Abstract
We have studied the in vivo replication properties of plasmids carrying deletion mutations within cloned adenovirus terminal sequences. Deletion mapping located the adenovirus DNA replication origin entirely within the first 67 bp of the adenovirus inverted terminal repeat. This region could be further subdivided into two functional domains: a minimal replication origin and an adjacent auxillary region which boosted the efficiency of replication by more than 100-fold. The minimal origin occupies the first 18 to 21 bp and includes sequences conserved between all adenovirus serotypes. The adjacent auxillary region extends past nucleotide 36 but not past nucleotide 67 and contains the binding site for nuclear factor I.
Full text
PDF

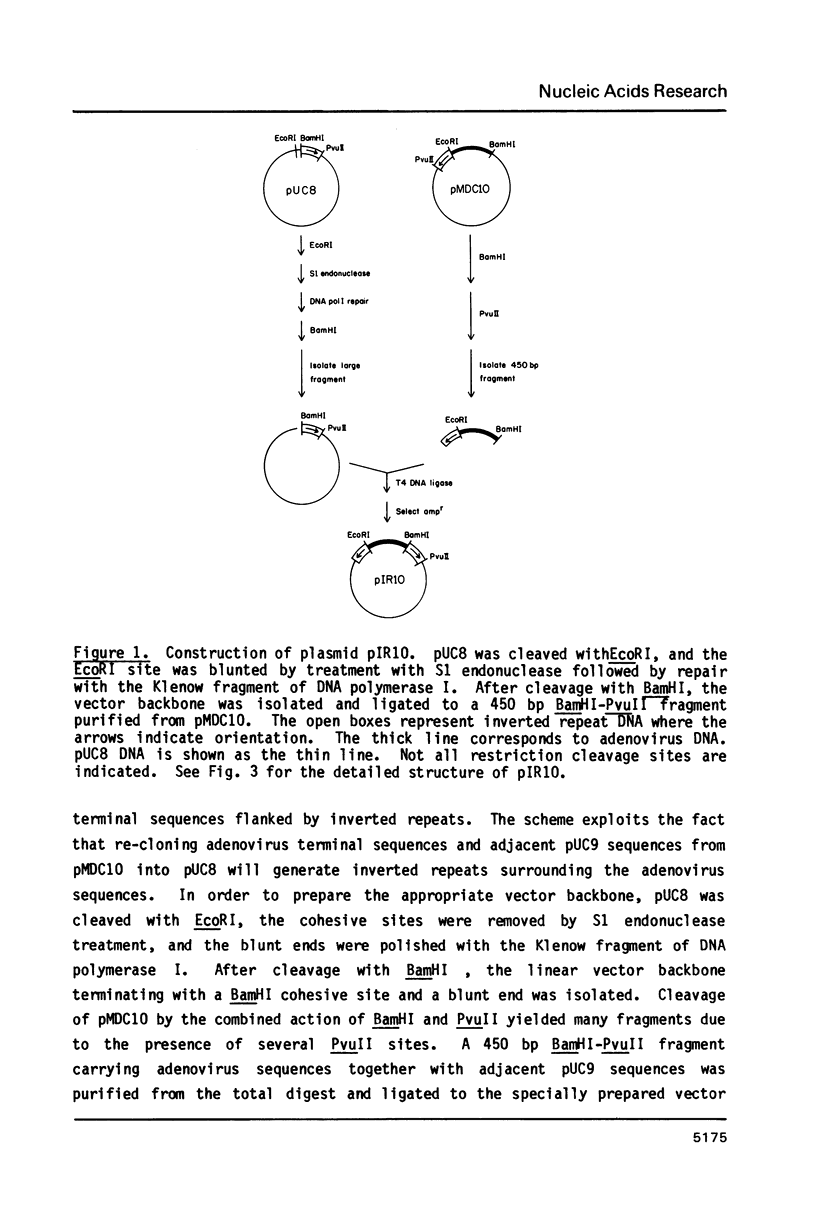
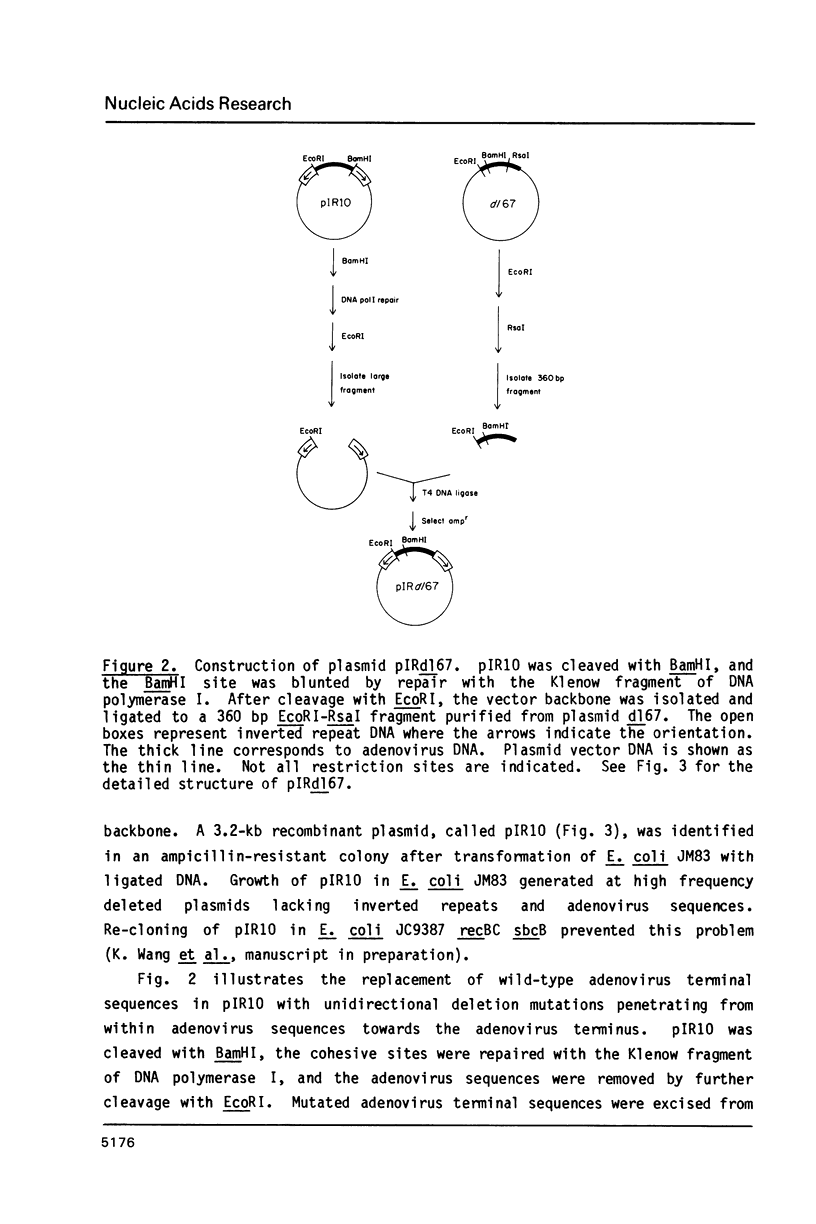

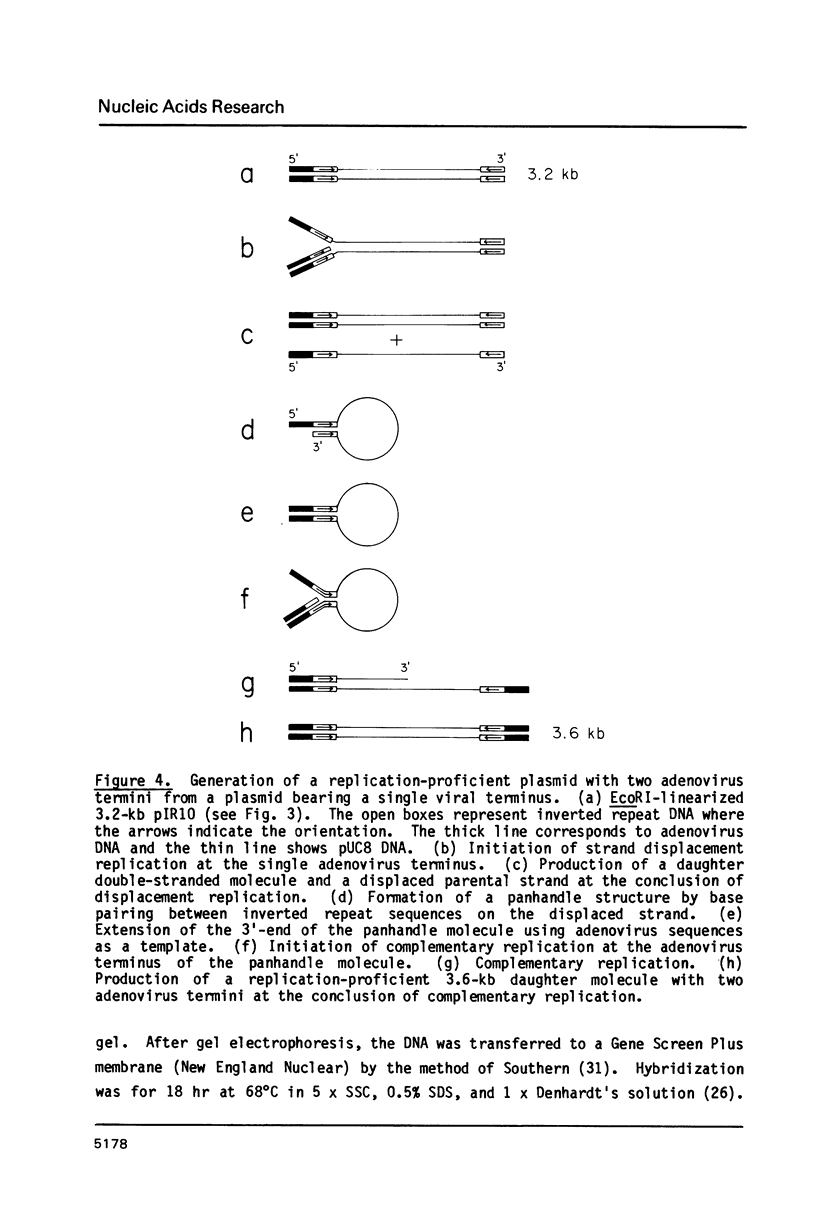









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Rawlins D. R. Template requirements for the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1984 Jan;81(1):100–104. doi: 10.1073/pnas.81.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Winnacker E. L. Adenovirus DNA replication. Curr Top Microbiol Immunol. 