Abstract
Background
Increased rates of RBC alloimmunization in patients with sickle cell disease may be due to transfusion frequency, genetic predisposition, or immune dysregulation. To test the hypothesis that sickle cell pathophysiology influences RBC alloimmunization, we utilized two transgenic mouse models of sickle cell disease.
Study Design and Methods
Transgenic sickle mice, which express human α and βS globin, were transfused with fresh or 14-day stored RBCs containing the HOD (hen egg lysozyme, ovalbumin, and human Duffyb) antigen; some recipients were inflamed with poly (I:C) prior to transfusion. Anti-HOD alloantibody responses were subsequently measured by ELISA and flow crossmatch; a cohort of recipients had post-transfusion serum cytokines measured by bead array.
Results
Both Berkeley and Townes homozygous (SS) and heterozygous (AS) mice had similar rates and magnitude of anti-HOD RBC alloimmunization following fresh HOD RBC transfusion compared with control animals; under no tested condition did homozygous SS recipients make higher levels of alloantibodies than control animals. Unexpectedly, homozygous SS recipients had blunted cytokine responses and lower levels of anti-HOD alloantibodies following transfusion of 14-day stored RBCs, compared with control animals.
Conclusions
In sum, homozygous βS expression and the ensuing disease state are not alone sufficient to enhance RBC alloimmunization to transfused HOD RBCs in 2 distinct humanized murine models of sickle cell disease under the conditions examined. These data suggest other factors may contribute to the high rates of RBC alloimmunization observed in humans with sickle cell disease.
Keywords: red blood cells (sickle cell disease), alloimmunization, transfusion medicine
Introduction
Human patients with sickle cell disease (hemoglobin SS) have rates of RBC alloimmunization approaching 25–50%1–3. This is substantially higher than RBC alloimmunization rate in the general transfused patient population, which approximates 3%4–7. The high rate of alloimmunization in sickle cell patients is problematic on multiple levels; RBC alloantibodies increase the risk of acute and delayed hemolytic transfusion reactions, increase the risk of hemolytic disease of the newborn, may be associated with the development of autoantibodies, and lead to costly evaluations by transfusion services and potential delays in providing compatible RBC units. In fact, some patients with sickle cell disease have so many RBC antibodies that compatible blood is not available for transfusion.
Strategies to prevent RBC alloimmunization in sickle cell patients are currently limited to avoiding exposure to the most immunogenic RBC alloantigens. Partial phenotypic matching of RBC units, although costly, decreases rates of RBC alloantibody formation in sickle cell patients8. However, the residual RBC alloimmunization rate in sickle cell patients receiving partially phenotypically-matched blood remains higher than that of patients without sickle cell disease8. Additionally, some transfusion services provide more extensively phenotypically-matched RBCs or partially genotypically-matched RBCs for “responder” patients with a history of RBC alloimmunization. However, it is impossible to match for the hundreds of described RBC antigens 9, and patients with sickle cell disease may still make antibodies against non-matched antigens despite matching strategies. Therefore, it would be desirable to develop an expanded understanding of potential underlying pathophysiologic processes that contribute to RBC alloimmunization, such that additional strategies can be developed to minimize alloimmunization.
The reason that sickle cell disease patients are more likely than other patient populations to make RBC alloantibodies is a matter of dispute. One hypothesis is that patients with sickle cell disease have a higher transfusion burden and are thus exposed to more RBC antigens than other patient populations. In addition, the transfused RBC units, which may come from racially-mismatched donors, often contain multiple antigens foreign to the transfusion recipients10. However, rates of RBC alloimmunization in sickle cell patients remain higher than that of the general population, even in countries with homogenous populations and racially-matched donors and recipients11,12. Another hypothesis is that the presence of the sickle globin (βS) gene itself, or an immunoregulatory gene close to this globin gene that is co-inherited with the globin gene13, predisposes sickle patients (or a subset of sickle patients) to RBC alloimmunization. Finally, it must also be considered that alloimmunization rates in non-sickle patient populations may be underestimated, due to lack of follow-up testing and/or antibody evanescence14,15.
An additional hypothesis is that the chronic baseline inflammation observed in patients with sickle cell disease enhances immune responses to transfused RBCs16. It is accepted that baseline states of inflammation and innate immune activation occur in patients with sickle cell disease; in particular, white blood cell counts and C-reactive proteins are often elevated. Additionally, increases in inflammatory cytokines and decreases in anti-inflammatory cytokines have been reported in patients with sickle cell disease17–21. Finally, patients with sickle cell disease also exhibit chronic vascular endothelial activation22–28. Thus, taken together with our prior reports that inflammation enhances RBC alloimmunization in a murine model29 and a report that inflammation may enhance RBC alloimmunization in humans30, it is possible that the baseline inflammation that occurs secondary to sickle cell disease pathophysiology may contribute to the high rates of RBC alloimmunization observed in patients with sickle cell disease.
To test the hypothesis that homozygous presence of the βS gene itself (and the ensuing pathophysiology of sickle cell disease, including hemolysis and inflammation) influences RBC alloimmunization, we utilized two distinct humanized murine models of sickle cell disease. Under no tested condition did transfusion recipients with homozygous sickle cell disease (expressing human α and human βS globin)31,32 have higher rates or magnitudes of RBC alloimmunization to HOD RBCs33, compared with control recipients. In sum, these data demonstrate that the homozygous βS genotype (and ensuing sickle cell pathophysiology) is not alone sufficient to enhance RBC alloimmunization in the described murine models, under the described tested conditions. These data thus suggest other factors may contribute to the high rates of RBC alloimmunization observed in humans with sickle cell disease.
Materials and Methods
Mice
C57BL/6 and Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). “Berkeley” transgenic knock-out sickle cell mice31 (generously provided initially by Drs. Paszty, Mohandas, and Rubin, and maintained at Emory since 2001), “Townes” knock-in sickle cell mice32 (developed by Dr. Townes in 2003 and generously provided for maintenance at Emory in 2009), and HOD transgenic mice33 (containing a RBC specific triple fusion recombinant protein with hen egg lysozyme, ovalbumin, and human Duffyb antigen sequences) were bred by the Emory University Department of Animal Resources. Both Berkeley and Townes mice with homozygous (SS) expression have human βS (mouse α−/−, mouse β−/−, Tg [human α, human βS]). “50%” AS Berkeley mice (which actually contain approximately 42% hemoglobin S) have human βS globin gene and murine β-globin gene (mouse α−/−, mouse β+/−, Tg [human α, human βS]). Townes mice have a 4.1 kb human βS fragment linked to a 5.6 kb human Aγ fragment, resulting in an HbF to HbS switch similar to that seen in humans with sickle cell disease34–36. The genetic background of Berkeley mice includes C57BL/6, Black Swiss, 129, FVB/N, and DBA/2, and that of Townes mice includes C57BL/6 and SJL. Sickle cell disease mice were selectively mated and expression of βS globin was confirmed by differential hemoglobin electrophoresis for Berkeley mice and by PCR for Townes mice. Transfusion recipient mice were used at 8–16 weeks of age, and all protocols were approved by the Emory University Institutional Animal Care and Use Committee.
