Abstract
A kaiABC clock gene cluster was previously identified from cyanobacterium Synechococcus elongatus PCC 7942, and the feedback regulation of kai genes was proposed as the core mechanism generating circadian oscillation. In this study, we confirmed that the Kai-based oscillator is the dominant circadian oscillator functioning in cyanobacteria. We probed the nature of this regulation and found that excess KaiC represses not only kaiBC but also the rhythmic components of all genes in the genome. This result strongly suggests that the KaiC protein primarily coordinates genomewide gene expression, including its own expression. We also found that a promoter derived from E. coli is feedback controlled by KaiC and restores the complete circadian rhythm in kaiBC-inactivated arrhythmic mutants, provided it can express kaiB and kaiC genes at an appropriate level. Unlike eukaryotic models, specific regulation of the kaiBC promoter is not essential for cyanobacterial circadian oscillations.
In various model organisms, the core mechanisms controlling circadian clocks that mark time in cells have been traced to transcription–translation feedback regulation of specific “clock genes” (1). In eukaryotic models, cis-acting elements and transacting factors controlling clock genes are thought to be clock-specific components. We previously identified a kaiABC clock gene cluster from the cyanobacterium Synechococcus elongatus PCC 7942 and proposed the feedback regulation of kai genes as the core mechanism governing circadian oscillations. In this model, kaiBC expression is regulated negatively by KaiC and positively by KaiA (2). However, the mechanism governing kaiBC gene expression is not yet well understood. Previously, we reported that the expression of every gene displays circadian transcription in this prokaryote (3), but the molecular mechanisms responsible for this phenomenon also remain unclear. In this study, we probed the nature of these regulations with following findings. Overexpression of kaiC repressed not only kaiBC but the majority of genes in the genome. When expressed under the control of a synthetic promoter (Ptrc) derived from Escherichia coli, kaiB and kaiC could restore the complete circadian rhythm in kaiBC-inactivated arrhythmic mutants. These results strongly suggest that the KaiC protein primarily coordinates genomewide gene expression, including its own expression, through a circadian feedback loop.
Materials and Methods
Bacterial Strains. A kaiC-inducible Synechococcus strain, used for promoter trap analysis, was generated by transformation of NUC42 (4) with pTS2KPtrc::kaiC (2). NUC43 was a kaiABC-deleted strain carrying a PkaiBC::luxAB reporter construct (4). NUC0202 is a wild-type reporter strain generated by restoring the kai gene cluster missing from NUC43 with pCkaiABC (2). The kaiBC-null mutant Synechococcus strain NUC0203 (PkaiBC::luxAB/kaiBC–) was generated by disruption of the kaiC gene in a kaiB-inactivated strain (ref. 2 and S.K., unpublished data). A DNA fragment containing a Shine–Dalgarno sequence and the ORF of kaiB was amplified by PCR using pTSKPtrc::kaiB as the template, then introduced into the SalI site of pTS2KPtrc::kaiC (pTS2KPtrc::kaiCB). NUC0203 was transformed with pTS2KPtrc::kaiCB to generate cells harboring an IPTG-inducible kaiCB cassette in the neutral site II site (NUC0204: PkaiBC::luxAB/kaiBC–+Ptrc::kaiCB). NUC0205 (complemented kaiABC), NUC0206 (kaiBC-inactivated), and NUC0207 (kaiBC-inactivated and Ptrc-driven kaiCB), all carrying the Ptrc::luxAB reporter in NSI, were constructed by the same methods used to create the PkaiBC::luxAB reporter strains. The Ptrc-driven, kaiA strain (NUC0209: kaiA–+Ptrc::kaiA) was generated by the transformation of a kaiA-inactivated strain (5) with pTS2KPtrc::GTGkaiA (S.K., unpublished data).