1984;111:41–64. doi: 10.1007/978-3-642-69549-0_2. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Stow N. D., McDougall I. M. Replication of adenovirus mini-chromosomes. J Mol Biol. 1984 Jun 5;175(4):493–510. doi: 10.1016/0022-2836(84)90181-5. [DOI] [PubMed] [Google Scholar]
- Lally C., Dörper T., Gröger W., Antoine G., Winnacker E. L. A size analysis of the adenovirus replicon. EMBO J. 1984 Feb;3(2):333–337. doi: 10.1002/j.1460-2075.1984.tb01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Chow K. C., Enns R. E., Ahern K. G., Corden J. L., Harpst J. A. In vitro replication directed by a cloned adenovirus origin. Gene. 1983 Sep;23(3):293–305. doi: 10.1016/0378-1119(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., O'Neill K. E., Yen C. T. DNA sequences from the adenovirus 2 genome. J Biol Chem. 1984 Nov 25;259(22):13968–13975. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Topp W. C., Engler J. A. Conserved sequences at the origin of adenovirus DNA replication. J Virol. 1982 Nov;44(2):530–537. doi: 10.1128/jvi.44.2.530-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D. The infectivity of adenovirus genomes lacking DNA sequences from their left-hand termini. Nucleic Acids Res. 1982 Sep 11;10(17):5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Initiation of adenovirus DNA replication in vitro requires a specific DNA sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6446–6450. doi: 10.1073/pnas.80.21.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Domingo E., Warner R. C. Properties of Azotobacter phage A14 and its DNA. Virology. 1973 Dec;56(2):523–531. doi: 10.1016/0042-6822(73)90055-x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M. The synthesis and substructure of herpesvirus DNA: the distribution of alkali-labile single strand interruptions in HSV-1 DNA. J Gen Virol. 1973 Dec;21(3):453–467. doi: 10.1099/0022-1317-21-3-453. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen B. G., van der Ley P. A., van Driel W., van Mansfeld A. D., van der Vliet P. C. Replication of origin containing adenovirus DNA fragments that do not carry the terminal protein. Nucleic Acids Res. 1983 Apr 11;11(7):1975–1989. doi: 10.1093/nar/11.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




