Abstract
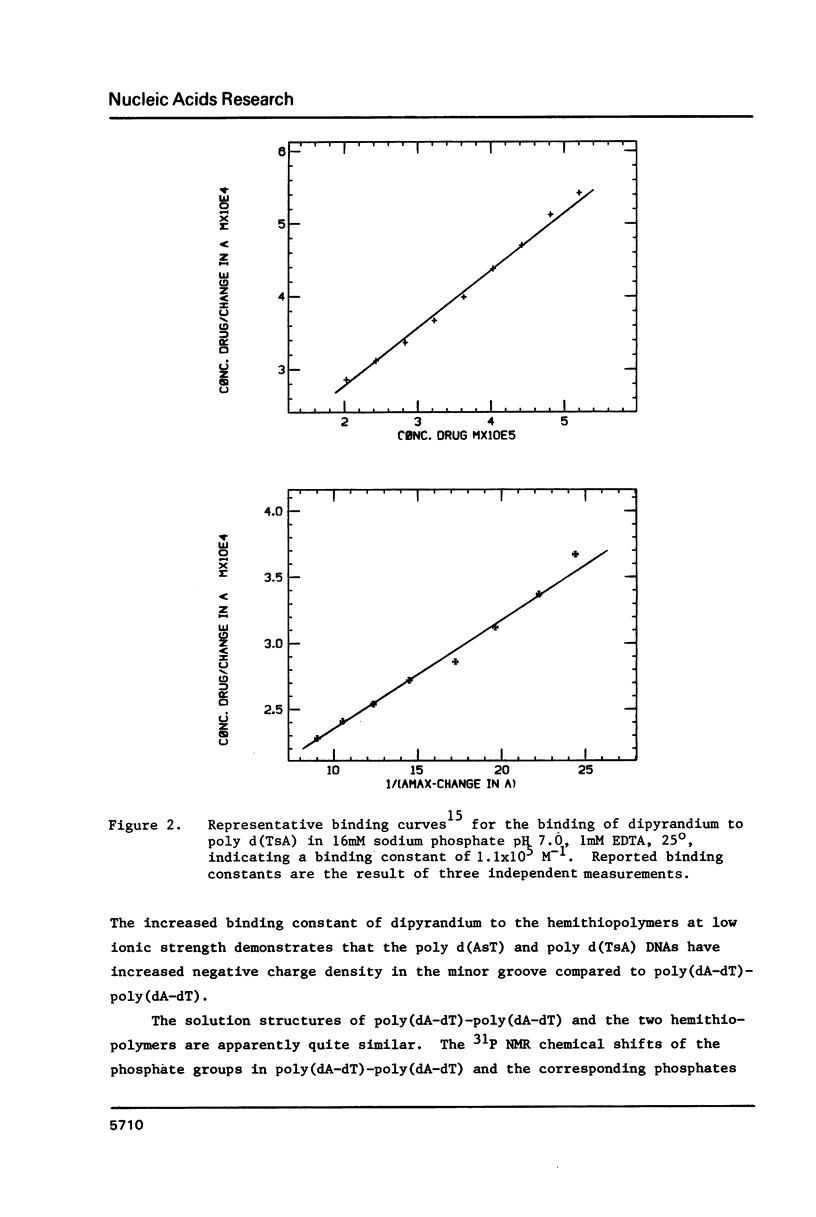
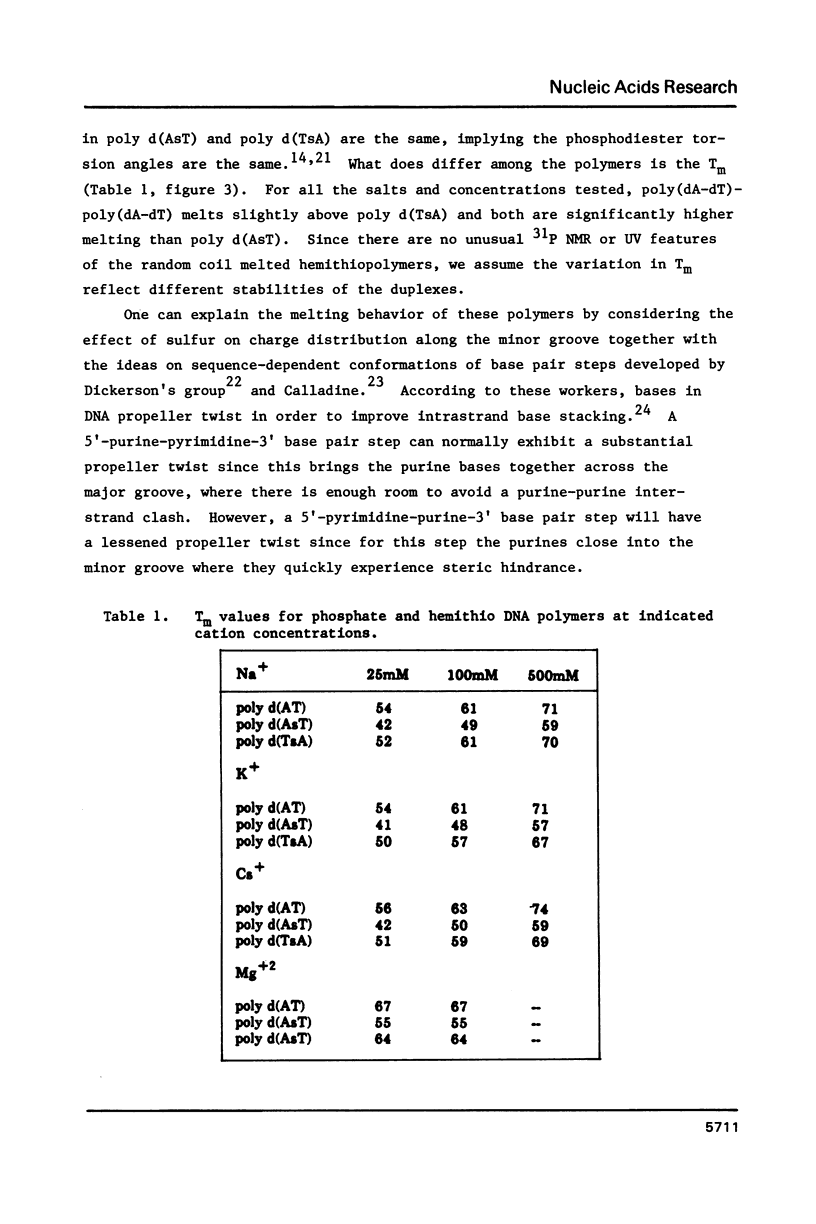
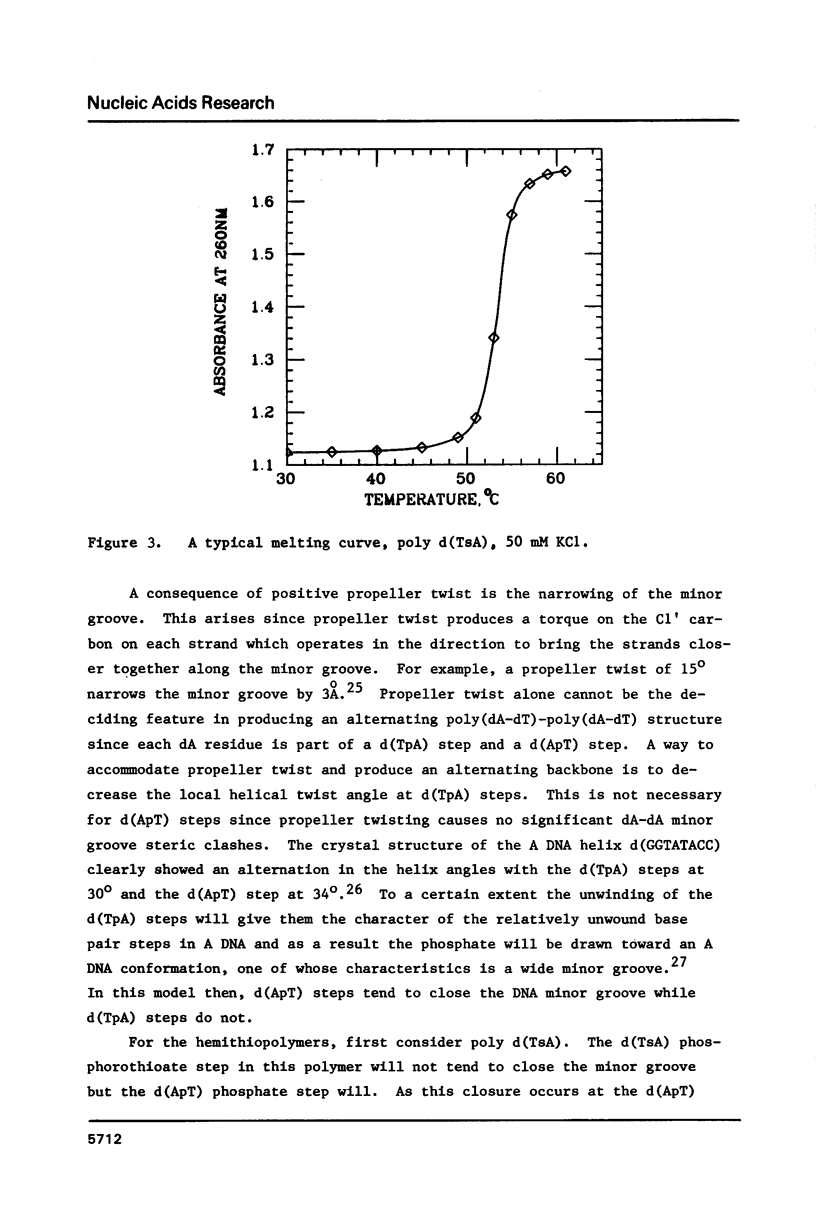
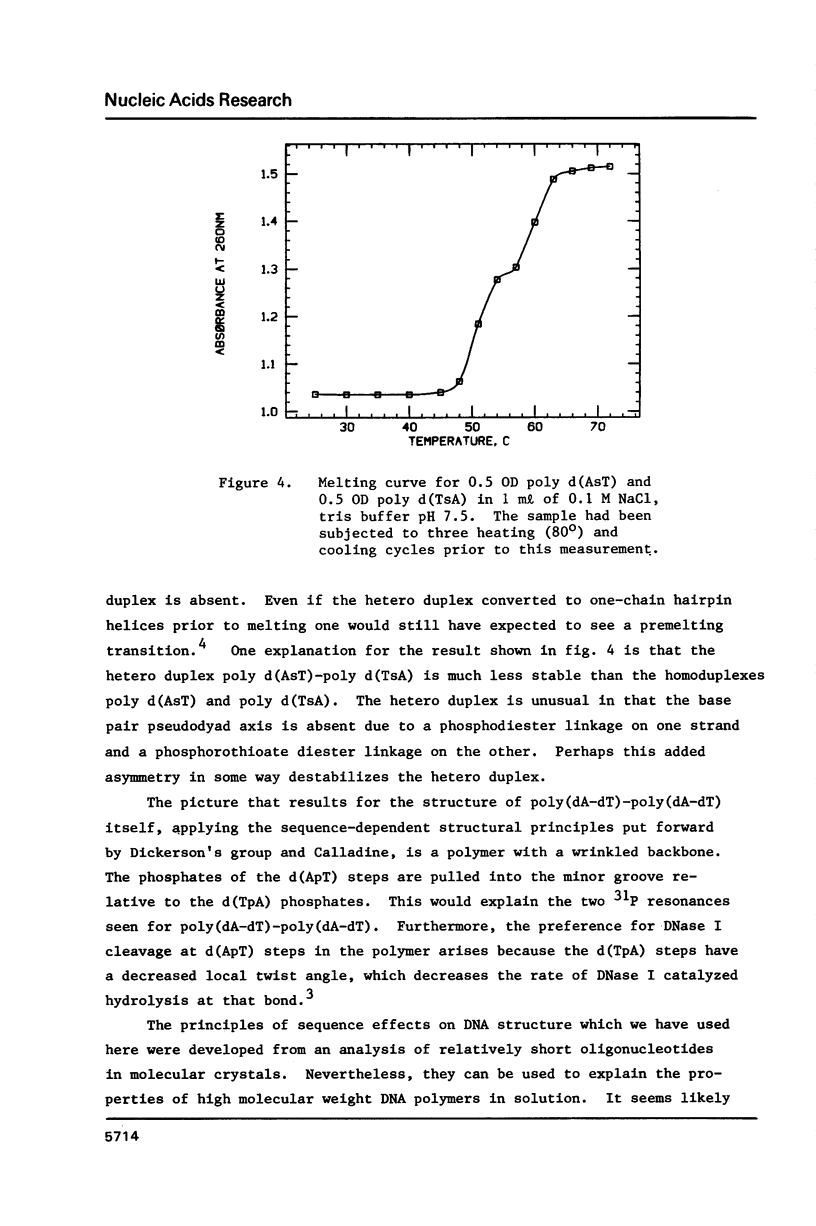
Analogs of alternating purine-pyrimidine DNA polymers such as poly(dA-dT)-poly(dA-dT) can be made with phosphorothioate groups in the DNA backbone. A phosphorothioate diester at the 5'-purine-pyrimidine-3' step causes a significant lowering of the polymer's melting temperature compared to a phosphorothioate diester at the 5'-pyrimidine-purine-3' step. This may occur because sulfur substitution increases anionic charge density in the DNA minor groove and 5'-purine-pyrimidine-3' steps narrow the minor groove. The ability to modulate charge density in the DNA backbone via sulfur substitution should prove useful in studies of sequence-dependent conformational changes in DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Puigjaner L. C., Walker J. K., Hall I. H., Birdsall D. L., Ratliff R. L. Wrinkled DNA. Nucleic Acids Res. 1983 Mar 11;11(5):1457–1474. doi: 10.1093/nar/11.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assa-Munt N., Kearns D. R. Poly(dA-dT) has a right-handed B conformation in solution: a two-dimensional NMR study. Biochemistry. 1984 Feb 28;23(5):791–796. doi: 10.1021/bi00300a001. [DOI] [PubMed] [Google Scholar]
