Abstract
Ecological studies have reported strong inverse correlations between indices of solar ultraviolet-B (UVB) doses and incidence and/or mortality rates for many types of cancer. Case-control studies (CCS) generally find inverse correlations between serum 25-hydroxyvitamin D [25(OH)D] concentration measured at time of diagnosis for cancer incidence, whereas nested case-control studies (NCCS), which involve a several-year follow-up time after serum sampling, generally do not. This paper examines the relation between follow-up interval and relative risk (RR) for breast, colorectal, and prostate cancer. I plot the RR versus serum 25(OH)D data as a function of follow-up time from the literature for each type of cancer. For breast cancer, RRs were significantly reduced only for follow-up periods less than 3 years. For colorectal cancer, RRs were generally significantly reduced for follow-up periods up to 12 years. For prostate cancer, RRs were not statistically significant from 4 years to 28 years. This study included no CCS. Follow-up periods after serum sampling should not be too long for breast cancer because once a tumor reaches a diameter of 1–3 mm, it requires angiogenesis to continue growing, and vitamin D reduces angiogenesis around tumors. Breast cancer diagnoses are more common in spring and fall than in summer or winter, indicating that they can grow rapidly if circulating 25(OH)D drops in the fall or melatonin levels drop in spring. Serum sampling should be conducted during the study, perhaps every 2 years, to overcome the problem of change of 25(OH)D concentration during cohort studies.
Key words: breast cancer, colorectal cancer, case-control studies, ecological studies, prostate cancer, ultraviolet-B, vitamin D
Introduction
The Institute of Medicine's (IOM) committee on dietary requirements for calcium and vitamin D recently released a report finding that vitamin D had scientifically justified benefits only for bones, with no demonstrated benefits for nonskeletal effects.1,2 This finding is at such odds with the growing body of literature on the health benefits of vitamin D3–5 that examining the review process seems worthwhile. The underlying document commissioned by the IOM for use by the committee6 indicates that federal agencies instructed the committee to include randomized controlled trials (RCTs) and prospective or retrospective observational studies [such as nested case-control studies (NCCS)] but to ignore ecological studies and CCS in which serum 25-hydroxyvitamin D [25(OH)D] was measured at the time of disease diagnosis. Because ecological studies generally find strong inverse correlations between solar ultraviolet-B (UVB) doses7–10 and CCS do likewise for serum 25(OH)D for breast cancer,11 the federal agencies may have effectively dictated the conclusion. The committee concluded that the literature offers conflicting results regarding vitamin D, and so ascribing health benefits for other than the bones was premature.
A recent review of observational studies of breast cancer incidence with respect to serum 25(OH)D levels offers a possible reason for the discrepancy: the summary relative risk (RR) for CCS was 0.59 [95% confidence interval (CI): 0.48, 0.73], whereas the RR for NCCS was 0.92 (95% CI: 0.82, 1.04).11 The difference between the two types of studies is the lag time between serum draw and cancer diagnosis: zero years for CCS and anywhere from 2 to 28 years for NCCS. There is the obvious concern that a single 25(OH)D level may lose predictive power over time. Indeed, two recent studies reported diminished correlations with either second serum 25(OH)D level measurements or cancer incidence rates. An observational study in Norway found that “depending on the method of adjusting for season, the correlation coefficient between serum 25(OH)D measurements from 1994 and 2008 ranged from 0.42 to 0.52.”12
In a 17.4-year follow-up study involving male smokers in Finland and incidence of non-Hodgkin's lymphoma (NHL) found the following:
The 25(OH)D-NHL association, however, differed by follow-up duration at diagnosis. Cases diagnosed less than 7 years from the baseline showed an inverse association (OR for highest vs. lowest tertile = 0.43; 95% CI: 0.23, 0.83; p for trend = 0.01), but not later diagnoses (OR = 1.52; 95% CI: 0.82, 2.80; p for trend = 0.17).13
Thus, investigating the relation between observational follow-up period and RR for cancer incidence seems worthwhile.
