 explanatory spots from RF in A; D: top 8 explanatory spots from RF in B. See text and Fig. 4 for protein spot identifications.
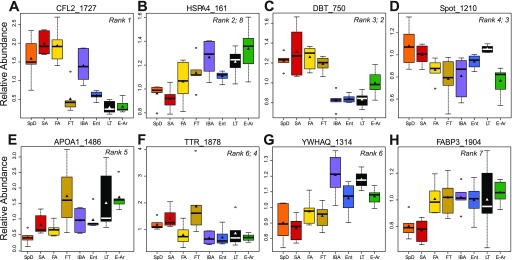
We examined the top 20 discriminating protein spots of the Random Forests classification and determined that using only the top six protein spots: 1) 1727, CFL2; 2) 161, heat shock 70 kDa protein 4 or HSPA4; 3) 750, dihydrolipoyl transacylase or DBT; 4) 1210, unidentified; 5) 1486, apolipoprotein A-I or APOA1; and 6) 1878, transthyretin or TTR (listed in order of importance; Fig. 4, A–F;) best clustered the summer and winter seasonal groups in the absence of fall (Fig. 3C). Using this limited dataset, we could also visualize distinct clusters that contained the IBA and LT states in addition to the seasonal separation seen by the initial analysis. When the fall samples were included (Fig. 3B), the best clustering occurred using the top eight protein spots (Fig. 3D). In this analysis, 5/6 FT individuals clustered together; the lone outlier was the individual with Tb at 37°C, again highlighting the importance of Tb in addition to season. The FA samples were also better separated from SA and SpD, and E-Ar clustered apart from the remaining winter groups. Although differing slightly in their order of importance, six of the top eight discriminating proteins for the Random Forests analysis including fall states were the same as in the analysis without fall. Not surprisingly, the abundance variation for several of these proteins supports the seasonal summer-winter switch, which is the dominant feature of these data. The remaining two discriminating proteins underlie the improved clustering of the fall groups. These eight discriminating protein spots were, in order of importance: 1) 1727, CFL2; 2) 750, DBT; 3) 1210; 4) 1878, TTR; 5) 1486, APOA1; 6) 1314, 14-3-3 protein θ or YWHAQ; 7) 1904, heart fatty acid-binding protein or FABP3; and 8) 161, HSPA 4 (Fig. 4).
Fig. 4.
Top RF protein discriminators of physiological and seasonal phenotypic states. Box plots showing relative abundances of proteins in each group. Colored boxes represent the region between the 25th and 75th percentiles, bold horizontal lines the median, triangles the mean, outside horizontal lines the 100th percentile and circles the outliers. Each panel plots data from 1 protein spot, labeled by gene symbol (if identified) and spot number. A–F: rank refers to the order of protein spot importance by RF analyses. For boxes with 2 ranks, the 1st number refers to RF analyses in Fig. 3A, while the 2nd number refers to Fig. 3B. For G and H, rank refers to Fig. 3B.
The top discriminating proteins for both of these analyses showed several different abundance level patterns among the groups, including seasonal changes in the proteins HSPA4, DBT, TTR, and FABP3 (Fig. 4, B, C, F, and H; mean fold changes are listed in Supplemental Table S2) as well as torpor-arousal cycle changes in CFL2, HSPA4, spot 1210, APOA1, and YWHAQ (Fig. 4, A, B, D, E, and G; Supplemental Table S3). The fall groups displayed intermediate patterns, such as protein spot abundance similar to SA and SpD but different from the winter groups (e.g., DBT; Fig. 4C) or similarity to winter groups but not to summer groups (e.g., FABP3; Fig. 4H). Finally, for CFL2, APOA1, and TTR, (Fig. 4, A, E, F) relative abundance levels were different between FA and FT. Such shifts in protein spot abundance among sampling states were therefore responsible for the separation and clustering of the two seasonal groups, the FT samples, and the limited intrawinter separation that was observed.
Seasonal Changes: Homeothermy vs. Heterothermy
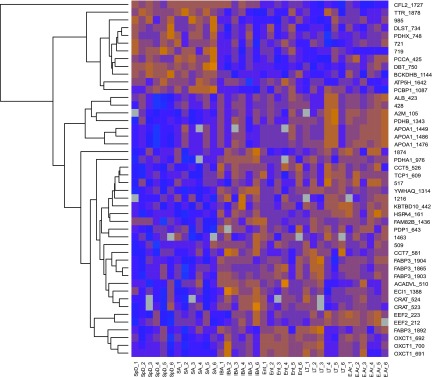
Because the Random Forests prominently classified the data by season, a Student's t-test was used to discern protein spots with an overall difference between the homeothermic (summer: SpD and SA) and heterothermic (winter: IBA, Ent, LT, and E-Ar) states. Significant seasonal changes occurred in 46 spots (q < 0.05, Fig. 5). Of these, the majority of spots (34 spots) were winter elevated (28 of these were identified with LC-MS/MS, 19 of these were unique proteins). Of the 12 summer-elevated protein spots, nine were identified as a single ID in the protein spot (Supplemental Table S2).
Fig. 5.
Most proteins that differ between summer homeotherms (SpD and SA) and winter heterotherms (IBA, Ent, LT, and E-Ar) are increased in winter. In this heat map, blue represents decreased intensity relative to the internal reference, while orange represents increased intensity. Grey represents an unmatched spot in that individual. Hierarchically clustered individual spots are displayed on the right vertical axis and are represented by their gene symbol, if known, and spot number. Individual samples are listed along the bottom by sampling state (summer = SA and SpD; winter = all others).
Seasonally elevated proteins were functionally clustered using DAVID. The summer-elevated proteins returned four functional annotation clusters (FACs): two were related to the mitochondrion, the other two terms were tricarboxylic acid cycle enzyme complex and lipoic acid binding. The valine, leucine, and isoleucine degradation pathway was also significantly summer-enriched (P < 0.001). This indicates that catabolism of essential branched-chain amino acids is downregulated during the hibernation season, a time when amino acid dietary intake ceases. FACs for the winter elevated proteins included the chaperonin-containing T-complex and fatty acid metabolism, indicating that pathways involved in protein folding and energy generation from fat oxidation are overrepresented in the winter cardiac phenotype.