- Calladine C. R. Mechanics of sequence-dependent stacking of bases in B-DNA. J Mol Biol. 1982 Oct 25;161(2):343–352. doi: 10.1016/0022-2836(82)90157-7. [DOI] [PubMed] [Google Scholar]
- Conner B. N., Takano T., Tanaka S., Itakura K., Dickerson R. E. The molecular structure of d(ICpCpGpG), a fragment of right-handed double helical A-DNA. Nature. 1982 Jan 28;295(5847):294–299. doi: 10.1038/295294a0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Gindl H. Polyribonucleotides containing a phosphorothioate backbone. Eur J Biochem. 1970 Apr;13(3):558–564. doi: 10.1111/j.1432-1033.1970.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Jovin T. M. Assignment of resonances in the phosphorus-31 nuclear magnetic resonance spectrum of poly[d(A-T)] from phosphorothioate substitution. Biochemistry. 1983 Sep 13;22(19):4546–4550. doi: 10.1021/bi00288a030. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D., Bertazzoni U., Bernardi G. The specificity of pancreatic deoxyribonuclease. Eur J Biochem. 1973 Dec 3;40(1):143–147. doi: 10.1111/j.1432-1033.1973.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Fratini A. V., Kopka M. L., Drew H. R., Dickerson R. E. Reversible bending and helix geometry in a B-DNA dodecamer: CGCGAATTBrCGCG. J Biol Chem. 1982 Dec 25;257(24):14686–14707. [PubMed] [Google Scholar]
