Abstract
The effect of hyperbaric oxygen treatment (HBOT) was examined using MSG mice, which are an animal model of obesity, hyperlipidemia, diabetes, and nonalcoholic fatty liver disease. Nineteen MSG male mice were divided into HBOT treated and control groups at 12 weeks of ages. The HBOT group was treated with hyperbaric oxygen from 12 to 14 weeks (first phase) and then from 16 to 18 weeks (second phase). Interestingly, the body weight of the HBOT group was significantly lower (P < 0.01) than that of the control group. In contrast, the serum lipid level did not show significant changes between the two groups. As for the effects of increasing oxidative stress, the liver histology of the HBOT group showed severer cellular damage and aberrant TNF-α expression. HBOT has the advantage of improving obesity in patients with metabolic syndrome, but the fault of causing organ damage by increasing oxidative stress.
Keywords: Nonalcoholic steatohepatitis, obesity, hyperbaric oxygen treatment, MSG mice
1. INTRODUCTION
“Metabolic syndrome” is serious current issue. Metabolic syndrome is called “visceral fat syndrome” because visceral fat accumulation and macrophage aggregations described as “crown-like structures” occur primarily and then cause various intractable disorders to the whole body [1, 2]. The liver phenotype of metabolic syndrome is called “nonalcoholic fatty liver disease (NAFLD)” and nonalcoholic steatohepatitis (NASH), which is the critical type of NAFLD, is a refractory progressive disease, which progresses to liver cirrhosis or hepatocellular carcinoma [3–5]. Although the elucidation of the mechanism behind the pathological condition remains controversial, the “two-hit theory” has support as the pathogenetic mechanism of NASH. It is suggested that simple steatosis arises as a result of the first hit and that the pathologic condition progresses due to a second hit such as oxidative stress [6]. This means that oxidative stress may be an exacerbating factor of liver disease caused by metabolic syndrome.
Hyperbaric oxygen therapy (HBOT) is a remedy in which an atmospheric pressure environment higher than the normal atmosphere is created artificially, and the patient inhales hyperbaric oxygen continuously in the environment in order to increase the level of dissolved oxygen in their blood, which promptly acts to improve systemic or local hypoxia [7]. This remedy is usually employed for conditions such as carbon monoxide poisoning, limb arterial occlusive diseases, diseases of the central nervous system, sudden deafness, and retinal arterial occlusion. Recently, its application has been expanded to multiple fields including orthopedics [8, 9] and sports medicine [10, 11]. We have proposed the possibility that HBOT would be effective in the treatment of autoimmune diseases and hematopoietic malignancies [12, 13]. These effects are supposedly caused by the strong oxidative stress that is produced as a side effect of HBOT. We hypothesized that oxidative stress predominantly affects autoreactive lymphoid and tumor cells. Various inflammatory cytokines play an important role in the onset and progression of metabolic syndrome and NASH [14]. The visceral fat cells of obese patients store several times more fat than those of nonobese patients. These cells are often enlarged, and their cell membranes frequently collapse causing the leakage of stored lipids. Subsequently, macrophages begin to cluster in order to process these lipids. These clustered macrophages are called “crown-like structures” and are frequently observed in the visceral fat of patients with metabolic syndrome [15, 16]. It is thought that the various cytokines and chemokines that are produced by these macrophages in visceral fat are closely related to the onset and exacerbation of the symptoms of metabolic syndrome. They are also considered to be involved in the formation of hepatic lesions through the portal vein. It is expected that when HBOT is used for the treatment of metabolic syndrome [17–19], these excessively activated cytokines may be inhibited, at the same time, as metabolism is accelerated by the more efficient oxygen supply. On the other hand, the risk that NASH will be worsened by increased oxidative stress is also taken into consideration [20–22]. In this study, using MSG mice with NASH and a background of obesity, hyperlipidemia, and type-2 diabetes which are all symptoms of metabolic syndrome, the effects of HBOT on obesity, hyperlipidemia, and NASH are examined [23, 24].
2. MATERIALS AND METHODS
2.1. Experimental Animals
The MSG mouse is an obese animal model, which is prepared by hypodermic injection of monosodium glutamate (MSG) into an ICR mouse immediately after birth. This mouse is reported to show symptoms of obesity, type-2 diabetes, hyperlipidemia, and non-alcoholic fatty liver disease after usual feeding without showing hyperphagia [23, 24]. Although it is known that MSG mice of both sexes demonstrate various symptoms of metabolic syndrome, males show more severe symptoms. MSG mice are usually prepared by administering a hypodermic injection of MSG of 2 mg per g of body weight for successive five days. Recently, Sasaki et al. reported that MSG mice that were prepared by one hypodermic injection of 4 mg MSG showed a more severe pathologic condition than those produced using the conventional method [25]. In present study, 19 MSG male mice were prepared according to the method of Sasaki et al.
2.2. Study Design
Nineteen MSG male mice were divided into the HBOT-treated group (n = 10) and the control group (HBOT-untreated group) (n = 9) at 12 weeks of age. The HBOT-treated group was treated with HBOT (2.5 ATA/60 min/d) over 2 weeks from 12 to 14 weeks of age for 1 hour a day (first phase). Then, after a two-week interval, the mice were treated with HBOT (2.5 ATA/60 min/d) for another two weeks from 16 to 18 weeks of ages for 1 hour a day (second phase). The control group was kept in a chamber under normal pressure instead of HBOT for 1 hour and supplied with a normal concentration of oxygen. The mice in each group were sacrificed after 12 hours of fasting after the last HBOT treatment, and their serum, liver, and visceral fat were extracted.