Promoter Trap. For promoter-trap experiments, ≈0.6-kb SauIIIAI-digested Synechococcus genomic DNA fragments were cloned into pNS2CmLuxTer4ΩPL-I, which contained a chloramphenicol-resistant (Cmr) gene and promoter-less luxAB genes in a neutral site II target sequence (H.M., unpublished data). The resulting 5.0 × 105 independent clones were used as a promoter-trap library. Wild-type Synechococcus and several clock mutants, in which the PpsbAI::luxAB reporter had been replaced with a kanamycin resistance gene, were transformed with library DNA. For promoter trap in kaiC-overexpresser cells (Fig. 1), ≈0.5- to 2.2-kb SauIIIAI-digested Synechococcus genomic DNA fragments were cloned into pAM717DdluxAB(+), a derivative of pAM1852 containing promoter-less luxAB and Cmr genes in an NSI targeting sequence (M.K., unpublished data). The resulting 2.7 × 105 independent clones were used as the second promoter-trap library. A kaiC-inducible strain, NUC0201, was generated by transformation of Synechococcus with pTS2Ptrc::kaiC (2). NUC0201 was then transformed with the second promoter-trap library. Bioluminescent colonies were screened by using a cooled charge-coupled device camera system (6). Among the 8,000 Cm-resistant colonies, 638 bright bioluminescent colonies were subjected to bioluminescence rhythm assays.
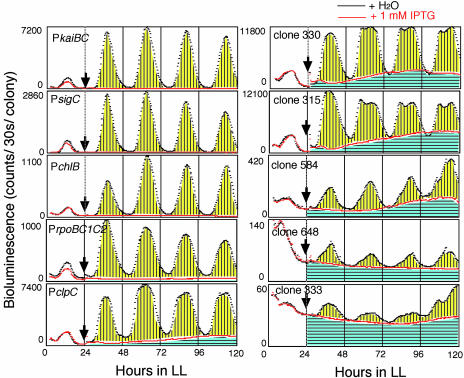
Fig. 1.
KaiC represses circadian rhythms of promoter activities. Approximately 640 clones carrying random promoter::luxAB reporters were isolated by promoter-trapping from IPTG-inducible kaiC cells. The circadian bioluminescence rhythms of these clones and the PkaiBC::luxAB reporter strain were analyzed on solid media under continuous light (LL) conditions of 46 μmol·m–2·s–1 at 30°C in the absence or presence of 1 mM IPTG (administered at the time point indicated by arrows). The bioluminescence profiles of nine representative clones are shown here. Maximum bioluminescence intensity during the experiment, normalized to the sample colony numbers, is shown for each rhythm. Rhythmic and nonrhythmic components are shown with yellow and blue backgrounds, respectively. Abscissa, hours in LL.
Assay of Bioluminescence. Bioluminescence assays were performed with the cooled charge-coupled device camera system and a photomultiplier-based quantitative system (6).
Western and Northern Analyses. Proteins and mRNA were extracted from cell grown in continuous or batch culture systems (see Fig. 3), then analyzed as described (5, 7).
Fig. 3.
Expression profiles of KaiC protein in kaiBC–+Ptrc::kaiCB cells. (A) Circadian rhythm of KaiC accumulation kaiBC–+Ptrc::kaiCB cells. Cells carrying a PkaiBC::luxAB reporter were grown in a continuous liquid culture system in the presence of 10 μM IPTG under LL conditions after a 12-h dark treatment, as described (2). (Upper) Bioluminescence from the PkaiBC reporter was measured every 30 min. Cells were harvested every 4 h, from hour 48 to hour 96 in LL. (Lower) Western blotting analysis was performed by using an anti-KaiC antiserum. P-KaiC and KaiC bands are phosphorylated KaiC and nonphosphorylated KaiC, respectively (5). (B) ΔkaiABC, ΔkaiABC+kaiABC, kaiBC–, and kaiBC–+Ptrc::kaiCB cells were grown in liquid BG-11 medium in LL of 30 μmol·m–2·s–1 at 30°C. IPTG (10 or 100 μM) was administered to kaiBC–+Ptrc::kaiCB cells as indicated. Total protein (5 μg), prepared from cells collected at hour 12 in LL after 12 h of darkness, was analyzed by Western blotting.