- Gorenstein D. G. Nucleotide conformational analysis by 31P nuclear magnetic resonance spectroscopy. Annu Rev Biophys Bioeng. 1981;10:355–386. doi: 10.1146/annurev.bb.10.060181.002035. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Green G., Goutarel R., Khuong-Huu Q. Nucleic acid-small molecule interactions. VII. Further characterization of deoxyribonucleic acid-diamino steroid complexes. Biochemistry. 1968 Apr;7(4):1568–1582. doi: 10.1021/bi00844a046. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Steroid diamine-nucleic acid interactions: partial insertion of dipyrandium between unstacked base pairs of the poly(dA-dT) duplex in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):24–28. doi: 10.1073/pnas.76.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Sarma R. H. Solution structure of poly(dA-dT).poly(dA-dT) in low and high salt: a 500 MHz 1H NMR study using one-dimensional NOE. J Biomol Struct Dyn. 1984 Jun;1(6):1423–1455. doi: 10.1080/07391102.1984.10507529. [DOI] [PubMed] [Google Scholar]
- Saucier J. M. Physicochemical studies on the interaction of irehdiamine A with bihelical DNA. Biochemistry. 1977 Dec 27;16(26):5879–5889. doi: 10.1021/bi00645a036. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by dAT oligomers. I. Hairpin and straight-chain helices. J Mol Biol. 1968 Sep 28;36(3):291–304. doi: 10.1016/0022-2836(68)90156-3. [DOI] [PubMed] [Google Scholar]