2.3. Measured Parameters
Weekly weight was measured from the start of the experiment until sacrifice. In serum, triglycerides (TG), total cholesterol (T-chol), very-low-density lipoprotein (VLDL-) chol, low-density lipoprotein (LDL-) chol, and high-density lipoprotein cholesterol (HDL-) chol were measured. The cholesterol and phospholipid profiles in serum lipoproteins were analyzed using a dual-detection HPLC system with two tandem-connected TSKgel LipopropakXL columns (300 mm × 7.8 mm; Tosoh, Tokyo, Japan) at Skylight Biotech (Akita, Japan).
Formalin-fixed paraffin-embedded specimens of the visceral fat and liver were prepared and analyzed pathomorphologically. Histopathological findings of the liver were scored using the NASH Clinical Research Network Scoring System based on four semiquantitative factors: steatosis [0–3], lobular inflammation [0–3], hepatocyte ballooning [0–2], and fibrosis [0–4] as previously described [26]. The NAFLD activity score (NAS) was defined as the unweighted sum of the scores for steatosis, lobular inflammation, and hepatocyte ballooning; thus, the scores ranged from 0 to 8. A NAS of 0 to 2 was considered not diagnostic of steatohepatitis, and scores of 5 or greater were taken as diagnostic of steatohepatitis.
2.4. Immunohistochemistry
Five µm-thick specimens from each sample were immunostained using standard immunostaining procedures. Rat monoclonal antibody against mouse MAC-2 (Galectin-3) (Cedarlane, Hornby, Ontario; 1 : 100 dilutions) was used as a marker of macrophages. Rabbit polyclonal antibody against mouse TNF-α (Monosan, Uden, The Netherlands; 1 : 20 dilutions) was also used. Histofine-peroxidase for rat primary antibody (Nichirei, Tokyo, Japan) and Envision-peroxidase (Envision-PO) (DAKO, Glostrup, Denmark) for rabbit primary antibodies were used as secondary antibodies. For the substrate peroxidase, 3,3′-diaminobenzidine (DAB) was applied. In all cases, optimal dilutions were used, and positive and negative samples were included in each assay.
2.5. Statistical Analysis
Statistical analysis was performed with Stat View version 5.0 (Abacus Concept, Berkley, Calif, USA). Data were expressed as mean ± SE. The differences between the control and HBOT groups were analyzed using the Student's t-test. A value of P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Change of Weight
The body weights of the male MSG mice prepared using a single subcutaneous injection of MSG at a dose of 4 mg per g body weight increased constantly until 20 weeks of age and then plateaued until 28 weeks of age. Between 12 and 18 weeks of age, the rate of weight gain hits its peak with a mean of about a 9 g increase in weight. The changes in body weight in this study are shown in Figure 1. In the control group, the mean weight was 56.0 g at 12 weeks old, 59.2 g at 14 weeks old, 61.8 g at 16 weeks old, and 64.5 g at 18 weeks old, showing a constant increase. On the other hand, in the HBOT-treated group, the mean weight was 56.8 g at 12 weeks old but decreased rapidly to 53.6 g at 14 weeks old, that is, immediately after completing the first phase of HBOT. The mean weight increased again in the period of HBOT pause, and the mean weight was 57.9 g at 16 weeks old; however, it was 51.7 g at 18 weeks old, which is after the second phase of HBOT. The weight of the HBOT group was significantly decreased compared with that in the control group (Figure 2).
Figure 1.
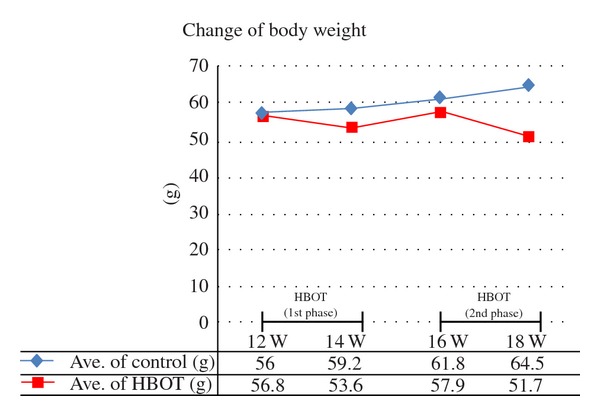
Change in body weight.
Figure 2.
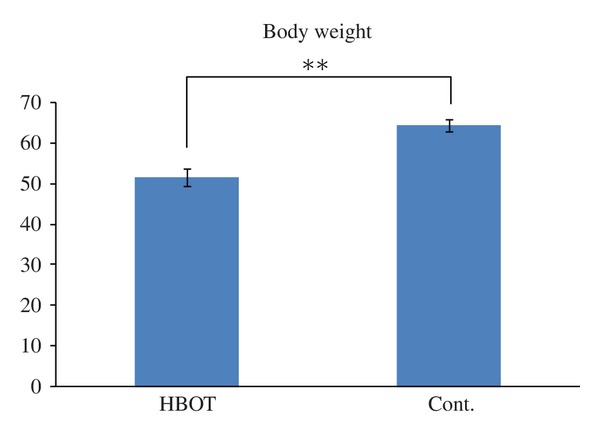
The weight of the HBOT group was significantly decreased compared with that in the control group (Student's t-test, **P < 0.01).