Results and Discussion
The kai Oscillator as the Dominant Oscillator. As every promoter assayed exhibited period lengths identical to that of the kai oscillator, we expected that the kai oscillator would dominate all gene expression rhythms. However, it remains possible that alternate oscillators coincide or synchronize their periods with that of the kai oscillator. A population of promoters exhibits an altered period length in a sigma factor mutant background (sigC–) (8), suggesting the possibility that another oscillator can regulate these promoters if linkage of SigC to the kai oscillator is disconnected. To address this issue, we applied a promoter trap to various kai mutants (Fig. 5 A and B, which is published as supporting information on the PNAS web site) and the ΔkaiABC (Fig. 5C) strain. Irrespective of the gene mutated, the bioluminescence profiles of each of the several hundred promoters examined displayed identical period lengths (Fig. 5B) and arrhythmia in all arrhythmic mutants (Fig. 5 A and C). These results indicate that the kai oscillator is indeed the dominant oscillator orchestrating gene expression in the whole genome of Synechococcus.
Global Gene Repression by KaiC. How does the kai oscillator influence all cellular promoters? To examine whether repression by KaiC is specific to the kaiBC promoter, we applied the promoter trap to a Synechococcus strain carrying a kaiC overexpression construct (Ptrc::kaiC). In the absence of inducer, all of the 640 bioluminescence transformants tested exhibited circadian rhythms of various bioluminescence intensities, phases, waveforms, and amplitudes, as reported (3). As expected from our clock model (2), kaiC overexpression by isopropyl-β-d-thiogalactopyranoside (IPTG) abolished the normal rhythms of all clones, while also severely lowering their bioluminescence.
Quantitative analyses of bioluminescence for representative promoters (Fig. 1) disclosed that Synechococcus promoters can be classified into high- and low-amplitude promoter groups. Five to 10 percent of the promoters (e.g., PkaiBC, PsigC, and PchlB) were classified as high-amplitude promoters, characterized by sharp bioluminescence peaks with complete extinction at trough phases. The remaining 90% of promoters were low-amplitude promoters exhibiting broader peaks; in this subset, a significant level of bioluminescence was retained even during trough phases. PkaiA, PclpC, and PpsbAI belong to this group. The activity of low-amplitude promoters exhibited both rhythmic and nonrhythmic components, whereas high-amplitude promoters possessed only the rhythmic component. Excess KaiC completely eliminated the rhythmic components of both groups, but did not affect the nonrhythmic ones (Fig. 1). Thus, excess KaiC presumably suppresses gene expression regulated by the circadian clock without affecting expression leading to nonrhythmic activity. Interestingly, Synechococcus grew well regardless of kaiC overexpression. The lack of growth impairment implies that the expression of the group of high-amplitude genes and the rhythmic component of the low-amplitude gene group are not essential for survival. It is likely that nonrhythmic expressions of low-amplitude genes unaffected by KaiC are sufficient for Synechococcus housekeeping metabolism.