Blood collection and transfusion
Mouse donor blood was collected in ACD or CPDA-1 and, in a subset of experiments, was leukoreduced using a Pall neonatal leukoreduction filter (East Hills, NY)29. Recipients (C57BL/6, SS, AS, or AA) were transfused via lateral tail vein with 100 µL (1 “unit”) of fresh or 14-day stored leukoreduced HOD RBCs37. In a subset of experiments, post-transfusion recovery of HOD RBCs in recipients was determined at 10 minutes, 90 minutes, and 24 hours, using MIMA 29 (an anti-Fy3 mouse monoclonal antibody, generously provided by Mr. Greg Halverson and Dr. Marion Reid of the New York Blood Center); logistic regression analysis was performed, with extrapolation to time 038. In some experiments, recipients were pre-treated intraperitoneally 4 hours prior to transfusion with 25 micrograms of poly (I:C) (Amersham, Piscataway, NY).
Splenocyte collection and injection
Splenocytes from Balb/c donors were collected, as previously described39. Splenocytes were enumerated using trypan blue exclusion, and 1 million total live splenocytes were injected via lateral tail vein.
Flow cytometry and antibody detection
Peripheral blood was collected 90 minutes after transfusion in some experiments, and cytokines (keratinocyte-derived chemokine/CXCL1 (i.e. KC/CXCL1), interleukin-6 (i.e. IL-6) and monocyte chemoattractant protein-1 (i.e. MCP-1)) were measured in serum by the Cytokine Bead Array assay (Mouse Flex Kit, BD Biosciences)40.
Anti-HEL responses were measured by HEL-specific ELISA, as previously described29, in serum collected 2 weeks following transfusion. In a subset of experiments, anti-HOD alloantibody responses were also measured by indirect immunofluorescence using HOD RBCs as targets and wild-type antigen negative FVB RBCs as controls37. Antibody binding was detected by flow cytometry using goat anti-mouse immunoglobulin conjugated to allophycocyanin (Becton Dickinson, San Jose, CA). Anti-Balb/c responses were determined at 1 and 2 weeks following splenocyte injection, using Balb/c splenocytes as targets and C57BL/6 splenocytes as negative controls. A CD3+ inclusion gate and a CD19+ exclusion gate were utilized, with goat anti-mouse immunoglobulin as the secondary antibody.
Statistical analysis
Statistical analysis and graphing was performed with Graph Pad Prism software (San Diego, CA). One way ANOVAs with Bonferroni post test or Mann-Whitney U tests were performed, with a statistically significant value defined as p<0.05.
Results
Berkeley homozygous SS mice do not have increased alloimmunization to transfused HOD RBCs
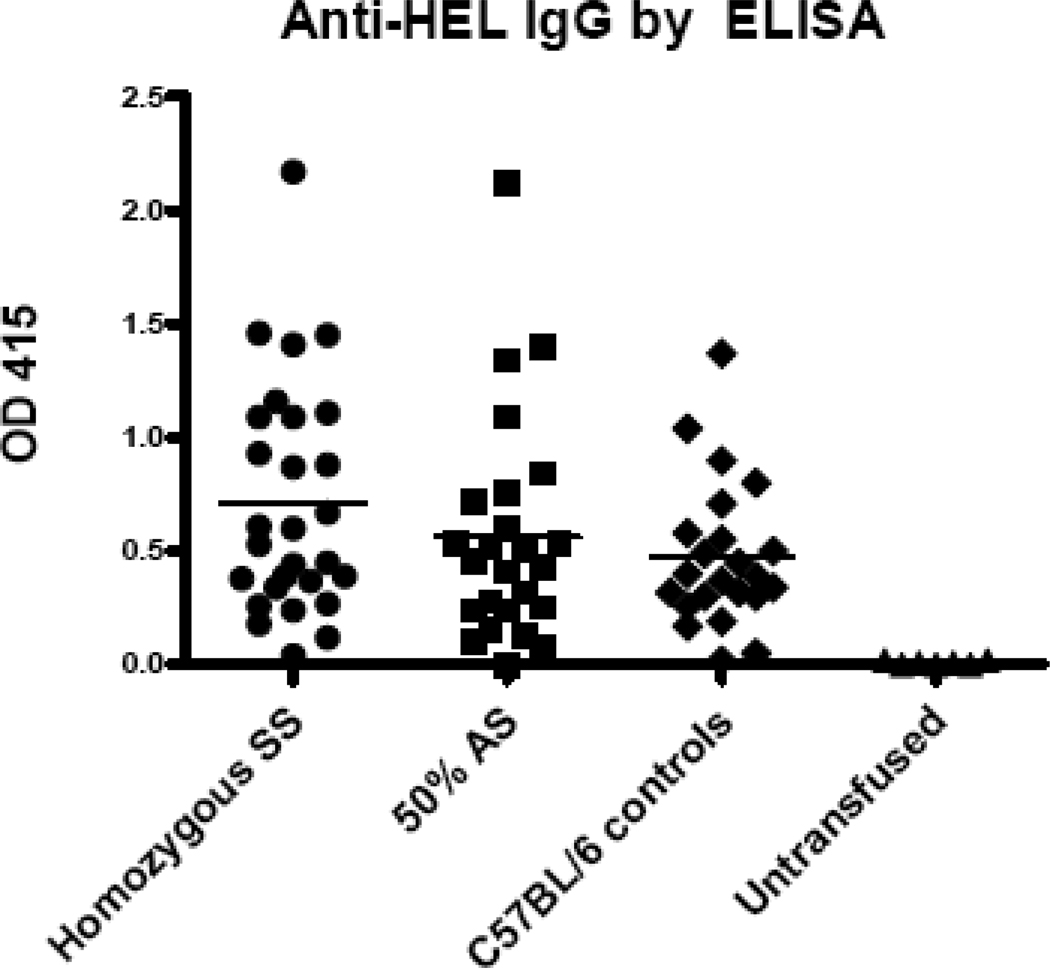
Berkeley transgenic homozygous SS mice, 50% AS mice, and C57BL/6 control mice were transfused via lateral tail vein with 100 µL (i.e. 1 “unit”) of HOD RBCs; additional control mice were left untransfused. Two weeks following transfusion, alloantibodies were measured by HEL-specific ELISA. In 6 of 6 experiments (n=76 mice total), more than 97% of transfused recipients mounted an anti-HEL IgG response, as defined by antibody activity exceeding 2 standard deviations of the mean background signal of untransfused animals (e.g. 28/28 SS recipients, 24/25 AS recipients, and 22/23 C57BL/6 recipients produced detectable anti-HEL IgG). However, there were no statistically significant differences in the magnitude or frequency of the anti-HEL response between SS, AS, or control mice (Fig. 1); p>0.05 by ANOVA with Bonferroni post-test.