3.2. Serum Lipid
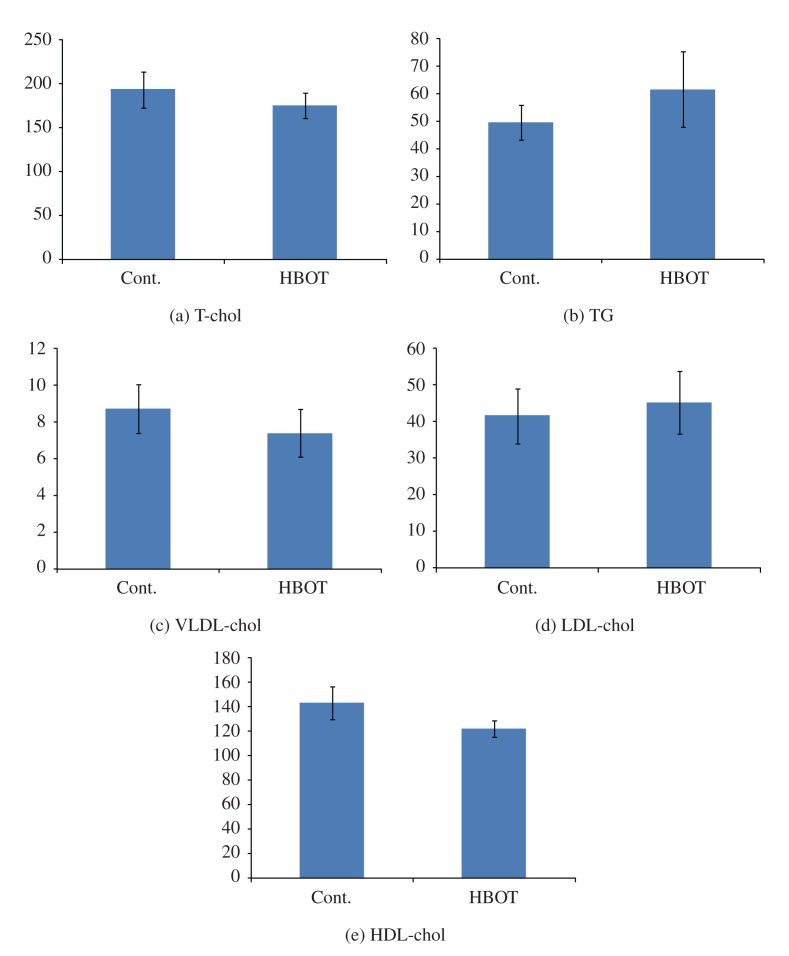
The serum lipid levels of each group are shown in Table 1 and Figure 3. In the control group, TG was 49.3 mg/dL, T-chol was 192.9 mg/dL, VLDL-chol was 8.7 mg/dL, LDL-chol was 41.5 mg/dL, and HDL-Chol was 142.7 mg/dL. On the other hand, in the HBOT-treated group, TG increased to 61.6 mg/dL. Although T-Chol declined slightly to 175.1 mg/dL, VLDL-chol was 7.4 mg/dL, LDL-chol was 45.1 mg/dL, and HDL-chol was 121.7 mg/dL. Although there were no statistical differences between the groups, LDL-chol was higher and HDL-chol was lower in the HBOT-treated group.
Table 1.
The serum lipid.
| Cholesterol (mg/dL) | TG (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Total | CM (>80 nm) |
VLDL (30–80 nm) |
LDL (16–30 nm) |
HDL (8–16 nm) |
Total | CM (>80 nm) |
VLDL (30–80 nm) |
LDL (16–30 nm) |
HDL (8–16 nm) |
| HBOT-1 | 217.70 | 3.06 | 15.48 | 52.19 | 146.98 | 165.50 | 27.59 | 84.87 | 19.04 | 34.00 |
| HBOT-2 | 155.22 | 0.53 | 4.36 | 26.41 | 123.92 | 36.12 | 2.95 | 17.80 | 10.21 | 5.16 |
| HBOT-3 | 111.52 | 1.05 | 6.32 | 15.74 | 88.41 | 51.20 | 6.58 | 27.34 | 9.82 | 7.47 |
| HBOT-4 | 107.11 | 1.13 | 9.94 | 13.90 | 82.14 | 90.72 | 9.38 | 59.87 | 13.56 | 7.90 |
| HBOT-5 | 166.60 | 0.88 | 11.72 | 37.44 | 116.56 | 78.42 | 5.79 | 49.83 | 15.12 | 7.67 |
| HBOT-6 | 205.66 | 0.39 | 3.28 | 66.57 | 135.42 | 24.46 | 1.44 | 9.68 | 7.69 | 5.64 |
| HBOT-7 | 207.75 | 0.25 | 3.72 | 59.95 | 143.82 | 22.55 | 0.86 | 9.86 | 7.63 | 4.20 |
| HBOT-8 | 193.78 | 0.43 | 3.82 | 57.63 | 131.90 | 21.62 | 0.74 | 9.65 | 6.40 | 4.84 |
| HBOT-9 | 245.03 | 1.73 | 9.21 | 98.57 | 135.53 | 71.69 | 12.76 | 36.07 | 12.66 | 10.20 |
| HBOT-10 | 140.91 | 0.40 | 5.65 | 22.38 | 112.48 | 53.57 | 3.08 | 30.37 | 14.58 | 5.54 |
|
| ||||||||||
| Ave | 175.13 | 0.99 | 7.35 | 45.08 | 121.72 | 61.59 | 7.12 | 33.53 | 11.67 | 9.26 |