Annual Changes and Fall Transition
We expanded our analysis to identify spots that were significantly affected by both seasonal and physiological state using all eight sampling groups, including FA and FT. A one-way ANOVA detected significant changes in 82 spots. Tukey's post hoc tests revealed that the majority of statistically significant changes occurred between the non-hibernating (SpD, SA) and hibernating groups (IBA, Ent, LT, E-Ar), while relatively fewer changes occurred among these groups (Table 1; see Supplemental Table S4 for Tukey P values of all pairwise comparisons). In agreement with the Random Forests analyses, FT differed from both groups and FA was more similar to the summer groups.
Table 1.
Counts of significant pairwise comparisons reveal that most protein adjustments occur between summer homeothermy and winter heterothermy
| SpD | SA | FA | FT | IBA | Ent | LT | |
|---|---|---|---|---|---|---|---|
| SA | 2 | ||||||
| FA | 8 | 2 | |||||
| FT | 28 | 10 | 14 | ||||
| IBA | 15 | 18 | 11 | 13 | |||
| Ent | 8 | 14 | 19 | 25 | 3 | ||
| LT | 24 | 23 | 28 | 21 | 3 | 0 | |
| E-Ar | 20 | 17 | 9 | 2 | 3 | 0 | 5 |
All significant Tukey tests (P < 0.05) for protein spots (n = 82) significant by ANOVA (q < 0.05) were counted. SpD, spring dark; SA, summer active; FA, fall active; FT, fall torpor; IBA, interbout aroused; Ent, entrance; LT, late torpor.
When picked for protein identification (Fig. 2), 70 of the 82 ANOVA significant (q < 0.05) spots were unambiguously identified, while 11 spots contained multiple proteins and one spot was not identified (Supplemental Table S1). The 70 positively identified spots (Supplemental Table S2) represented 50 unique genes.
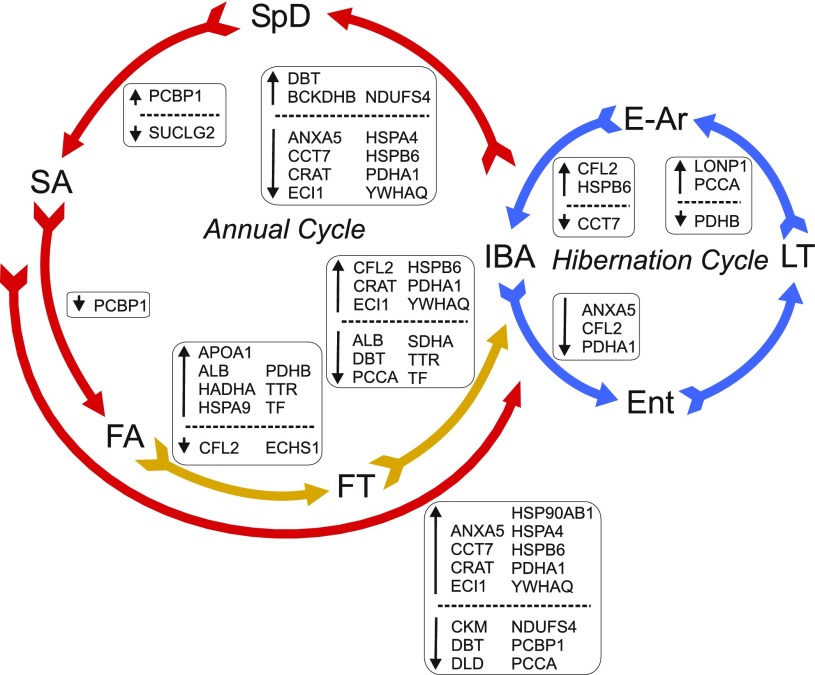
These 50 unique proteins were clustered using DAVID to highlight functional pathways that alter across the circannual rhythm of a hibernator. DAVID returned 17 FACs with an enrichment score ≥1.3 (Table 2). Many of the FACs included terms relating to metabolism such as mitochondrion, tricarboxylic acid cycle and lipid metabolism, demonstrating the adjustments made to the energy-generating pathways in the heart throughout the hibernator's year. Additionally, the terms “chaperone” and “response to heat stress” point to stress response or protein folding pathways as important for phenotypic changes that characterize the hibernator's heart. A step-wise analysis of significant Tukey pairwise protein differences that occur with each transition of the seasonal and torpor-arousal cycle (Fig. 6) not only provides further evidence that most proteins are regulated seasonally but also places these changes in the context of the full circannual rhythm of hibernation. For example, the abundances of heat shock proteins HSP90AB1 (heat shock protein 90-β), HSPA4, HSPB6 (heat shock protein β-6), and the protein chaperone CCT7 (T-complex protein 1 subunit η) all increased in the transition from summer (SA) to winter (IBA) and then decreased in the transition back to post-hibernation (IBA to SpD). Additionally, proteins involved in branched chain amino acid catabolism decreased in the transition to winter (SA to IBA; proteins DBT and DLD, dihydrolipoyl dehydrogenase) and increased in the transition back to summer (IBA to SpD; proteins DBT and BCKDHB, 2-oxoisovalerate dehydrogenase β). Protein levels altered in the fall as well; plasma transport proteins APOA1, ALB (albumin), TTR, and TF (transferrin) increased from FA to FT but fell in the transition to winter heterothermy (FT to IBA; proteins ALB, TTR, and TF). Finally, the proteins CRAT (carnitine O-acetyltransferase) and ECI1 (dodecenoyl-CoA isomerase), both involved in fatty acid β-oxidation, increased from SA through FT to IBA, remained elevated throughout the winter, and then subsequently decreased in the transition back to summer homeothermy (IBA to SpD). This visualization of significant pairwise comparisons confirmed the results of our seasonal t-test and Random Forests analyses: expression of chaperone and fatty acid metabolic proteins increased during the hibernation season while proteins involved in branched chain amino acid catabolism were concurrently downregulated. Furthermore, the FT group displayed a unique proteome of which plasma transport proteins comprised a substantial component.
Table 2.