Figure 1. Berkeley SS mice do not have increased alloimmunization to transfused HOD RBCs.
Berkeley SS or 50% AS recipients, as well as C57BL/6 recipients, were transfused with 1 “unit” (100 µL) of packed HOD RBCs. Anti-HEL IgG was measured by ELISA 2 weeks following transfusion. A compilation of data from 6 independent experiments (n=76 recipients total) is shown; p>0.05 by ANOVA for responses in SS, 50% AS, and C57BL/6 recipients.
Transfused HOD RBCs are cleared more rapidly in naïve SS than naïve control recipients
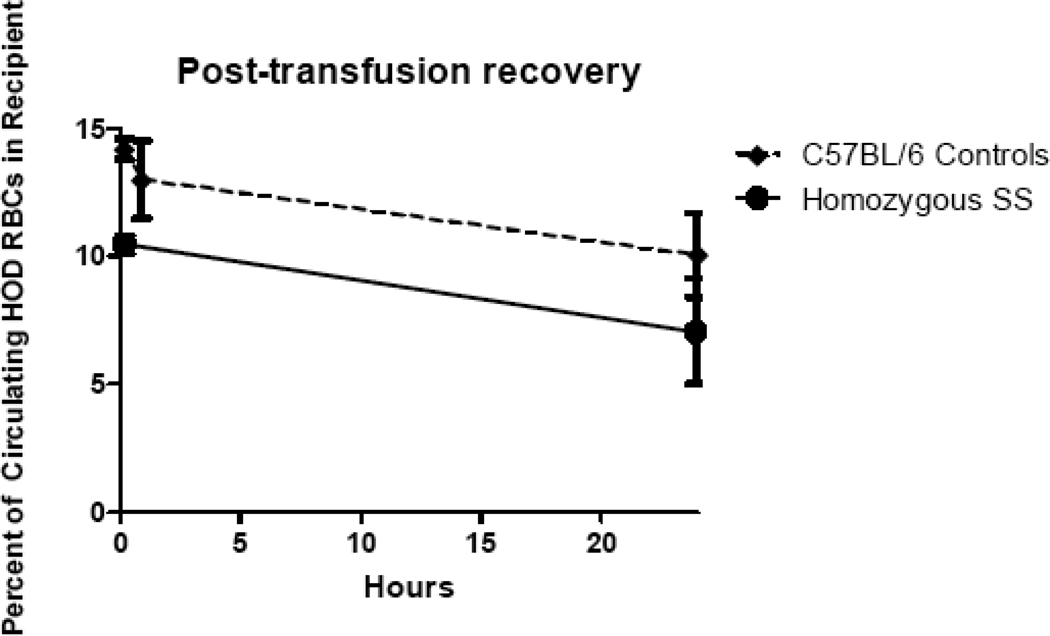
The spleens of mice with homozygous SS sickle cell disease are significantly enlarged as compared with non-sickle cell mice. Because transfused RBCs are cleared primarily by the spleen, we hypothesized that mice with sickle cell disease would have increased clearance rates of transfused RBCs. Establishing rate of RBC clearance could be important in RBC alloimmunogenicity, given recent reports that rapidly cleared murine RBCs are more immunogenic than those cleared at slower rates41. To test this hypothesis, Berkeley mice with homozygous sickle cell disease as well as control mice were transfused with 1 unit of HOD RBCs and samples were collected from recipients at defined timepoints post-transfusion. The samples were stained with MIMA 29 (anti-Fy3) to identify HOD RBCs, and 24-hour post-transfusion recovery curves were calculated. If clearance rates were equal, sickle recipients would be expected to have twice the percentage of circulating HOD RBCs as control recipients, taking into account the Hct differences between these animals (with sickle mice having an Hct that is approximately half that of control mice). However, sickle mice had a non-statistically significant decreased percentage of circulating HOD RBCs at all tested timepoints compared with C57BL/6 control recipients (Fig. 2). Thus, it is concluded that homozygous SS mice clear transfused HOD RBCs at higher rates than control recipients.
Figure 2. Berkeley SS mice clear transfused HOD RBCs more rapidly than control recipients.
Berkeley SS or C57BL/6 recipients were transfused with 1 unit of HOD RBCs, and recovery of the transfused RBCs was monitored by staining of recipient RBCs with MIMA 29 (anti-Fy3) at 10 minutes, 90 minutes, and 24 hours after transfusion (representative curves are shown).