Results
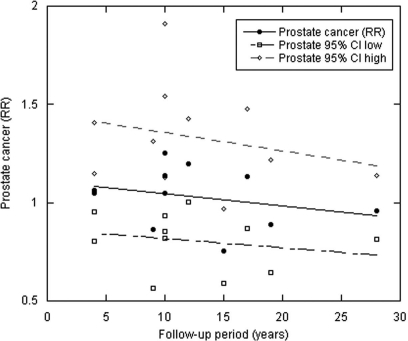
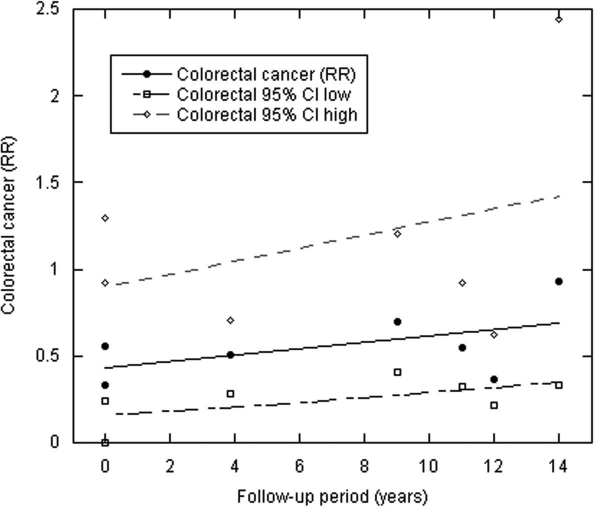
Figures 1–3 and Table 1 show the results. The zero year intercept is an estimate of the results of CCS with serum 25(OH)D level measured at time of cancer diagnosis. It is lowest for colorectal cancer, intermediate for breast cancer and above unity for prostate cancer. This order is consistent with ecological studies that find the strongest inverse correlation with indices of solar UVB for colon and rectal cancer; strong inverse correlations for breast cancer; and weak, if any, inverse correlation for prostate cancer.7,8,14,15 The slope is a measure of the change with respect to the length of the follow-up period. The steeper slopes may indicate that the particular type of cancer has a more rapid growth rate from undetectable to detectable size.
Figure 1.
Scatter plot of breast cancer RR with respect to follow-up period. The solid line is the linear fit to the RR; the dashed lines are the linear fit to the 95% CIs.
Figure 3.
Scatter plot of prostate cancer RR with respect to follow-up period as in Figure 1.
Table 1.
Regression results for cancer incidence as a function of years of follow-up after serum draw used for 25(OH)D level measurement
| Cancer | No. of studies | Zero year intercept (RR) | Slope of RR (per year) | R, adjusted r2, p |
| Breast | 9 | 0.62 | 0.046 | 0.70, 0.41, 0.04 |
| Colorectal | 7 | 0.43 | 0.018 | 0.52, 0.12, 0.24 |
| Prostate | 11 | 1.11 | −0.006 | Not meaningful |
The regression coefficients and p values for both breast and colorectal cancer support the interpretation that for these cancers, the correlation between serum 25(OH)D and cancer incidence decreases with increasing follow-up time. The results for prostate cancer are consistent with little effect of vitamin D for follow-up out to 28 years, although the findings suggest a hint of a small increased risk at shorter follow-up times.
Discussion
These results are consistent with a diminishing utility of one-time serum 25(OH)D measurements for determining the effect of vitamin D in reducing the risk of cancer as the follow-up time increases.