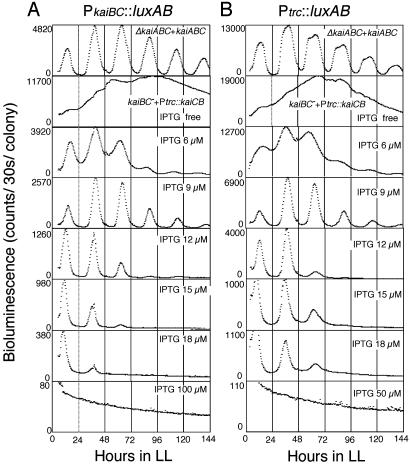
Circadian Oscillation by E. coli Promoter. Repression of high-amplitude promoters by KaiC raised the possibility that the cis-element(s) of the kaiBC promoter may be dispensable for rhythm generation. To test this possibility, we inactivated kaiBC, then introduced kaiC and kaiB back into the strain under the control of the E. coli trc promoter (kaiBC–+Ptrc::kaiCB). In this strain, kaiC and kaiB are inducible by IPTG in a dose-dependent manner. We monitored bioluminescence from a PkaiBC::luxAB reporter after treatment with variable IPTG concentrations (Fig. 2A). In the absence of IPTG, bioluminescence from the PkaiBC::luxAB reporter was stronger than that of control cells, presumably due to the absence of KaiC. Bioluminescence was reduced to the background levels by 100 μM IPTG treatment. Although bioluminescence profiles were completely arrhythmic in either the absence or presence of 100 μM IPTG, intermediate IPTG concentrations (from 6 to 15 μM) could restore circadian rhythms. The presence of 9 μM IPTG produced a restored rhythm very similar to that observed in control cells (ΔkaiABC+kaiABC).
Fig. 2.
Quantitative assay of IPTG dose-dependent restoration of the circadian rhythm in a kaiBC– strain. A kaiABC gene cluster was introduced back into a kaiABC-deleted strain (ΔkaiABC+kaiABC, top trace). Ptrc::kaiCB was introduced into a kaiBC-deleted strain (kaiBC– +Ptrc::kaiCB, lower traces). PkaiBC::luxAB (A) and Ptrc::luxAB (B) reporter cells are shown. Assays for bioluminescence rhythms and presentation of data are the same as described in Fig. 1. Note that the scale for the bioluminescence profile differs among panels. In the lower 12 panels, the concentrations of IPTG indicated in the figure were administered in the solid medium 48–60 h before experimental LL.
We monitored PkaiBC activity under conditions where Ptrc drives kaiCB transcription rather than PkaiBC (Fig. 2 A). To monitor expression of kaiC and kaiB in Ptrc::kaiCB strain, we replaced the PkaiBC::luxAB reporter with a Ptrc::luxAB reporter (Fig. 2B). As seen for all promoters, Ptrc::luxAB showed a circadian bioluminescence rhythm in the control cells (Fig. 2B, top trace). The broad peaks and relatively high expression levels evident during trough phase for Ptrc::luxAB indicated that this rhythm belonged to the low-amplitude group. As Ptrc is a strong promoter, kaiBC–+Ptrc::kaiCB cells exhibited significant bioluminescence even in the absence of IPTG induction. Elevation of IPTG to 50 μM lowered the average level of bioluminescence to background levels, as observed for PkaiBC::luxAB (Fig. 2 A). In the presence of 6–18 μM IPTG concentrations, a similar range to that required for rhythm restoration of the PkaiBC::luxAB reporter, the circadian rhythm was again restored. Surprisingly, at 9 μM IPTG, the waveform representing the Ptrc::luxAB rhythm changed from a low-amplitude type to a high-amplitude one, similar to the PkaiBC::luxAB reporter.
Feedback Regulation on the trc Promoter. We next examined Ptrc-driven accumulation of KaiC protein. In the presence of 10 μM IPTG, kaiBC–+Ptrc::kaiCB cells exhibited circadian rhythms of KaiC expression peaking at circadian time 16–20 (Fig. 3A), similar to the pattern seen in wild-type cells (5, 9). We also confirmed that KaiB and KaiC proteins accumulated in the kaiBC–+Ptrc::kaiCB cells in an IPTG-inducible, dose-dependent manner. KaiC levels in cells with the rhythm restored (by 10 μM IPTG) were similar to those in wild-type cells (Fig. 3B). The correlation of KaiC levels in the native and artificial oscillators suggests that appropriate KaiC levels coincide with the proper generation of circadian oscillation.