The response to transfused HOD RBCs in the presence of poly (I:C) is similar in Berkeley homozygous SS and control recipients
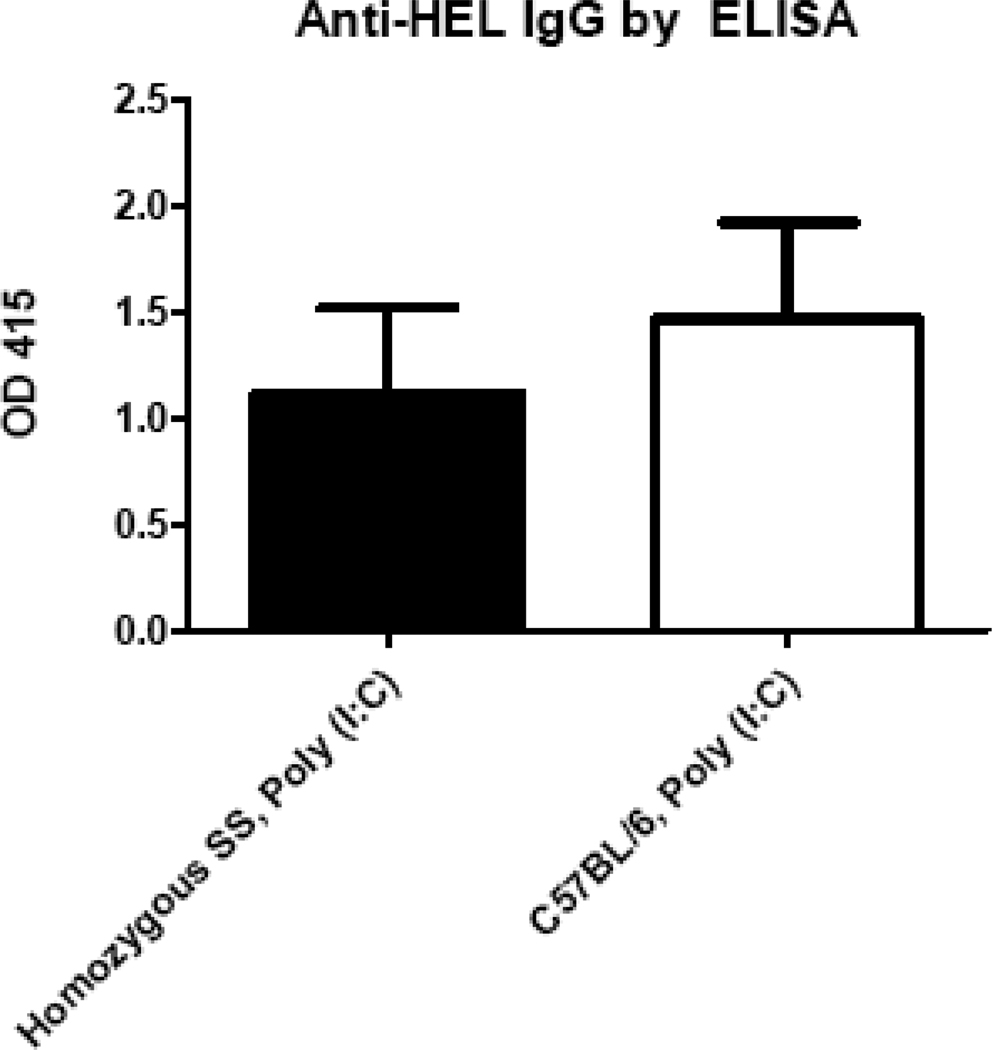
Although no differences in rates or magnitudes of anti-HOD RBC alloimmunization were observed in Berkeley mice with sickle cell disease compared to control recipients in their “baseline” state, it was possible that the alloimmunization responses would differ in acutely “inflamed” recipients. To test this, Berkeley homozygous SS and control mice were treated with 25 µg of the viral-like stimulus poly (I:C), 4 hours prior to transfusion of HOD RBCs. In 2 of 2 experiments (n=35 mice total), homozygous SS recipients had a slightly higher anti-HEL response in the presence of poly (I:C) compared to untreated SS mice. However, there were no statistically significant differences in anti-HEL responses between homozygous SS recipients treated with poly (I:C) prior to transfusion compared to control C57BL/6 recipients treated with poly (I:C) prior to transfusion (Fig. 3); p>0.05 by Mann-Whitney U test.
Figure 3. The response to transfused HOD RBCs in the presence of poly (I:C) is not greater in Berkeley SS than control recipients.
Berkeley SS or C57BL/6 recipients were pretreated with 25 micrograms of poly (I:C) i.p. and transfused 4 hours later with 1 unit of HOD RBCs; alloimmunization was measured by anti-HEL ELISA 2 weeks later. A representative experiment (n=16 recipients total) with mean +1SD is depicted; p>0.05 for groups of poly (I:C) transfused recipients by Mann-Whitney U test.
Berkeley homozygous SS recipients have a blunted pro-inflammatory cytokine and alloantibody response to stored HOD RBCs
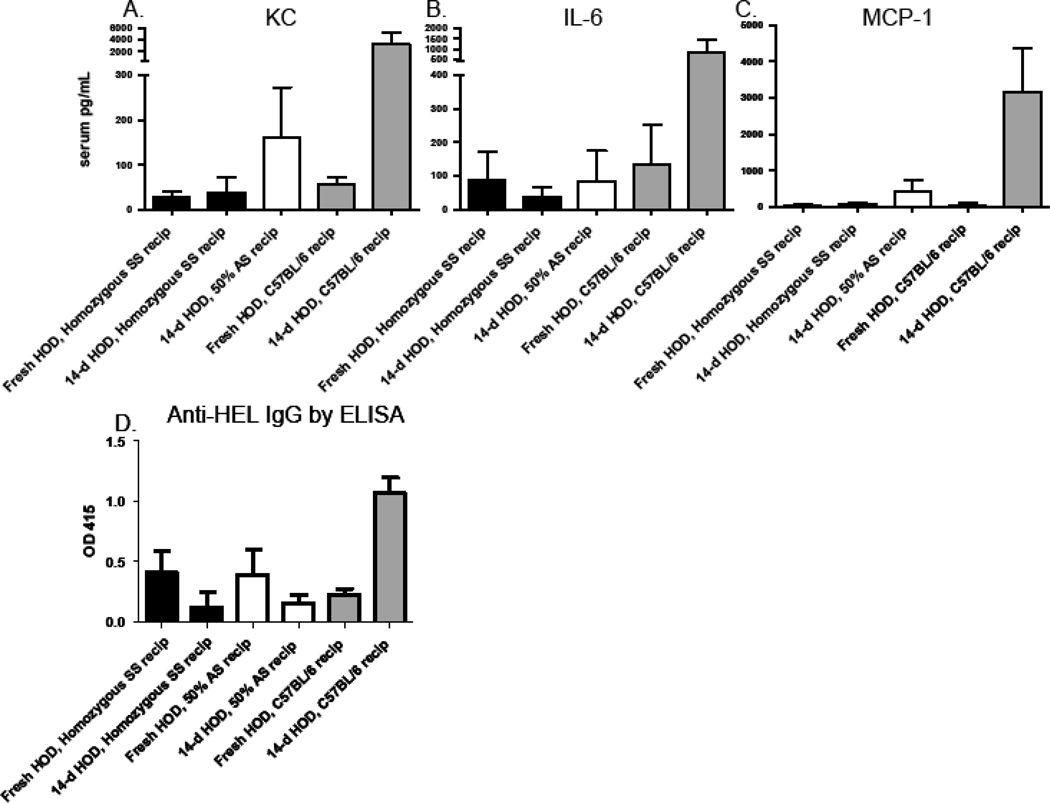
Few human transfusion recipients receive “fresh” RBCs. We have previously reported that transfusion of 14-day stored HOD RBCs induces a pro-inflammatory serum cytokine storm in C57BL/6 recipients40; these RBCs were also significantly more immunogenic than fresh RBCs37. In this context, we hypothesized that transfusing stored HOD RBCs would induce an amplified cytokine storm and increased anti-HEL response in sickle compared to control animals. Berkeley homozygous SS mice or control C57BL/6 mice were transfused with fresh or 14-day stored HOD RBCs and 50% AS mice were transfused with 14-day stored RBCs; serum levels of KC, IL-6, and MCP-1 were measured 90 minutes following transfusion and alloimmunization was assessed 2 weeks later. In agreement with previously published reports, control C57BL/6 recipients had robust increases in KC, IL-6, and MCP-1 levels following transfusion of 14-day stored, but not fresh HOD RBCs. However, in 2 of 2 experiments (n=38 recipients total), homozygous SS mice had negligible changes in serum levels of KC, IL-6, and MCP-1 following transfusion of 14-day stored HOD RBCs (Fig. 4A, B, C). 50% AS mice also had a blunted cytokine response, albeit to a lesser degree than the homozygous SS recipients. In 3 of 3 experiments (n=55 recipients total), homozygous SS and 50% AS mice produced low levels of anti-HEL following transfusion of 14-day stored HOD RBCs, compared to controls (Fig. 4D).