- Shakked Z., Rabinovich D., Kennard O., Cruse W. B., Salisbury S. A., Viswamitra M. A. Sequence-dependent conformation of an A-DNA double helix. The crystal structure of the octamer d(G-G-T-A-T-A-C-C). J Mol Biol. 1983 May 15;166(2):183–201. doi: 10.1016/s0022-2836(83)80005-9. [DOI] [PubMed] [Google Scholar]
- Shindo H. 13C NMR study of conformation and mobility of 145-base-pair poly(dA-dT) . poly(dA-dT) in solution. Eur J Biochem. 1981 Nov;120(2):309–312. doi: 10.1111/j.1432-1033.1981.tb05705.x. [DOI] [PubMed] [Google Scholar]
- Shindo H., Simpson R. T., Cohen J. S. An alternating conformation characterizes the phosphodiester backbone of poly(dA-dT) in solution. J Biol Chem. 1979 Sep 10;254(17):8125–8128. [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Gaillard C., Prunell A. Helical periodicity of DNA, Poly(dA) . poly(dT) and poly(dA-dT). poly(dA-dT) in solution. Eur J Biochem. 1981 Aug;118(2):215–222. doi: 10.1111/j.1432-1033.1981.tb06389.x. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Sklenár V. Salt-induced conformational transition of poly[d(A-T)] X poly[d(A-T)]. J Mol Biol. 1983 May 5;166(1):85–92. doi: 10.1016/s0022-2836(83)80052-7. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., Rich A. Right-handed and left-handed double-helical DNA: structural studies. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):33–44. doi: 10.1101/sqb.1983.047.01.006. [DOI] [PubMed] [Google Scholar]