|
| ||||||||||
| Cont-1 | 166.30 | 0.19 | 15.56 | 34.86 | 115.69 | 63.74 | 1.82 | 49.69 | 8.31 | 3.92 |
| Cont-2 | 128.11 | 0.03 | 3.61 | 24.80 | 99.67 | 28.63 | 0.36 | 18.14 | 6.81 | 3.32 |
| Cont-3 | 324.08 | 0.03 | 10.41 | 94.72 | 218.93 | 86.88 | 0.58 | 60.97 | 16.51 | 8.82 |
| Cont-4 | 189.40 | 0.04 | 7.25 | 32.47 | 149.63 | 52.36 | 0.39 | 34.64 | 11.99 | 5.34 |
| Cont-5 | 133.15 | 0.05 | 6.09 | 20.81 | 106.20 | 35.94 | 0.46 | 26.42 | 5.94 | 3.13 |
| Cont-6 | 165.41 | 0.14 | 10.74 | 33.28 | 121.25 | 40.28 | 0.80 | 28.52 | 8.25 | 2.72 |
| Cont-7 | 193.70 | 0.03 | 3.93 | 50.60 | 139.13 | 33.99 | 0.00 | 14.84 | 8.96 | 10.19 |
| Cont-8 | 183.32 | 0.02 | 7.60 | 34.33 | 141.38 | 37.74 | 0.03 | 25.99 | 7.88 | 3.84 |
| Cont-9 | 252.71 | 0.11 | 13.02 | 47.22 | 192.36 | 64.42 | 0.93 | 49.29 | 10.44 | 3.76 |
|
| ||||||||||
| Ave | 192.91 | 0.07 | 8.69 | 41.45 | 142.69 | 49.33 | 0.60 | 34.28 | 9.45 | 5.00 |
Figure 3.
The serum lipid levels of each group (Student's t-test).
3.3. Histopathological Imaging of Visceral Fat
In the control group, visceral fat was markedly enlarged, and crown-like structures were also observed in some places (Figure 4(a)). These represented MAC-2-positive macrophage aggregation (Figure 4(b)). On the other hand, in the HBOT-treated group, the lipid droplets were smaller, and the number of crown-like structures tended to be lower (Figures 4(c) and 4(d)).
Figure 4.
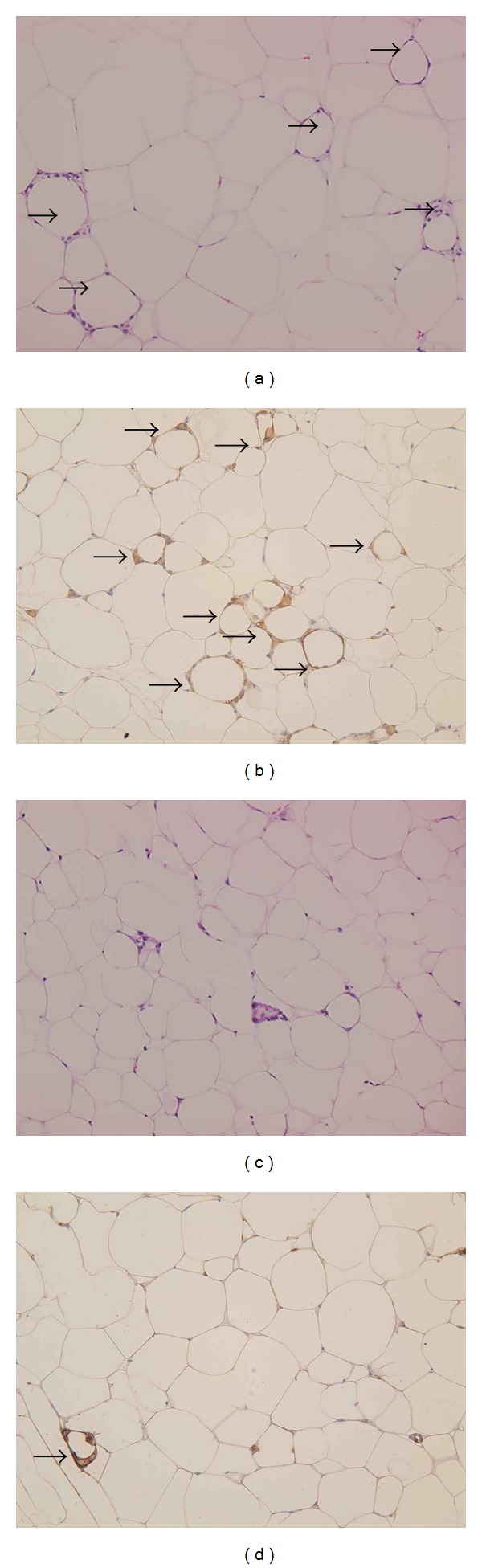
(a), (b) Visceral fat of control group. Adipocytes are markedly enlarged. Crown-like structures are frequently observed (arrows) (×200) ((a) H and E staining, (b) Mac-2 immunostaining as a macrophage marker) (c), (d) Visceral fat of HBOT group. Adipocytes are relatively small. Crown-like structures are rarely observed (arrows). (×200) ((c) H and E staining, (d) Mac-2 immunostaining as a macrophage marker).