Figure 4. Cytokine and alloimmunization responses to 14-day stored HOD RBCs are not greater in Berkeley SS than control recipients.
Berkeley SS, 50% AS, or C57BL/6 recipients were transfused with fresh or 14-day stored leukoreduced HOD RBCs. Serum KC, IL-6, and MCP-1 were measured 90 minutes after transfusion (A,B,C) and alloimmunization was measured 2 weeks later by ELISA (D). 2 representative experiments (with mean +1SD) are shown); this experiment has been repeated 2 times (n=38 recipients total) for cytokine analysis and 3 times (n=55 recipients total) for alloimmunization analysis, with similar results.
Berkeley homozygous SS mice and controls have similar responses to Balb/c splenocytes
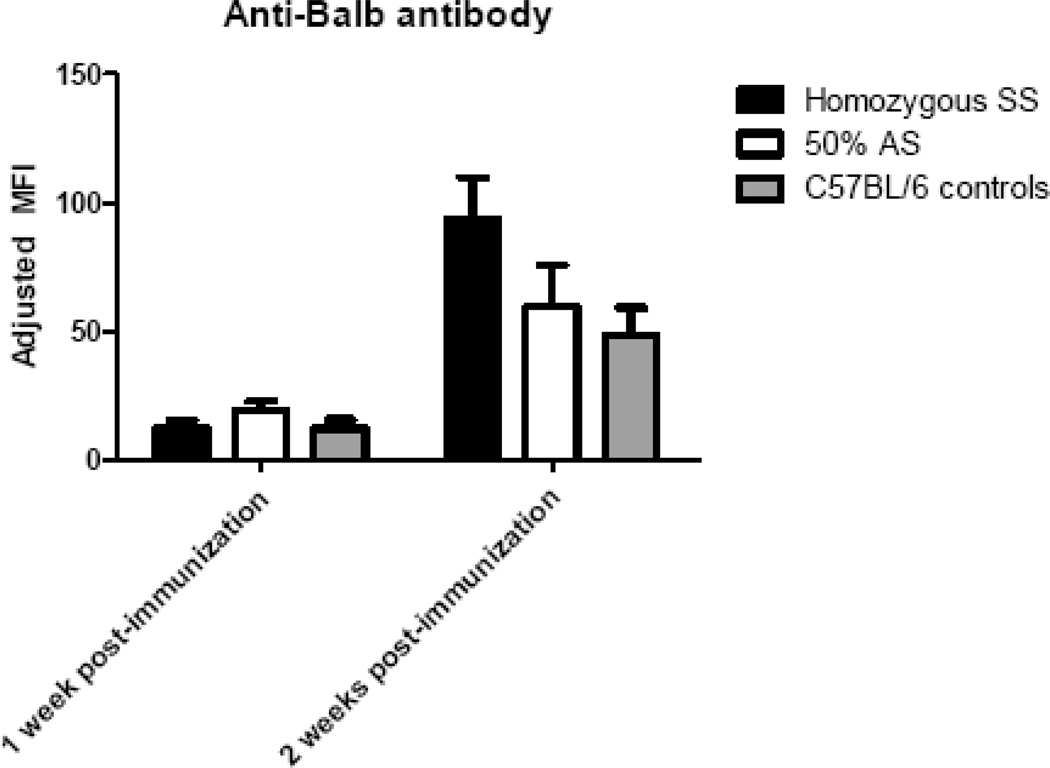
It was possible that the negligible cytokine response and lack of enhanced alloantibody response to transfused HOD RBCs in homozygous SS mice were due to an impaired ability of these animals to respond robustly to immune stimuli in general. To test this hypothesis, homozygous SS, 50% AS, or C57BL/6 control recipients were immunized intravenously with 1 million Balb/c splenocytes, and antibody responses were assessed at 1 and 2 weeks following infusion. In 2 of 2 experiments (n=26 recipients total), significant responses to Balb/c splenocytes were seen in all immunized animals (Fig 5); p>0.05 between groups at 1 and 2 weeks post-immunization by ANOVA with Bonferroni post-test.
Figure 5. Robust responses to Balb/c splenocytes are observed in Berkeley Homozygous SS, 50% AS, and C57BL/6 mice.
1 million Balb/c splenocytes were adoptively transferred to Berkeley SS (black bars), 50% AS (white bars), or C57BL/6 (grey bars) recipients, and anti-Balb antibodies were measured 1 and 2 weeks post-immunization by flow cytometric crossmatch using Balb/c or control C57BL/6 splenocyte targets. A representative experiment (n=15 recipients total) with mean +1SD is shown; p>0.05 by ANOVA with Bonferroni post-test at 1 and 2 week points; this experiment has been repeated twice with similar results.