DAVID FACs identify metabolism and energy generation as pathways having circannual regulation in 13-lined ground squirrels
| Cluster | Enrichment Score | Annotations, n | Genes, n |
|---|---|---|---|
| Mitochondrion | 20.24 | 4 | 23 |
| Mitochondrial lumen | 15.28 | 3 | 19 |
| Organelle lumen | 7.82 | 3 | 24 |
| Tricarboxylic acid cycle | 7.59 | 5 | 6 |
| Glucose catabolic process | 4.87 | 11 | 6 |
| Lipoic acid binding | 4.65 | 7 | 4 |
| Oxidoreductase activity | 4.41 | 3 | 4 |
| Mitochondrial alpha-ketoglutarate dehydrogenase complex | 3.78 | 4 | 3 |
| Lipid metabolism | 3.05 | 16 | 5 |
| Mitochondrial membrane | 2.56 | 7 | 8 |
| NAD or NADH binding | 2.27 | 4 | 4 |
| Oxidative phosphorylation | 1.76 | 10 | 4 |
| Cytoplasmic vesicle | 1.69 | 4 | 7 |
| Macromolecular complex assembly | 1.53 | 4 | 7 |
| ATP binding | 1.59 | 6 | 12 |
| Chaperone | 1.44 | 3 | 4 |
| Response to heat | 1.42 | 3 | 3 |
Functional Annotation Clusters (FACs) with an enrichment score >1.3 are listed for the 50 unique proteins found significant by ANOVA (q < 0.05) and identified by mass spectrometry. For each enriched FAC, the cluster term that provided the best biological meaning is presented.
Fig. 6.
Seasonal changes dominate the hibernator heart proteome. Schematic representation of the 2 cycles of a hibernator's year; the heart protein changes in each transition are labeled. Only identified proteins (indicated by gene symbols) that change significantly between states of the cycle (P < 0.05, Tukey) are shown. Large colored arrows reflect the state-to-state transitions within both annual and hibernation cycles. Small arrows show increased (↑) or decreased (↓) abundance for each transition. IBA is placed between the 2 cycles because it is the winter state that is the most physiologically similar to homeothermy and eliminates the confounding variable of reduced Tb.
CFL2
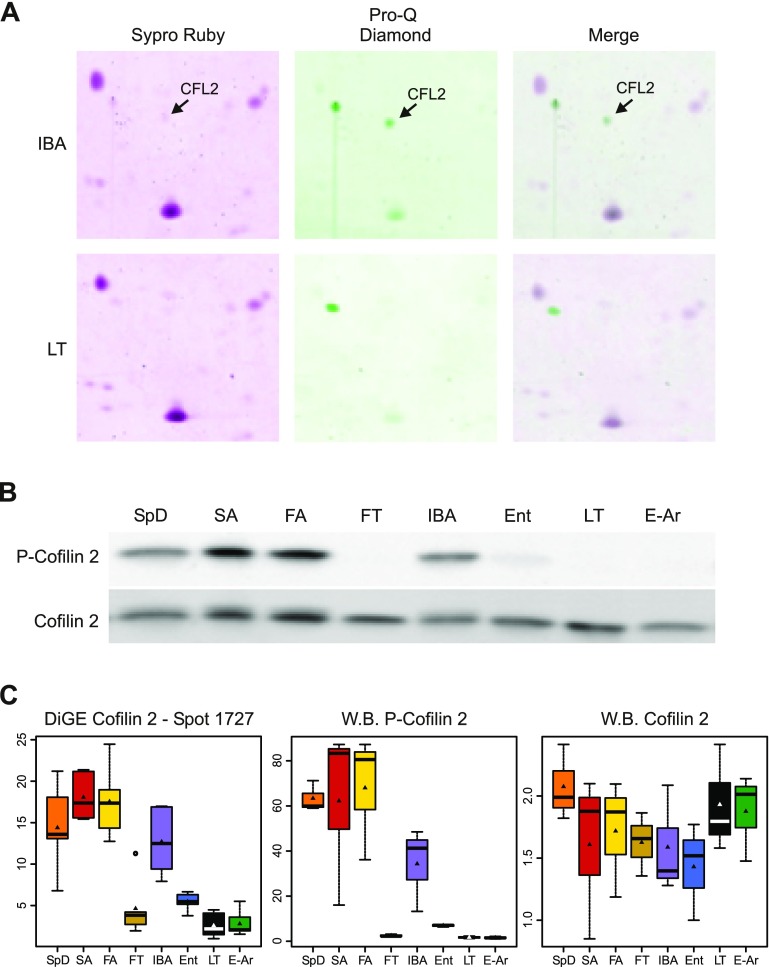
CFL2 (spot 1727) was selected for further analysis because it was the top discriminating protein for the Random Forests and showed the greatest fold change of all protein spots (∼7-fold; Supplemental Table S3). CFL2 also had the lowest q value of all spots examined (Supplemental Table S2). Phospho-staining indicated that spot 1727 (CFL2) was phosphorylated (Fig. 7A). We confirmed this result with Western blotting for both P-CFL2 and total CFL2. While total CFL2 was similarly abundant in all states, P-CFL2 decreased to near nondetectable levels in the FT, LT, and E-Ar samples (Fig. 7B). A one-way ANOVA to compare normalized band intensities confirmed that P-CFL2 fluctuated significantly among sampled states (F = 8.06, P = 0.0003), while total CFL2 was unchanged (F = 0.73, P = 0.65; Fig. 7C). Furthermore, the pattern among the sample groups of relative P-CFL2 protein abundance detected by Western blotting mimicked the pattern obtained by 2D DiGE (Fig. 7C). Hence, we concluded that spot 1727 was the phosphorylated form of CFL2.
Fig. 7.
Posttranslational modification of cofilin 2 by phosphorylation is state-dependent in the hibernator's heart. A: equivalent region from IBA (top) and LT (bottom) 2D gels stained with SYPRO Ruby (total protein, left panels), Pro-Q Diamond (phospho-protein, middle panels) and a merge of the 2 (right panels). Arrows mark CFL2 (spot 1727). B: Western blot developed with anti-phosphorylated cofilin 2 (top) and anti-cofilin 2 (bottom) from 1 heart extract representing each seasonal group. C: box plots showing the relative abundance of cofilin 2 from the 2-dimensional gel electrophoresis (2D DiGE) experiment (left), and from Western blotting of phospho-cofilin 2 (middle) and total cofilin 2 (right). Box plot symbols are as described in Fig. 4.