IPTG-induced KaiC accumulation (Fig. 3B) indicated that kaiCB was expressed as expected. Indeed, bioluminescence of the Ptrc::luxAB reporter increased in an IPTG dose-dependent manner in the absence of the Ptrc::kaiCB construct (unpublished data). However, trc promoter activity assayed by bioluminescence responded in an inverse manner in the presence of the Ptrc::kaiCB construct (Fig. 2B). As depicted by the scales of each diagram in Fig. 2B, bioluminescence of Ptrc::kaiCB was lowered as IPTG concentration was elevated. The apparent activity in the absence of the inducer (probably due to a leaky trc promoter) does not support KaiC accumulation. On the other hand, Ptrc-driven bioluminescence was greatly diminished by 50 μM IPTG treatment, whereas KaiC level (Fig. 3) increased in an IPTG dose-dependent manner.
This apparent contradiction can be reconciled by assuming negative feedback regulation of Ptrc by KaiC. Because we assayed the Ptrc activities from 48–60 h after an administration of IPTG, the initial response of Ptrc to IPTG was not monitored in our experiments. Instead, Fig. 2B should depict the activities attained by feedback regulation, if KaiC from the Ptrc::kaiCB construct affects the Ptrc activity. Under such tight feedback regulation, Ptrc activities that drive kaiCB would be lowered by the accumulated KaiC to a level that balances the positive (induction by IPTG) and negative (repression by KaiC) pressures. If KaiC could efficiently repress the promoter, then the stronger the IPTG induces the promoter, the stronger the promoter activity is repressed. In the presence of 50 μM IPTG, Ptrc would be permitted to drive the kaiCB gene only in sufficient amounts that compensate the degradation of kaiCB mRNA or KaiC. The response of Ptrc to IPTG, as shown in Fig. 2B, rather implies that Ptrc functions as a key component of the feedback loop.
Complementation of circadian oscillations through the introduction of per and tim clock genes under the control of constitutive promoters has been reported in clock-null mutants of Drosophila (10, 11). The generation of oscillations was thought to result from the interactions between synthesized clock proteins. Recently, Xu et al. (12) also reported complementation of 24-h oscillations in cyanobacteria by the Ptrc promoter fused to kaiBC. With quantitative analyses of Ptrc, we demonstrated that Ptrc, which is normally feed-forward regulated as a downstream promoter of the clock, switched to be feedback-controlled by promoting expression of the kaiCB genes. Moreover, it should be noted that the feedback loop generates a circadian oscillation at an appropriate level of IPTG induction (9 μM). Thus, any promoter, even promoters derived from E. coli, may function as a key regulator of circadian feedback loops, provided that it can express kaiB and kaiC genes at an appropriate level. We also determined that the kaiA promoter was replaceable by the Ptrc promoter. The circadian rhythm of kaiA-inactivated cells was complemented by the introduction of the Ptrc::kaiA construct (Fig. 6, which is published as supporting information on the PNAS web site). Thus, two of the kai cluster promoters can be replaced with the Ptrc promoter.
Circadian Characteristics of Ptrc Driven Rhythm. An important criterion of circadian rhythms is that the period length is not greatly altered by temperature changes (13); Synechococcus satisfies this criterion (14). The period lengths of kaiBC–+Ptrc::kaiCB cell rhythms were not altered at temperatures between 25–35°C (Fig. 7, which is published as supporting information on the PNAS web site). The Q10 values for kaiBC–+Ptrc::kaiCB cells were 0.95 and 0.97 for 6 μM and 9 μM IPTG, respectively, similar to the values observed for wild-type cells (≈1.0). Moreover, a 5-h pulse in darkness, resetting the phase of the Synechococcus rhythm, also shifted the phase of the Ptrc-driven rhythms (data not shown). These results indicate that kaiBC–+Ptrc::kaiCB cells generate circadian rhythms physiologically equivalent to those of the wild-type. Thus, the properties of circadian rhythm are not ascribed to the kai gene promoters but to the Kai proteins themselves.