A second humanized sickle mouse model (Townes “knock-in” model) also fails to show an enhanced alloantibody response to transfused HOD RBCs
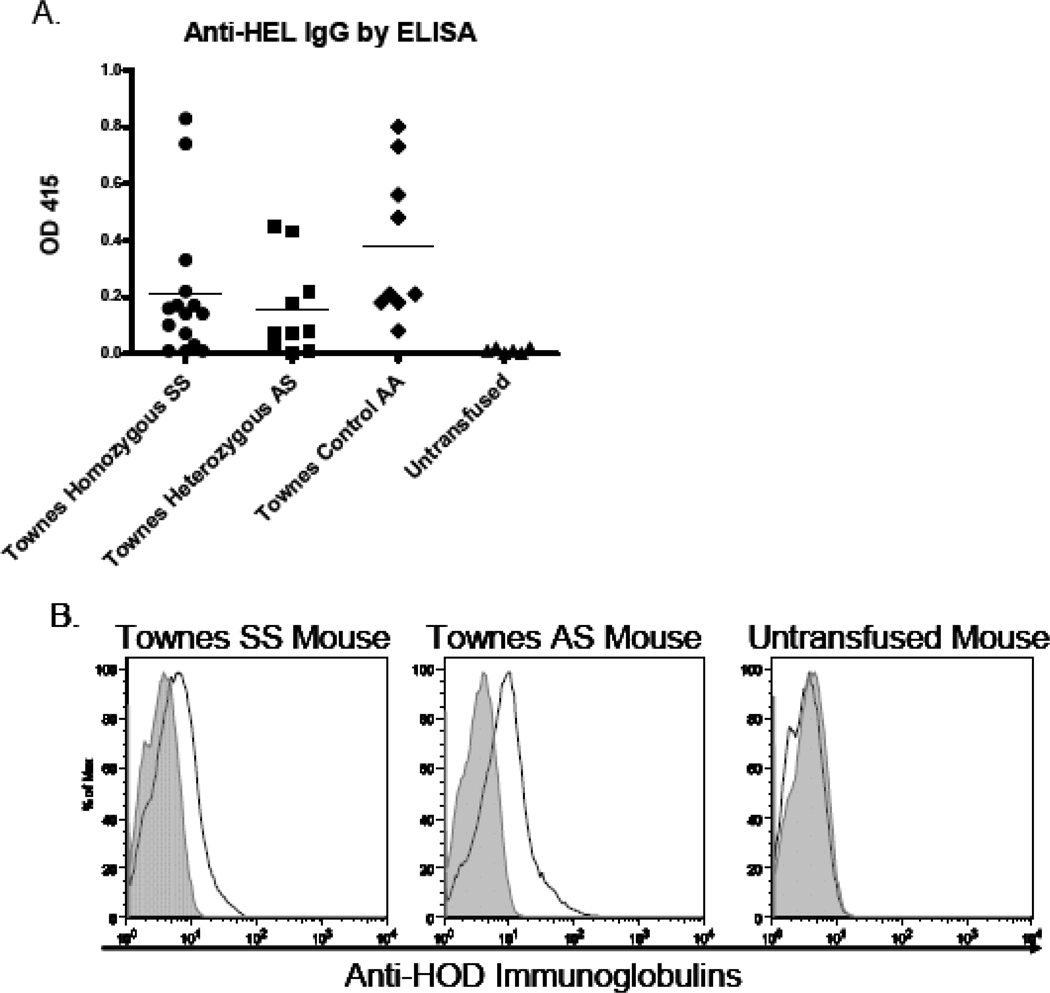
To determine if the lack of enhanced alloimmunization to HOD RBCs was recipient strain specific, a second humanized mouse model of sickle cell disease was studied. These mice were generated by Tim Townes using a “knock-in” method to express solely human α-globin, human βS-globin, and human Aγ globin32. Similar to the phenotype of Berkeley homozygous SS mice, the Townes “knock-in” SS mice (human α/human α, −1400 human γ- βS/−1400 human γ- βS) also have RBC sickling and develop severe hemolytic anemia, splenic infarcts, and urine concentrating defects32,42. Analogous to the experiments with the Berkeley recipients, Townes homozygous SS, Townes heterozygous AS, or Townes control AA mice were transfused via lateral tail vein with 1 unit (100 uL) of HOD RBCs. Two weeks following transfusion, alloimmunization was measured by a HEL-specific ELISA. In 3 of 3 experiments (n=37 mice total), 84% of transfused recipients mounted an anti-HEL IgG response (13/16 SS recipients, 8/11 AS recipients, and 10/10 AA recipients). Similar to what was observed with Berkeley sickle cell mice, there were no statistically significant differences in rates of response between SS, AS, or AA recipients, nor were there statistically significant differences in the magnitudes of anti-HEL responses between these recipients (Fig. 6A); p>0.05 by ANOVA with Bonferroni post-test. Flow cytometric crossmatching of immune sera with HOD or control FVB RBC targets was also completed to measure the ability of anti-HOD antibodies to bind the HOD antigen, with results similar to those seen by ELISA (e.g. no significant differences in the rate nor degree of anti-HOD response was observed between Townes homozygous SS, Townes heterozygous AS, or Townes control AA recipients (Fig. 6B shows a representative crossmatch)).
Figure 6. The rate and magnitude of response to transfused HOD RBCs are similar in Townes “knock-in” homozygous SS, heterozygous AS, and control AA recipients.
Townes SS, AS, or AA recipients were transfused with 1 unit of packed HOD RBCs. Anti-HEL IgG was measured by ELISA 2 weeks following transfusion (A). A compilation of data from 3 independent experiments (n=37 recipients total) is shown; p>0.05 by ANOVA with Bonferroni post-test for responses in SS, AS, and AA recipients. Representative flow cytometric histograms (B) with sera from representative transfused homozygous SS recipient (left panel), transfused heterozygous AS recipient (middle panel), and untransfused control (right panel), crossmatched with target HOD RBCs (black line) and control FVB RBCs (grey line).