3.4. Histopathological Imaging of the Liver
In the control group, fatty changes were recognized in the hepatocytes in the Zone3 to Zone2 regions. Ballooning degeneration was also found in various locations (Figure 5(a)). TNF-α expression was not seen in hepatocytes or Kupffer cells (Figure 5(b)). Although the emergence of lipogranuloma and focal necrosis was also observed and the image was considered to be equivalent to human NASH, no Mallory bodies or megamitochondria were evident and no fibrosis was seen either. On the other hand, in the HBOT-treated group, although the level of fatty change was slightly decreased compared with that in the control group, the ballooning degeneration of hepatocytes was more intensive and appeared extensively. Mallory bodies and the condensation of subcellular organelles were also sporadically observed (Figure 5(c) and its inset) as were small necrotic foci. No fibrosis was evident. In several cases, TNF-α expression was aberrantly observed in the hepatocytes of the perivenular and periportal areas; however, it was not seen in Kupffer cells (Figure 5(d)). Evaluation of liver pathology is shown in Table 2.
Figure 5.
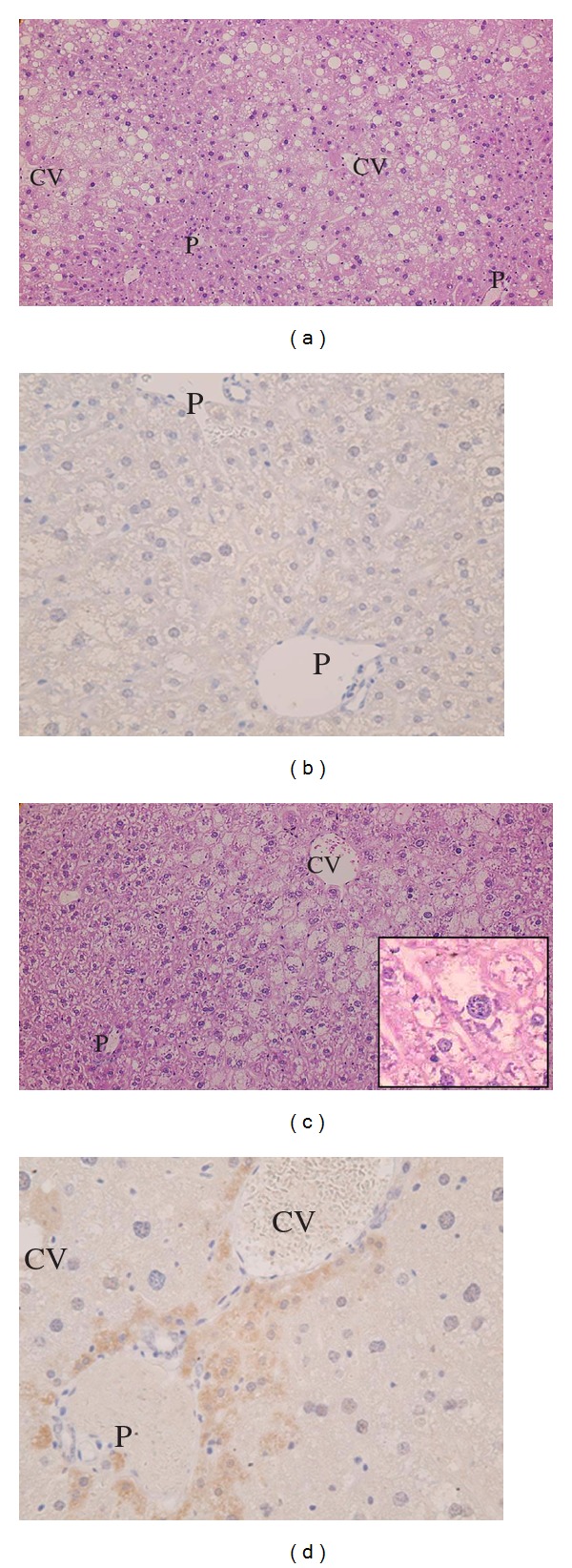
(a), (b) Liver of the control group. (a) H and E staining, ×200 magnification. In Zone3-Zone2, moderate fatty changes were observed. Ballooning degeneration was sporadically observed. (b) TNF-α immunostaining, ×400 magnification. No TNF-α expression was seen in hepatocytes or Kupffer cells. (P: portal tracts, CV: central vein) (c), (d) Liver of the HBOT group. (c) H and E staining, ×200 magnification. Fatty changes were less severe, while marked ballooning degeneration was observed. Mallory bodies were seen sporadically (inset). (d) TNF-α immunostaining, ×400 magnification. TNF-α expression was observed in the hepatocytes of the perivenular and periportal areas; however, it was not seen in Kupffer cells. (P: portal tracts, CV: central vein).