DISCUSSION
The present study quantified protein differences among eight distinct seasonal and physiological states in the heart of the 13-lined ground squirrel using 2D DiGE proteomics. We anticipated detection of proteins that contribute to the maintenance of cardiac function during winter heterothermy. Specifically, we hypothesized the identification of summer-winter seasonal proteomic changes that protect the heart during the low Tb of torpor and rapid rewarming of arousal and, by virtue of our unique winter season sampling strategy, recovery of at least some components of the torpor-arousal switch.
The dominant proteomic signature of the hibernator's heart that emerged from this analysis is one of a seasonal shift between the homeothermic (nonhibernating) and heterothermic (hibernating) portions of the year with relatively few changes detected within the hibernation cycle. This finding is in line with two previous heart screening studies that compared at least two heterothermic states and a homeothermic state (70, 72). The relative constancy of the cardiac proteome throughout the hibernation cycle is also consistent with the fact that the heart must continually function throughout torpor-arousal cycles. Moreover, new protein synthesis is an inefficient way to regulate heart function during the hibernation cycle, especially during its dramatic increase in activity from torpor to arousal. Peak metabolic rate in the heart occurs mid-arousal and is estimated to be 10× greater than in euthermia (32). As the animal is fully committed to rewarming during this period, heart rate is maximal in order to support whole body reperfusion (44). ATP generation and use may be completely directed at maintaining ion homeostasis and heart contractility during arousal instead of synthesizing and/or degrading proteins. Furthermore, initiation of protein translation is inhibited at Tb < 18°C (67), implying that de novo synthesis of any protein required to regulate heart function must occur during the IBA and Ent states. Yet, our pairwise Tukey tests revealed few differences among any of the winter states. Enhanced protection from protein degradation may be at least partly responsible for the constancy of the proteome throughout winter heterothermy, and the observed adjustments to relative protein spot abundance may in fact largely result from posttranslational modifications, a rapidly reversible and less energy-consuming form of protein regulation (11). The phosphorylation state of the winter cycling spot, 1727, or P-CFL2, was indeed responsible for explaining its robust change by DiGE. Regardless of whether synthesis, degradation, or posttranslational modifications is responsible for the detected spot changes in the hibernation cycle, it is important to note that only 10/432 spots differed significantly within winter, while a total of 82/432 spots differed among all groups. Taken together, the paucity of within-winter changes and the relatively large number of interseasonal changes in the heart proteome are consistent with the hypothesis that seasonal reprogramming of gene expression enables enhanced heart function during heterothermy (17, 26–27, 73), whereas sympathetic and parasympathetic innervation (47), likely signaling via posttranslational modifications of proteins to modulate activity, and Q10 effects (29) act concertedly to facilitate the winter cycle.
Metabolic Pathways
The cardiac proteomic cycles are dominated by representatives of pathways involved in metabolism (Fig. 6, Table 2). Functional annotation revealed overrepresentation of proteins related to the TCA cycle as well as to glucose and lipid metabolism. Our data support the well-documented enhancement of lipid metabolism during hibernation (reviewed by Refs. 2, 11) by a winter abundance increase in ECI1, CRAT, FABP3, and ACADVL (acyl-Coenzyme A dehydrogenase, very long chain) proteins. Additionally, OXCT1 (3-oxoacid-CoA transferase 1), which is involved in ketone catabolism, increased in winter, consistent with the study of Russeth et al. (57). Thus, our results agree with previous studies that highlight the fundamental importance of lipid metabolism as the main energy-generating source during hibernation.
Proteins involved in branched chain amino acid catabolism decreased in winter. Specifically, the catalytic subunits BCKDHB (E1 subunit), DBT (E2 subunit), and DLD (E3 subunit) that compose the branched chain α-ketoacid dehydrogenase complex (BCKDC) were all reduced compared with summer. BCKDC is involved in the breakdown of leucine, isoleucine, and valine by catalyzing a reaction that commits these essential amino acids to their degradation pathways (33). Branched chain amino acids are only available in the diet, and BCKDC downregulation does occur in response to long-term low-protein diet in rats (74). Because the 13-lined ground squirrel does not eat during the hibernation season, a downregulation of the BCKDC complex in response to the elimination of protein intake would be expected in order to spare essential amino acids. In fact, this response has been previously noted in the liver (22) and plasma (21) of this species. Furthermore, the mRNA of BCKDHB was also found to decrease in the heart of torpid versus summer active Arctic ground squirrels (72).
Chaperone and Heat Shock Proteins
A controlled stress response is a key aspect of the heart proteome in hibernating 13-lined ground squirrels. Functional annotation analysis of the protein spots that varied significantly by ANOVA for all sampling states highlighted the enrichment of FACs related to chaperone and heat stress responses. When protein spots that specifically increased in winter were examined, an enrichment of the chaperonin containing T-complex (abbreviated CCT or TRiC; 61) was detected. This apparent strong reliance on chaperones during winter heterothermy may be a mechanism by which protein damage is reduced or prevented in the hibernator's heart, thereby minimizing the requirement for new protein synthesis to replace degraded proteins.
The CCT is barrel-shaped and composed of two rings, each with eight subunits (65). All three of the CCT subunits recovered in our dataset, CCT5 (T-complex protein 1 subunit ε, spot 526), CCT7 (spot 581), and TCP1 (T-complex protein 1 subunit α, spot 609), increased in hibernation, indicating their importance for winter cardioprotection. CCT folds a subset of nascent cytosolic proteins, many of which are essential to the cell, including actin and tubulin (65); it specifically folds proteins with complex topologies that are prone to aggregation or have slow folding kinetics (71). The detected winter seasonal increase of CCT may regulate the replenishment of required proteins during IBAs by preventing nascent protein aggregation and ensuring that slowly folding proteins reach completion during this brief euthermic stage.