Discussion
Herein, we have shown that mice with homozygous SS sickle cell disease do not have higher rates or magnitude of alloimmunization to fresh or 14-day stored HOD RBCs than control animals, in the presence or absence of recipient pretreatment with poly (I:C). The lack of an enhanced alloantibody response was not due to the inability of sickle cell mice to process and present the HOD antigen, as most transfusion recipients produced low but detectable levels of anti-HOD alloantibodies. Furthermore, the lack of an enhanced anti-HOD response was not due to a decreased ability of the homozygous SS mice to respond to immune stimuli, because their responses to infused Balb/c splenocytes were robust. The reported findings are not due to an idiosyncrasy of one particular mouse model, because two independently developed mouse models containing human α and βS globin yielded similar results. In sum, these data support the conclusion that homozygous expression of βS globin (and the ensuing pathophysiology of sickle cell disease) is not alone sufficient for enhancement of anti-HOD RBC alloimmunization.
The described experiments test the effects of βS globin as an isolated independent variable. It is noteworthy in humans, however, that the βS globin gene is in linkage disequilibrium with a number of polymorphisms in adjacent genes. Furthermore, some of these gene polymorphisms presumably have effects on immune regulation. The current murine studies cannot address the possibility that polymorphisms of genes co-inherited with human βS globin influence RBC alloimmunization rates in humans13, since the background genes are the same in both the knock-out and knock-in sickle cell disease mouse models. Thus, these “negative” data raise the question of whether, in fact, genes co-inherited along with the βS globin gene in humans with sickle cell disease may play a role in the observed high rates of RBC alloimmunization.
In addition to lacking human genes other than α and βS globin, the pathophysiologic sequelae of the human βS globin gene in mice are similar but not identical to those found in humans. Like humans with homozygous sickle cell disease, SS mice have severe hemolytic anemia, splenic infarcts, vaso-occlusion, and renal concentrating defects32,42,43. Additionally, they exhibit vascular inflammation, with elevated white blood cell counts, activated platelets, and elevated levels of IL-6, serum amyloid P-component (SAP), and adhesion molecules44,45. Unlike humans with sickle cell disease, however, SS mice have massive splenomegaly and lack inflammatory/fibrotic lung changes, large vessel vasculopathy, and bone marrow infarcts. Furthermore, mice heterozygous for the human βS globin have more significant disease manifestations than humans with sickle cell trait,43 and this must be taken into consideration when comparing data between SS and heterozygote AS transfusion recipients. Thus, the described reductionist RBC alloimmunization studies are limited by the inherent differences observed in mice and humans with human βS globin expression.
Just as the manifestations of human and murine sickle cell disease are not identical, the RBC antigen (HOD) utilized in these studies has several features in common with human RBC antigens, but differs in others. HOD is a protein antigen with RBC specific expression within the hematopoietic compartment, like most human RBC antigens33. It contains the human Duffyb antigen within its construct, carried by the DARC protein that serves as a chemokine receptor capable of binding interleukin-8, MCP-1, and RANTES in humans9,46–48. Potential effects of chemokine binding in the current findings are not clear, however, whatever chemokine binding DARC may have, it is present on the majority of transfused human RBCs as well. By virtue of its presence on donor RBCs and absence on recipient RBCs, the HOD antigen more closely resembles the clinically significant human Rh(D) antigen than other RBC antigens, which may differ by a single amino acid polymorphism between donor and recipient10,49. Furthermore, the response rates to transfused HOD RBCs are similar to those observed to Rh(D) positive RBCs in Rh(D) negative recipients. In sum, HOD is a model RBC antigen, and the described rates and degrees of alloimmunization cannot necessarily be extrapolated to other RBC antigens.
Patients with sickle cell disease often require multiple RBC transfusions, exposing them to many RBC alloantigens, over the course of their lives. The current studies involved a single transfusion of RBCs expressing HOD, a design necessitated by the model system. Although the pathophysiology is not understood, the anti-HOD antibody response cannot be boosted by multiple transfusions in control or sickle cell disease mice (data not shown). Thus, the response of mice with sickle cell disease to repeat transfusions of RBC antigens known to be capable of inducing anamnestic responses is difficult to evaluate utilizing the HOD system. Of note, human data in non-sickle cell disease patients postulates that there may be genetically predisposed “responder” and “non-responder” patients, and suggests RBC alloimmunization is only weakly dependent on transfusion load50.
A large percentage of patients with sickle cell disease are in their “usual state of health” when receiving RBC transfusions (e.g. patients on a chronic RBC transfusion protocol for stroke prevention); however, others are quite ill at the time of transfusion. The described data showing no difference in RBC alloimmunization rates or magnitude between murine sickle cell disease and control recipients were obtained with animals transfused in their “baseline” status. Subsequent experiments using the double stranded RNA poly (I:C) as an acute “viral-like” recipient inflammatory stimulus failed to show a difference in response rates or magnitude of response between pretreated mice with sickle cell disease and pretreated control recipients. It must be emphasized, however, that poly (I:C) is but one type of inflammation. Thus, these results cannot necessarily be extrapolated to other states of inflammation (both acute as well as chronic) that sickle cell patients may be in during transfusion, including hypoxia, acute chest syndrome, splenic sequestration, acute ischemic stroke, or vaso-occlusive crisis.
We have recently reported that HOD RBCs stored for 14 days prior to transfusion induce a pro-inflammatory cytokine response in recipients, and are significantly more immunogenic than fresh RBCs37,40. Given that few human transfusion recipients (including those with sickle cell disease) receive “fresh” RBCs, we compared the rates and magnitude of alloimmunization in sickle cell disease and control recipients of fresh or 14-day stored HOD RBCs. To our surprise, both Berkeley and Townes mice with sickle cell disease failed to produce significant levels of pro-inflammatory cytokines or anti-HOD alloantibodies after transfusion of stored HOD RBCs. Possible explanations for these findings, among others, include the presence of baseline inflammation, chronic hemolysis and excess free heme, and the genetic background of the recipients. However, the latter is an unlikely explanation, because 2 murine models with different genetic backgrounds yielded similar results.
In summary, our results fail to support the hypothesis that homozygous expression of human βS globin alone, including the pathophysiological sequelae resulting from expression of this gene, influences anti-HOD RBC alloimmunization in murine transfusion recipients in their baseline states of health or after inflammation with poly (I:C). Although differences between mice and humans with sickle cell disease exist, these data reject the hypothesis that sickle cell disease results in an intrinsic “hyperimmunizable” state to HOD RBC transfusion in mice. These findings do not question the observation that humans with sickle cell disease generate more alloantibodies than other patient groups. They do, however, provoke the question of whether the observed high RBC alloimmunization rates in humans are due to the effects of the βS globin gene itself as opposed to other co-incident factors.