Table 2.
NAS score and fibrosis.
| Steatosis | Lobular inflammation | Hepatocyte ballooning | NAS | Fibrosis | |
|---|---|---|---|---|---|
| HBOT-1 | 1 | 2 | 2 | 5 | 0 |
| HBOT-2 | 1 | 1 | 1 | 3 | 0 |
| HBOT-3 | 1 | 1 | 2 | 4 | 0 |
| HBOT-4 | 0 | 1 | 2 | 3 | 0 |
| HBOT-5 | 1 | 1 | 2 | 4 | 0 |
| HBOT-6 | 1 | 1 | 1 | 3 | 0 |
| HBOT-7 | 1 | 1 | 2 | 4 | 0 |
| HBOT-8 | 2 | 1 | 2 | 5 | 0 |
| HBOT-9 | 1 | 1 | 1 | 3 | 0 |
| HBOT-10 | 2 | 1 | 1 | 4 | 0 |
|
| |||||
| Cont-1 | 1 | 1 | 1 | 3 | 0 |
| Cont-2 | 2 | 1 | 1 | 4 | 0 |
| Cont-3 | 1 | 1 | 0 | 2 | 0 |
| Cont-4 | 2 | 1 | 1 | 4 | 1A |
| Cont-5 | 1 | 1 | 2 | 4 | 0 |
| Cont-6 | 2 | 1 | 1 | 4 | 1A |
| Cont-7 | 1 | 1 | 1 | 3 | 0 |
| Cont-8 | 2 | 1 | 1 | 4 | 0 |
| Cont-9 | 2 | 1 | 0 | 3 | 0 |
4. DISCUSSION
Metabolic syndrome is a combination of disorders that increase the risk of developing cardiovascular disease and diabetes [1, 2]. It affects one in five people, and its prevalence increases with age. Some studies have estimated its prevalence in the USA to be up to 25% of the population. Metabolic syndrome is also known as metabolic syndrome X, insulin resistance syndrome, and visceral fat syndrome. Usually it involves the development of visceral fat, after which the adipocytes (fat cells) of the visceral fat increase the plasma levels of TNF-α and alter the levels of a number of other substances (e.g., adiponectin, resistin, PAI-1). TNF-α has been shown not only to cause the production of inflammatory cytokines but also to trigger cell signaling by interacting with the TNF-α receptor, which may lead to insulin resistance. The progression from visceral fat to increased TNF-α to insulin resistance has some parallels to the human development of metabolic syndrome [26, 27]. NASH is thought to be the phenotype of metabolic syndrome in the liver, and the number of patients with the condition is constantly increasing. In Japan, 10–30% of population is considered to have NAFLD accompanied by metabolic syndrome, and 10% is presumed to have NASH, a progressive pathologic lesion [5]. Although there are many unclear points about the pathogenetic mechanism of NASH, a two-hit theory in which fat is first accumulated in hepatocytes by overeating, hyperlipidemia, and so forth, then the pathologic condition is induced to progress by a second hit, such as oxidative stress, is widely accepted [4, 6]. No definite remedy for metabolic syndrome or NASH has been established, and dietary counseling and therapeutic exercise are considered as the first options [28, 29]. However, a continuous strict adaptation is difficult with remedies based on lifestyle improvement, and the response rate is not always high. Large-scale clinical trials of pioglitazone for patients with NASH caused by metabolic syndrome have been performed; however, the reported effects are contradictory. For example, simultaneous weight gains together with improvements in damage have been reported [30–32].
Hyperbaric oxygen therapy is a remedy that improves hypoxia by inhaling hyperbaric oxygen in an environment in which the atmospheric pressure is higher than that of the normal atmosphere. Although it is widespread as a remedy for carbon monoxide poisoning and sudden deafness, its application for other purposes, such as in the fields of orthopedics and sports medicine, is also being widely investigated [7–11]. We have reported that the oxidative stress raising effects of HBOT may affect the “pathogenic cells” involved in autoimmune disease or hematopoietic malignancies selectively, using animal models and cultured cells [12, 13]. This indicates the potential of HBOT being applied to various diseases through a mechanism other than medication. In this study, we proposed the hypothesis that HBOT improves various symptoms of metabolic syndrome and attempted in vivo verification in an animal model. The animal model used was the MSG mouse, which shows various symptoms of metabolic syndrome with near-natural development and is known to show the symptoms of central obesity, hyperlipidemia, type-2 diabetes, and NASH in succession after 12 weeks of age [23, 24]. In the current study, our research was limited to obesity, hyperlipidemia, and NASH, and the benefits of HBOT on metabolic syndrome were examined.