Heart proteins may be further preserved in hibernation by the chaperone and antiapoptotic activities of a number of heat shock proteins elevated in the transition from SA to IBA (Fig. 6). The small heat shock protein HSPB6 (spot 1709) is related in several ways to improved cardiac function; it is associated with enhanced myocardial contraction (13) as well as protection against ischemia-reperfusion injury (25). Furthermore, HSPB6 inhibits apoptosis by binding and stabilizing the actin cytoskeleton and by directly interacting with the proapoptotic protein BAX (24). HSPA4 (spot 161), a member of the HSP110 family, is induced under stressful conditions such as ischemia (41), radiation (37), and hepatocellular carcinoma (30). HSPA4 may depress apoptosis by inhibiting protein aggregation but is unable to fold proteins itself (30). Instead, it binds and holds substrates for refolding as well as serving as a nucleotide exchange factor for HSP70 (68). We also detected a winter increase, including a significant pairwise increase from SA to LT, in HSP90AB1 protein content (spot 285). HSP90AB1 stabilizes proteins, especially those involved in signal transduction and protein secretion (5). Furthermore, it plays an antiapoptotic role by complexing with AKT (protein kinase B), which leads to downstream activation of antiapoptotic NF-κB (4). Carey et al. (12) observed fluctuations in NF-κB levels of hibernating ground squirrel intestinal mucosa, with this transcription factor constitutively activated throughout the torpor-arousal cycle. The increase of HSP90AB1 protein during hibernation may represent a component of the antiapoptotic NF-κB regulatory pathway, which is in turn an important aspect of cardiac cellular defense strategies. Interestingly, HSP90AB1 mRNA was reported to be significantly underexpressed in the hearts of torpid versus summer active Arctic ground squirrels (72). The discrepancy between the mRNA and protein levels of this heat shock protein may represent bona fide species-specific differences but more likely serves to underscore the likely importance of mechanisms in addition to differential gene expression to modulate protein content and function throughout the hibernation cycle. Alternative mechanisms include protection of protein from degradation or posttranslational modifications for the regulation of HSP90AB1 specifically, but also as a general, low-energy means to achieve cellular protection in the hibernating heart.
Resistance to ischemia-reperfusion injury and the apoptosis that typically follows (60) is a remarkable feature of the hibernating phenotype. Despite some evidence for apoptotic pathway adjustments in the small intestine (e.g., Ref. 28), the regulation of apoptotic signaling pathways during hibernation is still poorly characterized (66). All three heat shock proteins that increased in our dataset during winter heterothermy, HSPB6, HSP90AB1, and HSPA4, have antiapoptotic properties. Moreover, we detected a winter seasonal increase in the antiapoptotic YWHAQ protein (i.e., spot 1314; Fig. 4G), which also binds to and sequesters BAX (50). These proteins suggest a link between ischemia-reperfusion tolerance and apoptosis inhibition, and further study of their functional roles in relation to hibernation is thus warranted. Nonetheless, our finding of heat shock and chaperone protein winter elevation along with previous reports of upregulated heat shock protein 27 (18) and several antioxidant peroxiredoxins (49) in hibernating ground squirrel hearts suggests that enhanced cardioprotection (15, 39) during heterothermy is a fundamental component of the winter phenotype.
CFL2
CFL2 is a muscle-specific protein that belongs to the actin depolymerization factor/cofilin family; it regulates actin dynamics by promoting actin filament turnover (51, 53). CFL2 binds actin; phosphorylation prevents actin binding (7). In hibernating ground squirrels, P-CFL2 is not detected during FT, LT, and E-Ar and only faintly detected during Ent (Fig. 7B). The quantity of total CFL2 remains unchanged (Fig. 7B), suggesting that the pool of CFL2 is mostly in its active form during these states. A similarly robust dephosphorylation of the cofilin pool occurs during conditions of ATP depletion and oxidative stress in cultured cells (63), neurons (48), and vascular smooth muscle (40). Moreover, Bernstein et al. (8) proposed that hyperactivating cofilin by dephosphorylation is a protective mechanism to preserve ATP as well as inhibit apoptosis during ATP-limited conditions such as ischemia. When the entire cofilin pool is dephosphorylated, it is sequestered into actin-cofilin rods; because cofilin is not able to participate in the ATP hydrolyzing activity of actin turnover, this sequestration prevents further declines in available ATP. Additionally, cofilin sequestration prevents its localization to the mitochondria where it plays a role in initiating apoptosis. Although the observed decrease of P-CFL2 in the hibernator's hearts (Fig. 7) could indicate that ATP availability is limited during the entrance, torpid, and early arousal periods, others have shown that tissue ATP levels (43, 58, 75) and/or energy charge (10, 19) are maintained throughout hibernation. Furthermore, the hibernator's heart is able to maintain ion homeostasis and contractility in the cold, therefore it is unlikely that ATP is depleted in the heart during these states. Instead, the dephosphorylation of CFL2 observed here likely represents a preprogrammed mechanism to maintain the energy charge and conserve ATP for essential functions during the depressed metabolic states of Ent, FT, and LT and during the E-Ar period, which is transiently ischemic (45). It may instead or in addition be a mechanism for the heart to suppress apoptosis during these states. We speculate that without dephosphorylation and sequestration of CFL2, ATP would deplete in the heart and compromise the maintenance of homeostasis.
Fall Transition
Two fall sampling groups were included in our proteomics screen: this is the first proteomics study that has specifically examined the seasonal transition undertaken by hibernators between homeothermy and heterothermy. The two-switch model of hibernation predicts that torpor-arousal cycles are only possible once the switch to a heterothermy-permissive winter physiology has occurred (58). Because the Tb of all squirrels classified as FT had previously reached at least 10°C, and 4/6 were torpid at the time of tissue collection, we hypothesized the FT animals would have switched to the winter proteome. Instead, Random Forests clustering and ANOVA revealed FT to be an intermediate phase between summer and winter. The euthermic FT squirrel clustered near the FA and homeothermic groups, and the five FT animals with lower Tb moved toward but did not merge with the winter groups (Fig. 3D). This result indicates that FT is neither analogous to SpD, SA, or FA nor to any of the winter groups. Furthermore, Tukey pairwise comparisons revealed numerous spot abundance differences between FT and sampling states considered both homeothermic (SpD and SA) and heterothermic (IBA, Ent, and LT; Table 1), which supports the idea that FT is not biochemically identical to either season.