The finding that a single reading is not an accurate measure of the effect of a particular cancer risk-modifying factor is not limited to vitamin D. For example, a study of dietary risk factors for pancreatic cancer in the United States found the following:
The positive association for available carbohydrate intake was observed during the first four years of follow-up [HR(<2 years) = 2.60, 95% CI: 1.34, 5.06; HR(2–<4 years) = 1.94, 95% CI: 1.06, 3.55)] but not subsequently (HR = 0.86, 95% CI: 0.52, 1.44); the opposite pattern was observed for total fat and saturated fat intake. Rather than being causal, the short-term increase in pancreatic cancer risk associated with high available carbohydrate and low fat intake may be capturing dietary changes associated with subclinical disease.16
These results also help clear up some of the confusion regarding the role of vitamin D in reducing the risk of cancer. Both breast and colorectal cancer have strong support from ecological studies,7,8,17 which this analysis supports. A recent study in France found a contribution of vitamin D from a combination of both oral intake and solar UVB irradiance.18
Tumor growth rates for breast cancer come from two papers. An early paper suggested a power law (exponential) growth rate for breast cancer with an exponent of 0.5.19 In that paper, the tumor volume is near 0 mL until 20 years and then increases to 2,200 mL by year 25. A more recent paper, based on mammography screening, estimated that tumors increase from 1.5 cm diameter to 4.0 cm in 1.5 years.20 Backtracking from 2 cm to 2 mm, we find that such growth would take about 1.7 years according to the Hart et al. value. Thus, there appears to be about a 1- to-2-year window to go from start of angiogenesis to detection of breast cancer. Further support for this finding is that breast cancer diagnoses have peaks in spring and fall, with the authors suggesting that vitamin D in summer and melatonin in winter reduce the breast tumor growth rate in those seasons.21
Prostate cancer, however, has mixed results with respect to solar UVB irradiance. In 1990, Schwartz and Hulke22 originally suggested that prostate cancer was vitamin D sensitive; Hanchette and Schwartz23 extended this finding. Recent work, however, noted that the geographical variation of prostate cancer mortality rates in the United States24 is highly correlated with ethnic background as found in the Atlas of Greatest Ancestry by County.25 Another study hypothesized that apolipoprotein E ε4 (ApoE4) might be a risk factor,15 in part because cholesterol is an important risk factor for high-grade prostate cancer.26 In a study in Japan, prostate cancer was the only type of cancer for which cholesterol was a risk factor.27 ApoE4 produces more cholesterol in the liver28 and insulin in the pancreas to facilitate storage of excess food as fat.29 Thus, current or recent hunter-gatherer populations have a higher prevalence of ApoE4 to protect them from starvation between feasts. A recent multicountry ecological study found that risk of prostate cancer was directly correlated with ApoE4 and inversely with fraction of energy supply derived from cereals and grains.15
On the other hand, several papers indicate a reduced risk of prostate cancer with respect to solar UVB irradiance. Two studies reported a reduced risk of prostate cancer incidence with respect to early-life and/or entire-life UVB irradiance.30,31 Other studies have reported similar findings for prostate cancer on the basis of diagnosis of nonmelanoma skin cancer.32–34
Breast, colon and prostate cancer apparently have different rates of growth from initiation to detection, with breast cancer the most likely to be diagnosed early in life and prostate cancer most likely to be diagnosed late in life. The mortality rate data for the United States in 2004 and the UK in 2005 were plotted versus age.35 The average exponent of the power-law fit to the data is 4.0 for breast cancer, 6.1 for colon cancer and 11.7 for prostate cancer. Larger exponents are associated with faster increases with respect to age.
The recent Vitamin D Pooling Project (VDPP) found no evidence of a beneficial role of vitamin D in reducing the risk of seven rarer cancers: endometrial, esophageal, gastric, kidney, NHL, ovarian and pancreatic.36 The median follow-up time for the 10 studies included in the VDPP ranged from 2.1 to 10.8 years, with a median value of 4–5 years. As seen from the analysis presented here, that is one problem with the VDPP study. A second problem is that the 95% CIs for the highest serum 25(OH)D quantile range are all greater than 0.5 because of the few cases in the studies. Thus, detecting a small effect would be extremely difficult.37
In the IOM report, the following statement summarized the committee's finding regarding colorectal cancer:1
Taken in aggregate, epidemiological studies examining associations between vitamin D status and colorectal cancer incidence generally support an inverse association, although the shape of the dose-response relationship curve over a wide range of vitamin D intake remains very speculative. The biological plausibility is supported by data from cell culture and rodents, with additional support from surrogate biomarker studies in humans. There remains a paucity of prospective randomized intervention studies, and those available have not shown a significant relationship at this time. Thus, the data are insufficient for the committee to utilize colon cancer as an outcome for establishment of vitamin D DRIs. The data for an effect of dietary calcium on colorectal cancer risk are also highly suggestive of a protective effect, but there are not sufficient data available on dose-response relationships to utilize colorectal cancer as a health outcome for DRI development.