As a result, although a weight gain of about 3 g in the first phase of the HBOT treatment period (two weeks) was seen in the control group, weight loss was observed with eight out of ten mice in the HBOT-treated group. The mean weight of the ten animals was 56.8 g before HBOT treatment, and it decreased to 53.6 g after HBOT treatment that is, a mean of 3.2 g weight loss. When HBOT treatment was stopped, the rate of weight increase of the mice recovered to the same extent as seen in the untreated mice; however, the mean body weight among ten mice decreased from 57.9 g to 51.7 g (by 6.2 g) during the second phase of HBOT treatment. Although we observed the body weight reduction in the 1st phase of HBOT, the weight of body developed slightly after two weeks of HBOT. Therefore, we performed another course of HBOT just as what we will try in the clinics for patients with different kinds of sickness. We expected further weight reduction to our mice, and the results were positive. However, the burst of oxidative stress might induce normal hepatocytes but not the fat cells undergoing apoptosis. This may be consistent with our previous experiments that cells with different origins have different threshold to oxidative stress-induced apoptosis [13]. Furthermore, the interesting point is that cells with higher proliferative rate such as cancer cells have a lower threshold to oxidative stress induced by hyperbaric oxygen treatment, and HBO2-induced apoptosis of cancer cells was through the intracellular accumulation of H2O2 and O2.− as well as the involvement of phosphorylation of p38 MAPK [13]. Our unpublished data further demonstrated that HBO2 treatment effectively suppressed tumor growth from lung cancer transferred SCID mice after 14 days (**P < 0.01) and 28 days (**P < 0.01) of tumor transfer compared with control mice. The survival rate of HBO2 treatment mice was increased significantly compared with control mice. Since oxidative stress is closely related to the development and progression of metabolic syndrome and cardiovascular disease; therefore, we should be more careful to improve this side effect: firstly, we could reduce the pressure and duration in the 2nd phase of HBOT; secondly, supplement of optimal antioxidants could be prescribed in the 2nd phase of HBOT. Future studies should be done in order to avoid the HBOT-mediated deterioration of liver histology.
Although the mechanism of obesity in MSG mice is not yet clear, weight gain due to a fall in energy consumption is suggested. In our study, it also turned out that, when MSG mice were bred with 75% of their usual food intake, the increase in weight and liver damage were identical to those seen in the mice with normal food intakes (unpublished data). Although a future detailed examination is required for the mechanism of weight loss induced by HBOT, one possibility is that rapid improvement in systemic metabolism due to the sufficient supply of oxygen to peripheral tissues induced the weight loss. Another possibility is that the prevention of overactivity of pathogenic cells in visceral fat and the induction of apoptosis cause the weight loss.
In test concerning the levels of lipid-associated molecules in serum, slight increases in triglyceride and LDL-chol were observed in the HBOT-treated group. Since the visceral fat and fat cumulative dosage in the liver decreased in the HBOT-treated group, the lipids may have migrated into the blood from the cells storing them. On the other hand, although the lipid cumulative dosage in hepatocyte was decreased in the liver, the grade of the hepatocellular damage became getting worse. While HBOT supplies sufficient oxygen to local sites, it is known to induce a strong oxidative stress. Oxidative stress is considered to be a key accelerator of cell damage and oncogenesis as the second hit of NASH [4–6]. It was supposed that oxidative stress also played a role in the hepatocellular damage observed in the present study. This indicates that HBOT is a “double-edged blade” as it has the advantage of improving obesity and the fault of causing organ damage by increasing oxidative stress.
Patients with metabolic syndrome usually have multiple diseases, and there are often many restrictions on the medication they can take. Although HBOT has the potential of becoming an effective remedy in support of diet restriction and therapeutic exercises, for practical use, further techniques, such as suppressing the harmful effects of oxidative stress, are indispensable. It is believed that the intake of antioxidants, such as polyphenol and vitamin E, is effective in reducing oxidative stress [33, 34]. It may be possible to extract only the benefits of HBOT by taking antioxidants as supplements, before and after HBOT treatment. It is important to examine different conditions of HBOT treatment in order to determine the most effective conditions for treating metabolic syndrome.
AUTHOR CONTRIBUTION
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the paper. K. Tsuneyama, Y. C. Chen, M. Kujimoto, and S. Y. Chen conducted the experiments, Y. Sasaki, W. Suzki, T. Shimada, S. Iizuka, M. Nagata, and M. Aburada supplied critical reagents and experimental animals, and K. Tsuneyama, Y. C. Chan, M. Fujimoto, and S. Y. Chen wrote the paper. Koichi Tsuneyama and Yen-Chen Chen contributed equally to this work.
ACKNOWLEDGMENTS
The authors thank Tokimasa Kumada and Takeshi Nishida for their technical assistance. This study was supported by a research grant from the Interchange association of JAPAN (2008).