The intermediate nature of the two fall sampling states further implies that the transition from summer to winter requires a protracted and perhaps sequential reprogramming phase rather than a simple rapid switch. This phenotypic transition period is evidenced by specific spot abundance patterns. For example, levels of DBT (Fig. 4C) and YWHAQ (Fig. 4G) were comparable between the fall (FA, FT) and summer (SA, SpD) groups. Similar patterns were noted for OXCT1 (spots 691, 692, and 700), PDHX (pyruvate dehydrogenase protein X component, spot 748), PARK7 (parkinson protein 7, Spot 1660), PCCA (propionyl-CoA carboxylase α chain, spot 425), MDH1 (cytosolic malate dehydrogenase, spot 1162), ECI1 (spot 1388), and CRAT (spot 524) (Supplemental Table S3). The involvement of CRAT and ECI1 in fatty acid catabolism and of OXCT1 in ketone body catabolism suggests that the seasonal switch to enhanced lipid metabolism is not yet complete in the heart when animals initiate their first torpor bout. In contrast, the proteins FABP3 (Fig. 4H) and PDP1 (pyruvate dehydrogenase phosphatase, spot 643; Supplemental Table S3) display comparable levels between fall (FA, FT) and winter (IBA, Ent, LT, and E-Ar) states. Because FABP3 promotes intracellular transport of long-chain fatty acids and other hydrophobic moieties (62), the heart is poised for increased delivery of fatty acid substrates to mitochondria or peroxisomes, although their subsequent catabolism is not fully optimized for winter. Increased FABP3 in the fall would also support transport of n-6 poly-unsaturated fatty acids (PUFA) as they accumulate in heart membrane phospholipids by a diet-independent mechanism during this period (3). This appears to involve a seasonal redistribution of n-6 PUFA from white adipose tissue to heart; elevated n-6 PUFA are hypothesized to boost the activity of the sarcoplasmic reticulum Ca2+-ATPase SERCA2a at low temperature (55), which in turn leads to enhanced reuptake of Ca2+ from the cytoplasm at low Tb (42). Protein differences between FA and FT were also observed, including P-CFL2 (Fig. 4A), APOA1 (Fig. 4E), and TTR (Fig. 4F). In particular, the plasma proteins APOA1 (spots 1449, 1476, and 1486), ALB (spots 423, 436, and 462), TF (spot 333), TTR (spot 1878), and A2M (α2-macroglobulin, spot 105) were uniformly more abundant in the cardiac proteome of FT versus FA animals. As some of these plasma proteins have transport roles, e.g., fatty acid transport by ALB, thyroid hormone and vitamin A by TTR, and cholesterol with APOA1, it is possible that augmenting the ability to manage and transport lipids precedes the switch to fatty acid catabolism characteristic of winter heterothermy. However, we cannot rule out the possibility that the detection of plasma proteins in these tissue samples may result from a perfusion artifact that is enhanced when animals are at low Tb. Investigations of heart metabolites would reveal if cholesterol and fatty acid levels are indeed elevated during this transition period.
Finally, the gradual nature of the fall transition is revealed by those proteins that demonstrated incremental adjustments in abundance from summer to winter via the two fall sampling periods. For example, HSPA4 (Fig. 4B) increased steadily from summer to winter, perhaps in response to mounting cellular stress encountered in the heart during the short fall torpor bouts (56). In contrast, BCKDHB (spot 1144) declines steadily through the fall transition, likely a result of eliminating dietary protein intake with the onset of fasting. As this is the rate-limiting subunit (E1) of the BCKDC complex (74), its abundance likely reflects the actual decline of BCKDC activity in the catabolism of branched-chain amino acids.
Conclusions and Perspectives
Our study revealed that the heart proteome underlying summer homeothermy differs markedly from that supporting winter heterothermy. The findings are consistent with a need to establish fully a winter protected phenotype before prolonged multiday torpor bouts ensue. Remarkably, despite dramatic oscillations in temperature and heart and metabolic rates over the torpor-arousal cycle, very few protein changes were detected among winter heterothermic states. Yet the maintenance of ATP homeostasis stands out in this dataset. Because the heart must function in both the metabolically depressed state of torpor and the oxygen-limited but metabolically intense rewarming during arousal, available ATP resources are likely prioritized for essential functions such as contractility and ion homeostasis. The general lack of protein abundance changes detected across the torpor-arousal cycle provides evidence that use of ATP for protein degradation and resynthesis is limited; the increased abundance of chaperone and heat shock proteins preserve existing protein integrity and protect against apoptosis. An additional example of an ATP conserving mechanism in the heart was the near complete dephosphorylation of CFL2 in all of the hibernation states except IBA. Dephosphorylated CFL2 conserves ATP by limiting actin turnover and illustrates a strategy for acute posttranslational protein regulation. Enhanced ATP conservation facilitates the ability of the hibernator's heart to maintain function under metabolically depressed and/or oxygen-limited conditions. Moreover, the unique cardioprotected physiology of hibernators may be derived through a novel form of preconditioning, initiated by endogenous mechanisms in a constant environment (e.g., FA and FT animals, Fig. 4H). This preconditioning is modulated (Fig. 4A) and amplified (Fig. 4B) by short torpor bouts in fall (56) prior to achieving the deep extended torpor of hibernation. Therefore, the initial fall torpor bouts may be particularly stressful to the heart. Further study of this intermediate phase is imperative to characterize the mechanisms that permit survival of prolonged torpor in hibernators.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-089049 to S. L. Martin, R01GM-083649 to A. Karimpour-Fard, R01LM-008111 and R01LM-009254 to L. E. Hunter. The Institutional Proteomics Mass Spectrometry Facility is supported in part by NIH Grants to the Colorado Clinical and Translational Science Institute (UL1-RR025780) and the University of Colorado Cancer Center (P30-CA-046934).