Thus, the reliance on NCCS and exclusion of CCS ecological studies by the IOM's committee on dietary requirements for calcium and vitamin D seem to be important reasons why the committee failed to find health benefits of vitamin D for colorectal and other cancers. There being only one RCT that found a beneficial effect of vitamin D in reducing the risk of cancer38 is due to two important factors: that most RCTs on cancer to date used a vitamin D dosage of 400 IU/d39,40 and that funding for RCTs involving vitamin D has been limited until recently.
Another limitation of NCCS and CCS is that they are not sensitive to early-life cancer risk-modifying factors. A classical example is the effect of diet on cancer risk. In 1975, Armstrong and Doll41 published their classical ecological multicountry study showing that “dietary variables were strongly correlated with several types of cancer, particularly meat consumption with cancer of the colon and fat consumption with cancers of the breast and corpus uteri.” For many years thereafter, NCCS could not verify that finding.42,43 Eventually it was realized that ecological studies integrate dietary intake over the entire lifetime and that the effects of high-fat diets on risk of cancer might be related to early life rather than late life. When younger women were enrolled in the cohort studies, findings indicated that meat and animal fat were risk factors for breast cancer.44 Other studies associated soy consumption in adolescence with reduced risk of breast cancer.45
This review has several implications regarding how to determine the effect of vitamin D status on risk of cancer:
In NCCS, having multiple serum draws would be helpful, perhaps every 2 years and in different seasons, so that the variability of serum 25(OH)D can be assessed.
CCS should be used rather than NCCS. Although concern exists that disease state can affect serum 25(OH)D level, we need a way to ascertain whether any change in vitamin D intake, body mass index and lifestyle occurred over the past year or so that would affect UVB irradiance and vitamin D production.
In vitamin D RCTs, measuring serum 25(OH)D levels is important; individual response to oral vitamin D intake varies considerably.46 Part of the reason is the inverse relation between serum 25(OH)D and body weight.47
Any study that measures 25(OH)D should use a reliable assay as well as NIST standards.48–50
Such reviews should take into account viewpoints of experts on the topic, fully consider all valid epidemiological approaches, resolve discrepancies between different types of studies, conduct the review in an open fashion and respond to peer review. The IOM review1 did not follow these guidelines, which severely undercuts its credibility and validity.
Materials and Methods
I chose three cancers with several CCS and/or NCCS for this study: breast, colorectal and prostate cancer. The RR data came primarily from several recent reviews in which the authors determined the RR for an interval change in serum 25(OH)D level: breast,11 colorectal51 and prostate cancer.52 The papers by Yin et al. were the primary source; I also consulted the paper by Gandini et al. and added three more colorectal cancer studies.54–56 This analysis omitted papers presenting data on colon or rectal cancer without the combination, colorectal cancer. This analysis combines the data for colorectal cancer for males and females by Otani et al. into one value for males plus females. The values used in this review are given in Table 2.
Table 2.