Acknowledgments
This work was supported in part by grants from the NIH (HL092959) and the American Society of Hematology (to JEH)
Footnotes
Author Contributions: JEH, EAH, LSK, SLS, and JCZ designed the experiments; JEH, EAH, JP, PC, and OA performed the experiments; SFOA and DRA provided critically-important animals and reagents, and all authors assisted with data interpretation and manuscript editing.
Disclosures of conflicts of interest: The authors declare they have no conflicts of interest relevant to this manuscript.
References
- 1.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42(1):37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7):1431–1437. [PubMed] [Google Scholar]
- 3.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood.[see comment] New England Journal of Medicine. 1990;322(23):1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 4.Heddle NM, Soutar RL, O'Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion.[see comment] British Journal of Haematology. 1995;91(4):1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Archives of Pathology & Laboratory Medicine. 1995;119(1):42–45. [PubMed] [Google Scholar]
- 6.Schonewille H, van de Watering LM, Loomans DS, Brand A. Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006;46(2):250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 7.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48(10):2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 8.Vichinsky EP, Luban NL, Wright E, et al. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial.[see comment] Transfusion. 2001;41(9):1086–1092. doi: 10.1046/j.1537-2995.2001.41091086.x. [DOI] [PubMed] [Google Scholar]
- 9.Reid MECL-F. The Blood Group Antigen Facts Book. ed 2nd. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- 10.Blood Banking and Transfusion Medicine. 2nd edition. Philadelphia: Churchill Livingstone, Elsevier; 2007. [Google Scholar]
- 11.Natukunda B, Schonewille H, Ndugwa C, Brand A. Red blood cell alloimmunization in sickle cell disease patients in Uganda. Transfusion. 2010;50(1):20–25. doi: 10.1111/j.1537-2995.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 12.Bashawri LA. Red cell alloimmunization in sickle-cell anaemia patients. East Mediterr Health J. 2007;13(5):1181–1189. doi: 10.26719/2007.13.5.1181. [DOI] [PubMed] [Google Scholar]
- 13.Tatari-Calderone Z, Minniti CP, Kratovil T, et al. rs660 polymorphism in Ro52 (SSA1; TRIM21) is a marker for age-dependent tolerance induction and efficiency of alloimmunization in sickle cell disease. Mol Immunol. 2009;47(1):64–70. doi: 10.1016/j.molimm.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg N, Peck K, Ross K, Avila E. Immune response to chronic red blood cell transfusion. Vox Sang. 1983;44(4):212–217. doi: 10.1111/j.1423-0410.1983.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 15.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49(3):505–512. doi: 10.1111/j.1537-2995.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 16.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115(23):4635–4643. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 17.Brittain JE, Parise LV. Cytokines and plasma factors in sickle cell disease. Current Opinion in Hematology. 2007;14(5):438–443. doi: 10.1097/MOH.0b013e3282a4a673. [DOI] [PubMed] [Google Scholar]
- 18.Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol. 1994;87(3):592–597. doi: 10.1111/j.1365-2141.1994.tb08318.x. [DOI] [PubMed] [Google Scholar]
- 19.Hibbert JM, Hsu LL, Bhathena SJ, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Experimental Biology & Medicine. 2005;230(1):68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makis AC, Hatzimichael EC, Bourantas KL. The role of cytokines in sickle cell disease. Ann Hematol. 2000;79(8):407–413. doi: 10.1007/s002770000173. [DOI] [PubMed] [Google Scholar]
- 21.Pathare A, Kindi SA, Daar S, Dennison D. Cytokines in sickle cell disease. Hematology. 2003;8(5):329–337. doi: 10.1080/10245330310001604719. [DOI] [PubMed] [Google Scholar]
- 22.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–2459. [PubMed] [Google Scholar]
- 23.Blum A, Yeganeh S, Peleg A, et al. Endothelial function in patients with sickle cell anemia during and after sickle cell crises. J Thromb Thrombolysis. 2005;19(2):83–86. doi: 10.1007/s11239-005-1377-7. [DOI] [PubMed] [Google Scholar]
- 24.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 25.Jison ML, Munson PJ, Barb JJ, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104(1):270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nath KA, Shah V, Haggard JJ, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 27.Shiu YT, Udden MM, McIntire LV. Perfusion with sickle erythrocytes up-regulates ICAM-1 and VCAM-1 gene expression in cultured human endothelial cells. Blood. 2000;95(10):3232–3241. [PubMed] [Google Scholar]
- 28.Vordermeier S, Singh S, Biggerstaff J, et al. Red blood cells from patients with sickle cell disease exhibit an increased adherence to cultured endothelium pretreated with tumour necrosis factor (TNF) Br J Haematol. 1992;81(4):591–597. doi: 10.1111/j.1365-2141.1992.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 29.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46(9):1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 30.Yazer MH, Triulzi DJ, Shaz B, Kraus T, Zimring JC. Does a febrile reaction to platelets predispose recipients to red blood cell alloimmunization? Transfusion. 2009;49(6):1070–1075. doi: 10.1111/j.1537-2995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 31.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 32.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108(4):1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114(11):2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behringer RR, Ryan TM, Palmiter RD, Brinster RL, Townes TM. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- 35.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278(5339):873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 36.Ryan TM, Townes TM, Reilly MP, et al. Human sickle hemoglobin in transgenic mice. Science. 1990;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- 37.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50(3):642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilson CR, Kraus T, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cells storage and post-transfusion in vivo survival. Transfusion. 2009;48(9):1456–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110(7):2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 40.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrickson J, Hod EA, Cadwell CM, Eisenbarth SC, Speigel DA, Tormey CA, Spitalnik SL, Zimring JC. Rapid clearance of murine RBCs is associated with recipient cytokine storm and enhanced alloimmunogenicity. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03162.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 43.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107(4):1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Archer DR, Stiles JK, Newman GW, et al. C-reactive protein and interleukin-6 are decreased in transgenic sickle cell mice fed a high protein diet. J Nutr. 2008;138(6):1148–1152. doi: 10.1093/jn/138.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 46.Darbonne WC, Rice GC, Mohler MA, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88(4):1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JS, Wurfel MM, Matute-Bello G, et al. The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. J Immunol. 2006;177(11):8086–8094. doi: 10.4049/jimmunol.177.11.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangalmurti NS, Xiong Z, Hulver M, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mollison's Blood Transfusion in Clinical Medicine. ed 11th edition. Blackwell Publishing; 2005. [Google Scholar]
- 50.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders.[see comment] Blood. 2008;112(6):2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]