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K. R. G., L. E. E., and S. L. M. conception and design of research; K. R. G. and L. E. E. performed experiments; K. R. G., A. K. -F., and L. E. H. analyzed data; K. R. G., L. E. E., and S. L. M. interpreted results of experiments; K. R. G. and A. K. -F. prepared figures; K. R. G. drafted manuscript; K. R. G., A. K. -F., L. E. E., A. G. H., and S. L. M. edited and revised manuscript; K. R. G., L. E. E., A. G. H., L. E. H., and S. L. M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Martin laboratory including R. Russell and N. Heidlebaugh for assistance with experimental procedures and P. O'Neill for helpful comments. We also thank the members of the University of Colorado School of Medicine Mass Spectrometry and Proteomics Core Facility for technical help.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Agrawal GK, Thelen JJ. Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics 5: 4684–4688, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays 29: 431–440, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Arnold W, Ruf T, Frey-Roos F, Bruns U. Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator. PLoS One 6: e18641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci 32: 595–610, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Barginear MF, Van Poznak C, Rosen N, Modi S, Hudis CA, Budman DR. The heat shock protein 90 chaperone complex: an evolving therapeutic target. Curr Cancer Drug Targets 8: 522–532, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57: 289–300, 1995. [Google Scholar]
- 7. Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol 20: 187–195, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernstein BW, Chen H, Boyle JA, Bamburg JR. Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. Am J Physiol Cell Physiol 291: C828–C839, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Breiman L. Random forests. Mach Learn 45: 5–32, 2001. [Google Scholar]
- 10. Burlington RF, Meininger GA, Thurston JT. Effect of low temperatures on high energy phosphate compounds in isolated hearts from a hibernator and a nonhibernator. Comp Biochem Physiol B 55: 403–407, 1976. [DOI] [PubMed] [Google Scholar]
- 11. Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NK-kappaB in ground squirrel intestine. J Comp Physiol B 170: 551–559, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC, Maggio JE, Xiao RP, Kranias EG. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res 94: 184–193, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Colugnati DB, Arida RM, Cravo SL, Schoorlemmer GH, de Almeida AC, Cavalheiro EA, Scorza FA. Hibernating mammals in sudden cardiac death in epilepsy: what do they tell us? Med Hypotheses 70: 929–932, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res 70: 254–263, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol 133: 483–492, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Duker GD, Olsson SO, Hecht NH, Senturia JB, Johansson BW. Ventricular fibrillation in hibernators and nonhibernators. Cryobiology 20: 407–420, 1983. [DOI] [PubMed] [Google Scholar]
- 18. Eddy SF, McNally JD, Storey KB. Up-regulation of a thioredoxin peroxidase-like protein, proliferation-associated gene, in hibernating bats. Arch Biochem Biophys 435: 103–111, 2005. [DOI] [PubMed] [Google Scholar]
- 19. English TE, Storey KB. Enzymes of adenylate metabolism and their role in hibernation of the white-tailed prairie dog, Cynomys leucurus. Arch Biochem Biophys 376: 91–100, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Epperson LE, Dahl TA, Martin SL. Quantitative analysis of liver protein expression during hibernation in the golden-mantled ground squirrel. Mol Cell Proteomics 3: 920–933, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Metabolic cycles in a circannual hibernator. Physiol Genomics 43: 799–807, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Epperson LE, Rose JC, Carey HV, Martin SL. Seasonal proteomic changes reveal molecular adaptations to preserve and replenish liver proteins during ground squirrel hibernation. Am J Physiol Regul Integr Comp Physiol 298: R329–R340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Epperson LE, Rose JC, Russell RL, Nikrad MP, Carey HV, Martin SL. Seasonal protein changes support rapid energy production in hibernator brainstem. J Comp Physiol B 180: 599–617, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med 15: 138–141, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation 111: 1792–1799, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Fedorov VV, Glukhov AV, Sudharshan S, Egorov Y, Rosenshtraukh LV, Efimov IR. Electrophysiological mechanisms of antiarrhythmic protection during hypothermia in winter hibernating versus nonhibernating mammals. Heart Rhythm 5: 1587–1596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fedorov VV, Li L, Glukhov A, Shishkina I, Aliev RR, Mikheeva T, Nikolski VP, Rosenshtraukh LV, Efimov IR. Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: possible role of Cx43 and Cx45 up-regulation. Heart Rhythm 2: 966–975, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Fleck CC, Carey HV. Modulation of apoptotic pathways in intestinal mucosa during hibernation. Am J Physiol Regul Integr Comp Physiol 289: R586–R595, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66: 239–274, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Gotoh K, Nonoguchi K, Higashitsuji H, Kaneko Y, Sakurai T, Sumitomo Y, Itoh K, Subjeck JR, Fujita J. Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett 560: 19–24, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Hampton M, Andrews MT. A simple molecular mathematical model of mammalian hibernation. J Theor Biol 247: 297–302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hampton M, Nelson BT, Andrews MT. Circulation and metabolic rates in a natural hibernator: an integrative physiological model. Am J Physiol Regul Integr Comp Physiol 299: R1478–R1488, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun 313: 391–396, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 35. Johansson BW. The hibernator heart–nature's model of resistance to ventricular fibrillation. Cardiovasc Res 31: 826–832, 1996. [DOI] [PubMed] [Google Scholar]
- 36. Kamm KE, Zatzman ML, Jones AW, South FE. Maintenance of ion concentration gradients in the cold in aorta from rat and ground squirrel. Am J Physiol Cell Physiol 237: C17–C22, 1979. [DOI] [PubMed] [Google Scholar]
- 37. Kang CM, Park KP, Cho CK, Seo JS, Park WY, Lee SJ, Lee YS. Hspa4 (HSP70) is involved in the radioadaptive response: results from mouse splenocytes. Radiat Res 157: 650–655, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Kortner G, Geiser F. The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiol Int 17: 103–128, 2000. [DOI] [PubMed] [Google Scholar]