Data used in this study
| Cancer | RR for 20 ng/mL (95% CI) | Lag (years) | Reference |
| Breast (RR from Ref. 11) | 0.60 (0.47, 0.77) | 0 | 58 |
| 0.43 (0.35, 0.54) | 0 | 59 | |
| 0.67 (0.53, 0.85) | 0 | 60 | |
| 0.74 (0.61, 0.89) | 0 | 61 | |
| 0.60 (0.54, 0.67) | 2.67 | 62 | |
| 1.05 (0.83, 1.33) | 3.9 | 63 | |
| 1.02 (0.82, 1.27) | 6.0 | 64 | |
| 0.85 (0.71, 1.01) | 6.5 | 65 | |
| 0.82 (0.65, 1.04) | 7.0 | 66 | |
| Colorectal (RR from Ref. 53) | 0.56 (0.24, 1.30) | 0 | 54 |
| 0.33 (0, 0.93) | 0 | 55 | |
| 0.51 (0.28, 0.71) | 3.85 | 56 | |
| (RR from Ref. 51) | 0.70 (0.41, 1.20) | 9 | 67 |
| 0.55 (0.24, 1.30) | 11 | 68 | |
| 0.37 (0.22, 0.63) | 12 | 40 | |
| 0.69 (0.28, 1.68) | 14 | 57 (M) | |
| 1.21 (0.45, 3.27) | 14 | 57 (F) | |
| 0.93 (0.33, 2.44) | 14 | 57 (M+F) | |
| RR for 10 ng/mL | |||
| Prostate (10 ng/mL) (RR from Ref. 52) | 1.07 (0.81, 1.41) | 4 | 69 |
| 1.05 (0.95, 1.15) | 4 | 70 | |
| 0.86 (0.57, 1.31) | 9 | 71 | |
| 1.05 (0.94, 1.18) | 10 | 72 | |
| 1.14 (0.86, 1.51) | 10 | 73 | |
| 1.25 (0.82, 1.91) | 10 | 74 | |
| 1.20 (1.01, 1.43) | 12 | 74 | |
| 0.76 (0.57, 0.97) | 15 | 72 | |
| 1.13 (0.87, 1.48) | 17 | 75 | |
| 0.89 (0.65, 1.22) | 19 | 76 | |
| 0.96 (0.81, 1.14) | 28 | 77 |
F, females, M, males; RR, relative risk for incremental serum 25(OH)D.
The mean follow-up times came from the original papers. When a report gave a range of years for serum collection, I used the midpoint of that range. On the assumption that cancer incidence rates in any given study were uniform, the mean lag between serum 25(OH)D measurement and cancer incidence is half the mean follow-up time.
Since the 25(OH)D quantiles varied by study and since nonlinear variations occur in the 25(OH)D level-cancer incidence relation,76 some variability is introduced into the values due to different lowest quantile mean 25(OH)D level; however, this analysis ignores that effect.
Figure 2.
Scatter plot of colorectal cancer RR with respect to follow-up period as in Figure 1.
Disclosure
I receive or have received funding from the UV Foundation (McLean, VA), the Sunlight Research Forum (Veldhoven), BioTech-Pharmacal (Fayetteville, AR), the Vitamin D Council (San Luis Obispo, CA) and the Danish Sunbed Federation (Middelfart).
References
- 1.IOM (Institute of Medicine), author Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491–499. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, editor. Vitamin D; Physiology, Molecular Biology and Clinical Applications. Second Edition. Springer NY: Humana Press; 2010. p. 1155. [Google Scholar]
- 5.Holick MF. Vitamin D: Evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009:1–420. [PMC free article] [PubMed] [Google Scholar]
- 7.Grant WB, Garland CF. A critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer. 2004;48:115–123. doi: 10.1207/s15327914nc4802_1. [DOI] [PubMed] [Google Scholar]
- 8.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States 1993–2000. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr SB. A brief history of vitamin D and cancer prevention. Ann Epidemiol. 2009;19:79–83. doi: 10.1016/j.annepidem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Grant WB, Mohr SB. Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol. 2009;19:446–454. doi: 10.1016/j.annepidem.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 13.Lim U, Freedman DM, Hollis BW, Horst RL, Purdue MP, Chatterjee N, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124:979–986. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant WB. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UV irradiance and smoking. Int J Cancer. 2007;120:1123–1127. doi: 10.1002/ijc.22386. [DOI] [PubMed] [Google Scholar]
- 15.Grant WB. A multicountry ecological study of risk-modifying factors for prostate cancer: Apolipoprotein E ε4 as a risk factor and cereals as a risk reduction factor. Anticancer Res. 2010;30:189–199. [PubMed] [Google Scholar]
- 16.Meinhold CL, Dodd KW, Jiao L, Flood A, Shikany JM, Genkinger JM, et al. Available carbohydrates, glycemic load and pancreatic cancer: is there a link? Am J Epidemiol. 2010;171:1174–1182. doi: 10.1093/aje/kwq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 18.Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Joint effect of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:187–196. doi: 10.1158/1055-9965.EPI-10-1039. [DOI] [PubMed] [Google Scholar]