References
- 1.Guize L, Pannier B, Thomas F, Bean K, Jégo B, Benetos A. Recent advances in metabolic syndrome and cardiovascular disease. Archives of Cardiovascular Diseases. 2008;101(9):577–583. doi: 10.1016/j.acvd.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocrine Reviews. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berk PD. Regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, and metabolic syndrome. Hepatology. 2008;48(5):1362–1376. doi: 10.1002/hep.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. Journal of Clinical Gastroenterology. 2006;40(supplement 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 6.Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. American Journal of Clinical Pathology. 2007;128(5):837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 7.D’Agostino Dias M, Fontes B, Poggetti RS, Birolini D. Hyperbaric oxygen therapy: types of injury and number of sessions—a review of 1506 cases. Undersea & Hyperbaric Medicine. 2008;35(1):53–60. [PubMed] [Google Scholar]
- 8.Huang KC, Hsu WH, Peng KT, Huang TJ, Hsu RWW. Hyperbaric oxygen therapy in orthopedic conditions: an evaluation of safety. Journal of Trauma. 2006;61(4):913–917. doi: 10.1097/01.ta.0000196702.26858.56. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima M, Tamura H, Nagayoshi I, Takao K, Yoshida K, Yamaguchi T. Hyperbaric oxygen therapy in orthopedic conditions. Undersea and Hyperbaric Medicine. 2004;31(1):155–162. [PubMed] [Google Scholar]
- 10.Germain G, Delaney J, Moore G, Lee P, Lacroix V, Montgomery D. Effect of hyperbaric oxygen therapy on exercise-induced muscle soreness. Undersea and Hyperbaric Medicine. 2003;30(2):135–145. [PubMed] [Google Scholar]
- 11.Babul S, Rhodes EC. The role of hyperbaric oxygen therapy in sports medicine. Sports Medicine. 2000;30(6):395–403. doi: 10.2165/00007256-200030060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Chen SY, Chen YC, Wang JK, et al. Early hyperbaric oxygen therapy attenuates disease severity in lupus-prone autoimmune (NZB × NZW) F1 mice. Clinical Immunology. 2003;108(2):103–110. doi: 10.1016/s1521-6616(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Chen SY, Ho PS, et al. Apoptosis of T-leukemia and B-myeloma cancer cells induced by hyperbaric oxygen increased phosphorylation of p38 MAPK. Leukemia Research. 2007;31(6):805–815. doi: 10.1016/j.leukres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. Journal of Gastroenterology. 2008;43(11):811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 15.Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. Journal of Lipid Research. 2008;49(7):1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 17.Unfirer S, Kibel A, Drenjancevic-Peric I. The effect of hyperbaric oxygen therapy on blood vessel function in diabetes mellitus. Medical Hypotheses. 2008;71(5):776–780. doi: 10.1016/j.mehy.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Al-Waili NS, Butler GJ, Beale J, et al. Influences of hyperbaric oxygen on blood pressure, heart rate and blood glucose levels in patients with diabetes mellitus and hypertension. Archives of Medical Research. 2006;37(8):991–997. doi: 10.1016/j.arcmed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda K, Aoki N, Adachi T, et al. Hyperbaric exposure with high oxygen concentration inhibits growth-associated increase in the glucose level of diabetic Goto-Kakizaki rats. Diabetes, Obesity and Metabolism. 2006;8(6):714–715. doi: 10.1111/j.1463-1326.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- 20.Nomoto K, Tsuneyama K, Takahashi H, Murai Y, Takano Y. Cytoplasmic fine granular expression of 8-hydroxydeoxyguanosine reflects early mitochondrial oxidative DNA damage in nonalcoholic fatty liver disease. Applied Immunohistochemistry and Molecular Morphology. 2008;16(1):71–75. doi: 10.1097/PAI.0b013e31803156d5. [DOI] [PubMed] [Google Scholar]
- 21.Kadokawa Y, Ohba K, Omagari K, et al. Intracellular balance of oxidative stress and cytoprotective molecules in damaged interlobular bile ducts in autoimmune hepatitis and primary biliary cirrhosis: in situ detection of 8-hydroxydeoxyguanosine and glutathione-S-transferase-pi. Hepatology Research. 2007;37(8):620–627. doi: 10.1111/j.1872-034X.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 22.Victor VM, De La Fuente M. Several functions of immune cells in mice changed by oxidative stress caused by endotoxin. Physiological Research. 2003;52(6):789–796. [PubMed] [Google Scholar]
- 23.Nagata M, Suzuki W, Iizuka S, et al. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Experimental Animals. 2006;55(2):109–115. doi: 10.1538/expanim.55.109. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi Y, Tsuneyama K, Fujimoto M, et al. Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. Journal of Autoimmunity. 2008;30(1-2):42–50. doi: 10.1016/j.jaut.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki Y, Suzuki W, Shimada T, et al. Dose dependent development of diabetes mellitus and non-alcoholic steatohepatitis in monosodium glutamate-induced obese mice. Life Sciences. 2009;85(13-14):490–498. doi: 10.1016/j.lfs.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Copaci I, Micu L, Voiculescu M. The role of cytokines in non-alcoholic steatohepatitis. A systematic review. Journal of Gastrointestinal and Liver Diseases. 2006;15(4):363–373. [PubMed] [Google Scholar]
- 28.Rafiq N, Younossi ZM. Effects of weight loss on nonalcoholic fatty liver disease. Seminars in Liver Disease. 2008;28(4):427–433. doi: 10.1055/s-0028-1091986. [DOI] [PubMed] [Google Scholar]
- 29.Cave M, Deaciuc I, Mendez C, et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. Journal of Nutritional Biochemistry. 2007;18(3):184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto M, Tsuneyama K, Kainuma M, et al. Evidence-based efficacy of kampo formulas in a model of non alcoholic fatty liver. Experimental Biology and Medicine. 2008;233(3):328–337. doi: 10.3181/0707-RM-207. [DOI] [PubMed] [Google Scholar]
- 32.Balas B, Belfort R, Harrison SA, et al. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. Journal of Hepatology. 2007;47(4):565–570. doi: 10.1016/j.jhep.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Reboul E, Thap S, Tourniaire F, et al. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamins C and E) on lutein absorption. British Journal of Nutrition. 2007;97(3):440–446. doi: 10.1017/S0007114507352604. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. Journal of Nutrition. 2001;131(11):2837–2842. doi: 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]