- 39. Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res 51: 637–646, 2001. [DOI] [PubMed] [Google Scholar]
- 40. Lee CK, Park HJ, So HH, Kim HJ, Lee KS, Choi WS, Lee HM, Won KJ, Yoon TJ, Park TK, Kim B. Proteomic profiling and identification of cofilin responding to oxidative stress in vascular smooth muscle. Proteomics 6: 6455–6475, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Lee MY, Choi YS, Choi JS, Min DS, Chun MH, Kim ON, Lee SB, Kim SY. An immunohistochemical study of APG-2 protein in the rat hippocampus after transient forebrain ischemia. Brain Res 924: 237–241, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Liu B, Belke DD, Wang LC. Ca2+ uptake by cardiac sarcoplasmic reticulum at low temperature in rat and ground squirrel. Am J Physiol Regul Integr Comp Physiol 272: R1121–R1127, 1997. [DOI] [PubMed] [Google Scholar]
- 43. Lust WD, Wheaton AB, Feussner G, Passonneau J. Metabolism in the hamster brain during hibernation and arousal. Brain Res 489: 12–20, 1989. [DOI] [PubMed] [Google Scholar]
- 44. Lyman CP. Hibernation and Torpor in Mammals and Birds. New York: Academic, 1982. [Google Scholar]
- 45. Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 289: R1297–R1306, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Martin SL, Epperson LE, Rose JC, Kurtz CC, Ane C, Carey HV. Proteomic analysis of the winter-protected phenotype of hibernating ground squirrel intestine. Am J Physiol Regul Integr Comp Physiol 295: R316–R328, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Milsom WK, Zimmer MB, Harris MB. Regulation of cardiac rhythm in hibernating mammals. Comp Biochem Physiol A Mol Integr Physiol 124: 383–391, 1999. [DOI] [PubMed] [Google Scholar]
- 48. Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol 2: 628–636, 2000. [DOI] [PubMed] [Google Scholar]
- 49. Morin P, Jr, Storey KB. Antioxidant defense in hibernation: cloning and expression of peroxiredoxins from hibernating ground squirrels, Spermophilus tridecemlineatus. Arch Biochem Biophys 461: 59–65, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y. 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278: 2058–2065, 2003. [DOI] [PubMed] [Google Scholar]
- 51. Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton (Hoboken) 67: 677–692, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orr AL, Lohse LA, Drew KL, Hermes-Lima M. Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol 153: 213–221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papalouka V, Arvanitis DA, Vafiadaki E, Mavroidis M, Papadodima SA, Spiliopoulou CA, Kremastinos DT, Kranias EG, Sanoudou D. Muscle LIM protein interacts with cofilin 2 and regulates F-actin dynamics in cardiac and skeletal muscle. Mol Cell Biol 29: 6046–6058, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pengelley ET, Fisher KC. Locomotor activity patterns and their relation to hibernation in the golden-mantled ground squirrel. J Mammal 47: 63–73, 1966. [PubMed] [Google Scholar]
- 55. Ruf T, Arnold W. Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am J Physiol Regul Integr Comp Physiol 294: R1044–R1052, 2008. [DOI] [PubMed] [Google Scholar]
- 56. Russell RL, O'Neill PH, Epperson LE, Martin SL. Extensive use of torpor in 13-lined ground squirrels in the fall prior to cold exposure. J Comp Physiol B 180: 1165–1172, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Russeth KP, Higgins L, Andrews MT. Identification of proteins from non-model organisms using mass spectrometry: application to a hibernating mammal. J Proteome Res 5: 829–839, 2006. [DOI] [PubMed] [Google Scholar]
- 58. Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31: 15–24, 2007. [DOI] [PubMed] [Google Scholar]
- 59. Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J. Shotgun proteomics analysis of hibernating arctic ground squirrels. Mol Cell Proteomics 9: 313–326, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sobel BE, Zaman T, Budd RC, Schneider DJ, Taatjes DJ. Attenuation of apoptosis and the eye of the beholder. Coron Artery Dis 19: 55–58, 2008. [DOI] [PubMed] [Google Scholar]
- 61. Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol 14: 598–604, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta 1486: 28–44, 2000. [DOI] [PubMed] [Google Scholar]
- 63. Suurna MV, Ashworth SL, Hosford M, Sandoval RM, Wean SE, Shah BM, Bamburg JR, Molitoris BA. Cofilin mediates ATP depletion-induced endothelial cell actin alterations. Am J Physiol Renal Physiol 290: F1398–F1407, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2006. [Google Scholar]
- 65. Valpuesta JM, Martin-Benito J, Gomez-Puertas P, Carrascosa JL, Willison KR. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett 529: 11–16, 2002. [DOI] [PubMed] [Google Scholar]
- 66. van Breukelen F, Krumschnabel G, Podrabsky JE. Vertebrate cell death in energy-limited conditions and how to avoid it: what we might learn from mammalian hibernators and other stress-tolerant vertebrates. Apoptosis 15: 386–399, 2010. [DOI] [PubMed] [Google Scholar]
- 67. van Breukelen F, Martin SL. Translational initiation is uncoupled from elongation at 18 degrees C during mammalian hibernation. Am J Physiol Regul Integr Comp Physiol 281: R1374–R1379, 2001. [DOI] [PubMed] [Google Scholar]
- 68. Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 47: 7001–7011, 2008. [DOI] [PubMed] [Google Scholar]
- 69. Wang SQ, Zhou ZQ. Medical significance of cardiovascular function in hibernating mammals. Clin Exp Pharmacol Physiol 26: 837–839, 1999. [DOI] [PubMed] [Google Scholar]
- 70. Williams DR, Epperson LE, Li W, Hughes MA, Taylor R, Rogers J, Martin SL, Cossins AR, Gracey AY. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics 24: 13–22, 2005. [DOI] [PubMed] [Google Scholar]
- 71. Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol 15: 1255–1262, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008. [DOI] [PubMed] [Google Scholar]
- 73. Yatani A, Kim SJ, Kudej RK, Wang Q, Depre C, Irie K, Kranias EG, Vatner SF, Vatner DE. Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am J Physiol Heart Circ Physiol 286: H2219–H2228, 2004. [DOI] [PubMed] [Google Scholar]
- 74. Zhao Y, Popov KM, Shimomura Y, Kedishvili NY, Jaskiewicz J, Kuntz MJ, Kain J, Zhang B, Harris RA. Effect of dietary protein on the liver content and subunit composition of the branched-chain alpha-ketoacid dehydrogenase complex. Arch Biochem Biophys 308: 446–453, 1994. [DOI] [PubMed] [Google Scholar]
- 75. Zimny ML, Gregory R. High energy phosphates during hibernation and arousal in the ground squirrel. Am J Physiol 195: 233–236, 1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.