- 19.Hart D, Shochat E, Agur Z. The growth law of primary breast cancer as inferred from mammography screening trials data. Br J Cancer. 1998;78:382–387. doi: 10.1038/bjc.1998.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10:41. doi: 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh EY, Ansell C, Nawaz H, Yang CH, Wood PA, Hrushesky WJ. Global breast cancer seasonality. Breast Cancer Res Treat. 2010;123:233–243. doi: 10.1007/s10549-009-0676-7. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- 23.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Devesa SS, Grauman DJ, Blot WJ, Pennello GA, Hoover RN, Fraumeni JF., Jr . Atlas of Cancer Mortality in the United States 1950–1994. 1999. [March 9, 2010]. NIH Publication No. 99-4564, http://www3.cancer.gov/atlasplus/new.html. [DOI] [PubMed] [Google Scholar]
- 25.Brittingham A, de la Cruz GP. Ancestry 2000. Census 2000 Brief C2KBR-35. Vol. 9. Washington DC: US Dept. of Commerce Census Bureau; 2004. [July 18, 2010]. http://www.census.gov/prod/2004pubs/c2kbr-35.pdf. [Google Scholar]
- 26.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S, JPHC Study Group Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009;125:2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 28.Morbois-Trabut L, Chabrolle C, Garrigue MA, Lasfargues G, Lecomte P. Apolipoprotein E genotype and plasma lipid levels in Caucasian diabetic patients. Diabetes Metab. 2006;32:270–275. doi: 10.1016/s1262-3636(07)70279-3. [DOI] [PubMed] [Google Scholar]
- 29.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 30.Rukin NJ, Luscombe C, Moon S, Bodiwala D, Liu S, Saxby MF, et al. Prostate cancer susceptibility is mediated by interactions between exposure to ultraviolet radiation and polymorphisms in the 5′ haplotype block of the vitamin D receptor gene. Cancer Lett. 2007;247:328–335. doi: 10.1016/j.canlet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 31.John EM, Koo J, Schwartz GG. Sun exposure and prostate cancer risk: Evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev. 2007;16:1283–1286. doi: 10.1158/1055-9965.EPI-06-1053. [DOI] [PubMed] [Google Scholar]
- 32.de Vries E, Soerjomataram I, Houterman S, Louwman MW, Coebergh JW. Decreased risk of prostate cancer after skin cancer diagnosis: A protective role of ultraviolet radiation? Am J Epidemiol. 2007;165:966–972. doi: 10.1093/aje/kwk084. [DOI] [PubMed] [Google Scholar]
- 33.Tuohimaa P, Pukkala E, Scelo G, Olsen JH, Brewster DH, Hemminki K, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: Vitamin D as a possible explanation. Eur J Cancer. 2007;43:1701–1712. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Grant WB. The effect of solar UVB doses and vitamin D production, skin cancer action spectra and smoking in explaining links between skin cancers and solid tumours. Eur J Cancer. 2008;44:12–15. doi: 10.1016/j.ejca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization, author. World Health Statistics Annual 1996. Geneva: WHO; 1998. [Google Scholar]
- 36.Helzlsouer KJ. For the VDPP Steering Committee. Overview of the cohort consortium vitamin D pooling project of rarer cancers. Am J Epi. 2010;172:4–9. doi: 10.1093/aje/kwq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant WB. An ecological study of cancer mortality rates in the United States with respect to solar ultraviolet-B doses, smoking, alcohol consumption and urban/rural residence. Deramatoendocrinol. 2010;2:68–76. doi: 10.4161/derm.2.2.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 39.Grant WB, Garland CF. A critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer. 2004;48:115–123. doi: 10.1207/s15327914nc4802_1. [DOI] [PubMed] [Google Scholar]
- 40.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 42.Holmes MD, Hunter DJ, Colditz GA, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA. 1999;281:914–920. doi: 10.1001/jama.281.10.914. [DOI] [PubMed] [Google Scholar]
- 43.Willett WC. Diet and cancer: one view at the start of the millennium. Cancer Epidemiol Biomarkers Prev. 2001;10:3–8. [PubMed] [Google Scholar]
- 44.Linos E, Willett WC, Cho E, Colditz G, Frazier LA. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2146–2151. doi: 10.1158/1055-9965.EPI-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitamin D Action. [February 13, 2011]. http://GrassrootsHealth.net.