Genome-Based Clock Model. In this study, we revealed that the Kai-based oscillator is the dominant circadian oscillator functioning in cyanobacteria. KaiC represses not only kaiBC but also the rhythmic components of all genes in the genome. Surprisingly, an E. coli-derived promoter can close the Kai-based feedback loop to function in the same manner as the endogenous circadian oscillator. These results imply that specific regulation of the kaiBC promoter is not essential for circadian oscillation, provided that a kaiBC promoter supports sufficient RNA polymerase activity. Promoter-specific regulation, however, may be important for low-amplitude genes that respond to noncircadian factors or genes that display circadian rhythms of diverse phase relationship (ref. 3 and this study). We propose a revised model of circadian system regulation in cyanobacteria (Fig. 4). All of the genes in the genome can be divided into high- and low-amplitude gene groups. In addition to kai promoters, all promoters undergo transcriptional repression by KaiC (or a KaiC-associated complex). KaiC likely binds to DNA structures to regulate transcription by changing the condensation and/or supercoiling status of Synechococcus chromosomes. Because KaiC belongs to the superfamily of bacterial DNA recombinases, including RecA and the DnaB DNA helicase (15), we predict an interaction between KaiC and DNA. Mori et al. (16) recently reported that KaiC binds to DNA in vitro. In addition, DNA supercoiling in Chlamydomonas chloroplast changes with a diurnal rhythm in cells growing in alternating 12-h dark-12-h light periods (17), and histone acetylation has been implicated in circadian gene regulation in mammals (18).
Fig. 4.
The circadian system of cyanobacteria with a genomewide oscillator by Kai proteins. The circadian system of cyanobacteria proposed from the current study is illustrated schematically.
Recent cDNA microarray studies in Drosophila (19, 20), mouse (21, 22), Neurospora (23), Arabidopsis (24), and Lingulodinium (formerly Gonyaulax) (25) revealed that considerable numbers of genes (2–7% of total genes) are controlled by the circadian clock in eukaryotes. A recent enhancer trap experiment also revealed that one-third of Arabidopsis genes are clock-regulated (26). Thus, circadian regulation is far more extensive than previously expected. In cyanobacteria, our results imply that the circadian oscillator and the core metabolism are highly interdependent, that is, the oscillator regulates or influences whole cellular metabolism and the circadian oscillator is based on an integration of cellular metabolism.
Supplementary Material
Acknowledgments
We thank Dr. M. Ishiura (Nagoya University) for construction of the promoter trap library used in Fig. 5. This research was supported in part by Japanese Ministry of Education, Culture, Sports, Science, and Technology Grants-in-Aid 11233203, 15GS0308, and COE 14COEA01 (to T.K.), and Japanese Society for Promotion of Science Grants-in-Aid 13680778 and 15370074 (to H.I.) and 200203019 (to Y.N.).
Abbreviations: IPTG, isopropyl-β-d-thiogalactopyranoside; LL, continuous light.
