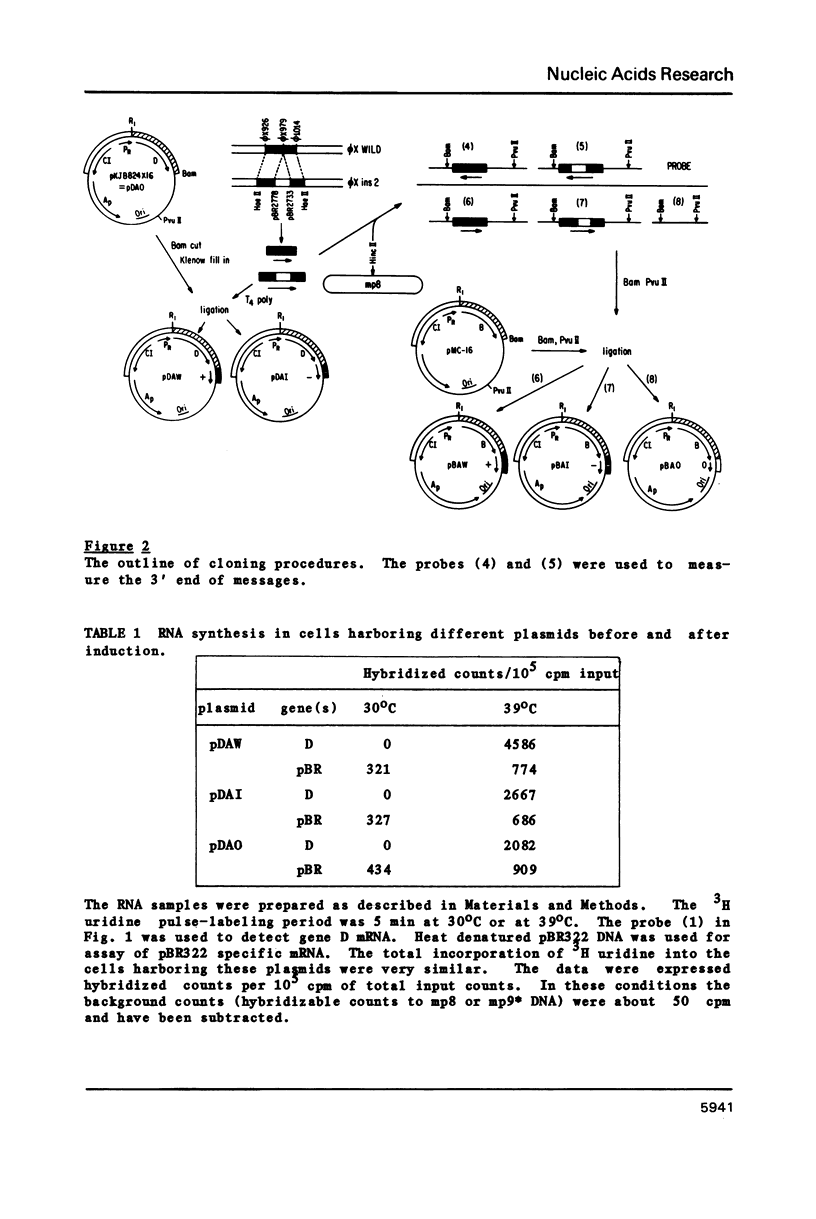
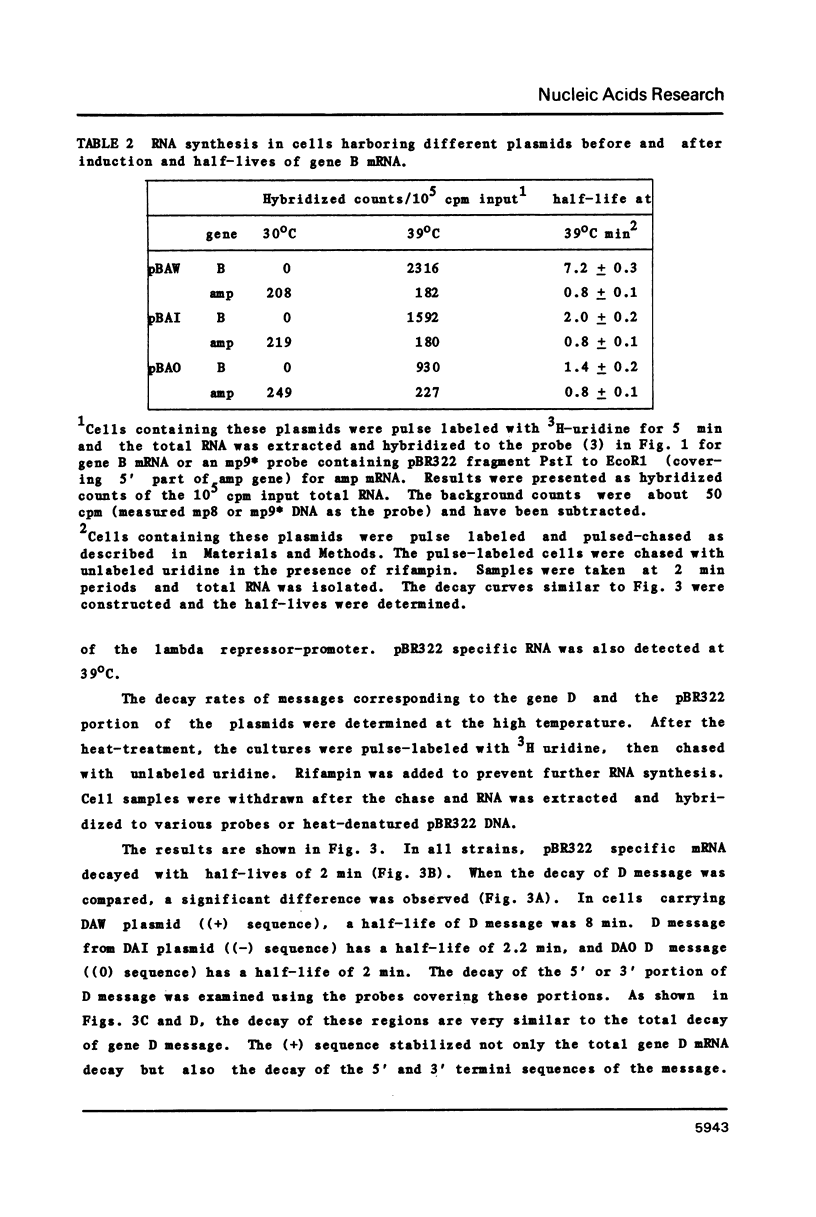
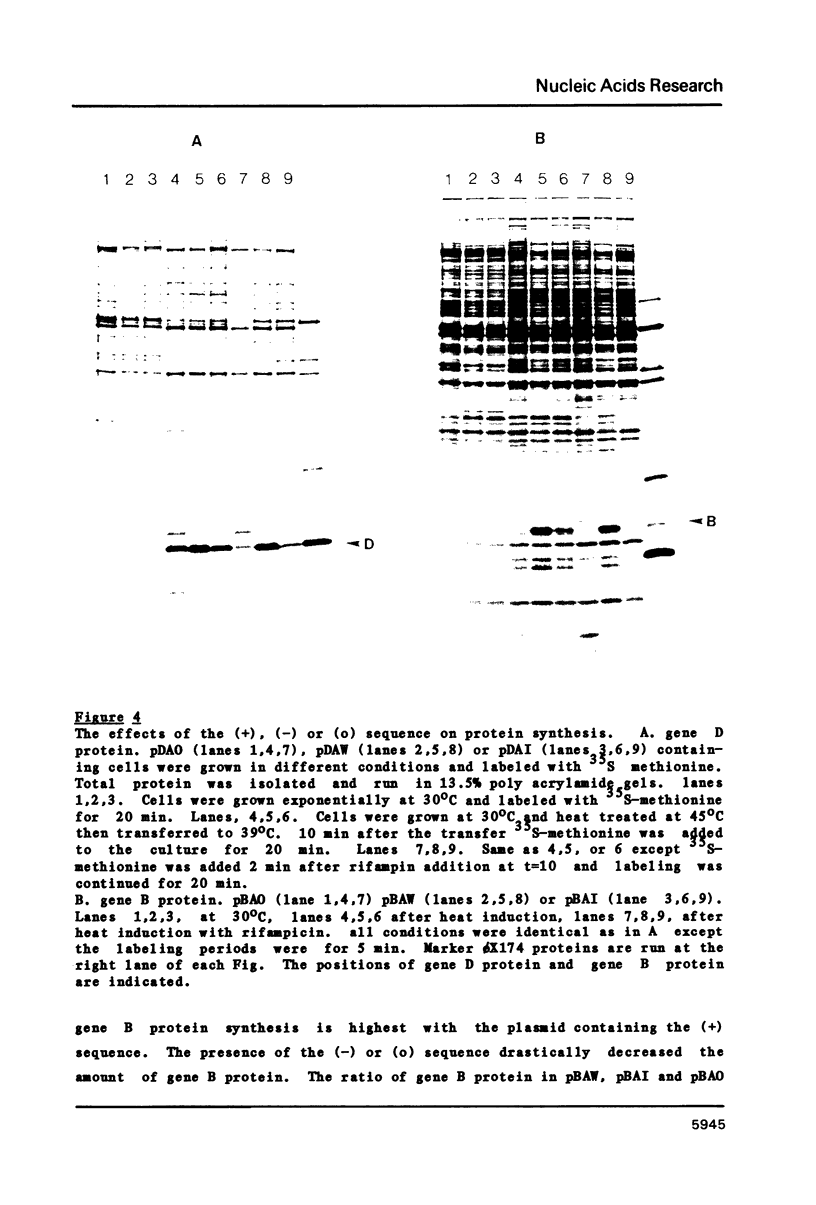
Abstract
The stability of two species of phi X174 polycistronic mRNA in vivo can be altered by mutating sequences existing immediately upstream of a termination site. The wild type phage contains an mRNA stabilizing sequence ((+) sequence), while the same sequence mutated by insertion ((-) sequence) reduces the stability of the mRNAs. These two sequences were cloned at the 3' ends of gene D or gene B of phi X174 in a pBR322 derivative plasmid. The cloned sequences were functional. The (+) sequence stabilized gene B or gene D mRNA; half-lives of these mRNAs were 7 to 8 min. When the (+) sequence is eliminated ((o) sequence) or replaced with the (-) sequence, the half-lives of the mRNA were reduced to about 1 to 2 min. The stabilization of mRNAs caused an increased production of these proteins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala J. A., Bassett C. L., Giles N. H., Kushner S. R. Increased expression of a eukaryotic gene in Escherichia coli through stabilization of its messenger RNA. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5774–5778. doi: 10.1073/pnas.76.11.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M., Müller U. R. Role for the J-F intercistronic region of bacteriophages phi X174 and G4 in stability of mRNA. J Virol. 1983 Oct;48(1):186–196. doi: 10.1128/jvi.48.1.186-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Stability of bacteriophage phi X174-specific mRNA in vivo. J Virol. 1981 Jan;37(1):506–510. doi: 10.1128/jvi.37.1.506-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mukai R., Hamatake R. K., Hayashi M. Isolation and identification of bacteriophage phi X174 prohead. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4877–4881. doi: 10.1073/pnas.76.10.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]