- 47.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 48.Edlich RF, Mason SS, Reddig JS, Gubler K, Long Iii WB. Revolutionary advances in the diagnosis of vitamin D deficiency. J Environ Pathol Toxicol Oncol. 2010;29:85–89. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i2.20. [DOI] [PubMed] [Google Scholar]
- 49.Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54:1062–1071. doi: 10.1002/mnfr.200900468. [DOI] [PubMed] [Google Scholar]
- 50.Binkley N, Krueger DC, Morgan S, Wiebe D. Current status of clinical 25-hydroxyvitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta. 2010;411:1976–1982. doi: 10.1016/j.cca.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 52.Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: Serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33:435–445. doi: 10.1016/j.canep.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 54.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8:615–625. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]
- 55.Yaylim-Eraltan I, Arzu Ergen H, Arikan S, Okay E, Ozturk O, Bayrak S, et al. Investigation of the VDR gene polymorphisms association with susceptibility to colorectal cancer. Cell Biochem Funct. 2007;25:731–737. doi: 10.1002/cbf.1386. [DOI] [PubMed] [Google Scholar]
- 56.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97:446–451. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss E, Flesch-Janys D, et al. Serum 25-hydroxyvitamin D and risk of postmenopausal breast cancer—results of a large case-control study. Carcinogenesis. 2008;29:93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 60.Abbas S, Chang-Claude J, Linseisen J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2009;124:250–255. doi: 10.1002/ijc.23904. [DOI] [PubMed] [Google Scholar]
- 61.Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–2156. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, et al. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev. 2009;18:2655–2660. doi: 10.1158/1055-9965.EPI-09-0531. [DOI] [PubMed] [Google Scholar]
- 63.Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, et al. Serum levels of vitamin D metabolites and breast cancer risk in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11:64. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 66.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A Nested case-control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 68.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 69.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 70.Travis RC, Crowe FL, Allen NE, Appleby PN, Roddam AW, Tjønneland A, et al. Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Epidemiol. 2009;169:1223–1232. doi: 10.1093/aje/kwp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faupel-Badger JM, Diaw L, Albanes D, Virtamo J, Woodson K, Tangrea JA. Lack of association between serum levels of 25-hydroxyvitamin D and the subsequent risk of prostate cancer in Finnish men. Cancer Epidemiol Biomarkers Prev. 2007;16:2784–2786. doi: 10.1158/1055-9965.EPI-07-0672. [DOI] [PubMed] [Google Scholar]
- 72.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, et al. Serum vitamin D concentration and prostate cancer risk: A nested case-control study. J Natl Cancer Inst. 2008;11:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baron JA, Beach M, Wallace K, Grau MV, Sandler RS, Mandel JS, et al. Risk of prostate cancer in a randomized clinical trial of calcium supplementation. Cancer Epidemiol Biomarkers Prev. 2005;14:586–589. doi: 10.1158/1055-9965.EPI-04-0319. [DOI] [PubMed] [Google Scholar]
- 74.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108:104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 75.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Prostate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States) Cancer Causes Control. 1995;6:235–239. doi: 10.1007/BF00051795. [DOI] [PubMed] [Google Scholar]
- 76.Jacobs ET, Giuliano AR, Martinez ME, Hollis BW, Reid ME, Marshall JR. Plasma levels of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and the risk of prostate cancer. J Steroid Biochem Mol Biol. 2004;89-90:533–537. doi: 10.1016/j.jsbmb.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 77.Nomura AM, Stemmermann GN, Lee J, Kolonel LN, Chen TC, Turner A, et al. Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States) Cancer Causes Control. 1998;9:425–432. doi: 10.1023/a:1008875819232. [DOI] [PubMed] [Google Scholar]