References
- 1.Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 9, 702–715. [DOI] [PubMed] [Google Scholar]
- 2.Ishiura, M., Kutsuna, S., Aoki, S., Iwasaki, H., Andersson, C. R., Tanabe, A., Golden, S. S., Johnson, C. H. & Kondo, T. (1998) Science 281, 1519–1523. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Y., Tsinoremas, N. F., Johnson, C. H., Lebedeva, N. V., Golden, S. S., Ishiura, M. & Kondo, T. (1995) Genes Dev. 9, 1469–1478. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura, H., Nakahira, Y., Imai, K., Tsuruhara, A, Kondo, H., Hayashi, H., Hirai, M., Saito, H. & Kondo, T. (2002) Microbiology 148, 2903–2909. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki, H., Nishiwaki, T., Kitayama, Y., Nakajima, M. & Kondo, T. (2002) Proc. Natl. Acad. Sci. USA 99, 15788–15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo, T., Tsinoremas, N. F., Golden, S. S., Johnson, C. H., Kutsuna, S. & Ishiura, M. (1994) Science 266, 1233–1236. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki, H., Williams, S. B., Kitayama, Y., Ishiura, M., Golden, S. S. & Kondo, T. (2000) Cell 101, 223–233. [DOI] [PubMed] [Google Scholar]
- 8.Nair, U., Ditty, J. L., Min, H. & Golden, S. S. (2002) J. Bacteriol. 184, 3530–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu, Y., Mori, T. & Johnson, C. H. (2000) EMBO J. 19, 3349–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, E. Y., Bae, K., Ng, F. S., Glossop, N. R., Hardin, P. E. & Edery, I. (2002) Neuron 28, 69–81. [DOI] [PubMed] [Google Scholar]
- 11.Yang, Z. & Sehgal, A. (2001) Neuron 29, 453–467. [DOI] [PubMed] [Google Scholar]
- 12.Xu, Y., Mori, T. & Johnson, C. H. (2003) EMBO J. 22, 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings, J. W., Boulos, Z. & Rusak, B. (1991) in Neural and Integrative Animal Physiology, ed. Prosser, C. L. (Wiley Interscience, New York), pp. 435–546.
- 14.Kondo, T., Strayer, C. A, Kulkarni, R. D., Taylor, W., Ishiura, M, Golden, S. S. & Johnson, C. H. (1993) Proc. Natl. Acad. Sci. USA 90, 5672–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leipe, D. D., Aravind, L., Grishin, N. V. & Koonin, E. V. (2000) Genome Res. 10, 5–16. [PubMed] [Google Scholar]
- 16.Mori, T., Saveliev, S. V., Xu, Y., Stafford, W. F., Cox, M. M., Inman, R. B. & Johnson, C. H. (2002) Proc. Natl. Acad. Sci. USA 99, 17203–17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvador, M. L., Klein, U. & Bogorad, L. (1998) Mol. Cell. Biol. 18, 7235–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etchegaray, J. P., Lee, C., Wade, P. A. & Reppert, S. M. (2003) Nature 421, 177–182. [DOI] [PubMed] [Google Scholar]
- 19.Ueda, H. R., Matsumoto, A., Kawamura, M., Iino, M., Tanimura, T. & Hashimoto, S. (2002) J. Biol. Chem. 277, 14048–14052. [DOI] [PubMed] [Google Scholar]
- 20.McDonald, M. J. & Rosbash, M. (2001) Cell 107, 567–578. [DOI] [PubMed] [Google Scholar]
- 21.Ueda, H. R., Chen, W., Adachi, A., Wakamatsu, H., Hayashi, S., Takasugi, T., Nagano, M., Nakahama, K., Suzuki, Y., Sugano, S., et al. (2002) Nature 418, 534–539. [DOI] [PubMed] [Google Scholar]
- 22.Duffield, G. E., Best, J. D., Meurers, B. H., Bittner, A., Loros, J. J. & Dunlap, J. C. (2002) Curr. Biol. 12, 551–557. [DOI] [PubMed] [Google Scholar]
- 23.Loros, J. J. & Dunlap, J. C. (2001) Annu. Rev. Physiol. 63, 757–794. [DOI] [PubMed] [Google Scholar]
- 24.Harmer, S. L., Hogenesch, J. B., Straume, M., Chang, H. S., Han, B., Zhu, T., Wang, X., Kreps, J. A. & Kay, S. A. (2000) Science 290, 2110–2113. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto, O. K. & Hastings, J. W. (2003) J. Phycol. 39, 1–9. [Google Scholar]
- 26.Michael, T. P. & McClung, C. R. (2003) Plant Physiol. 132, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.