Abstract
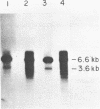
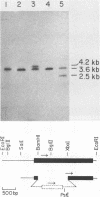
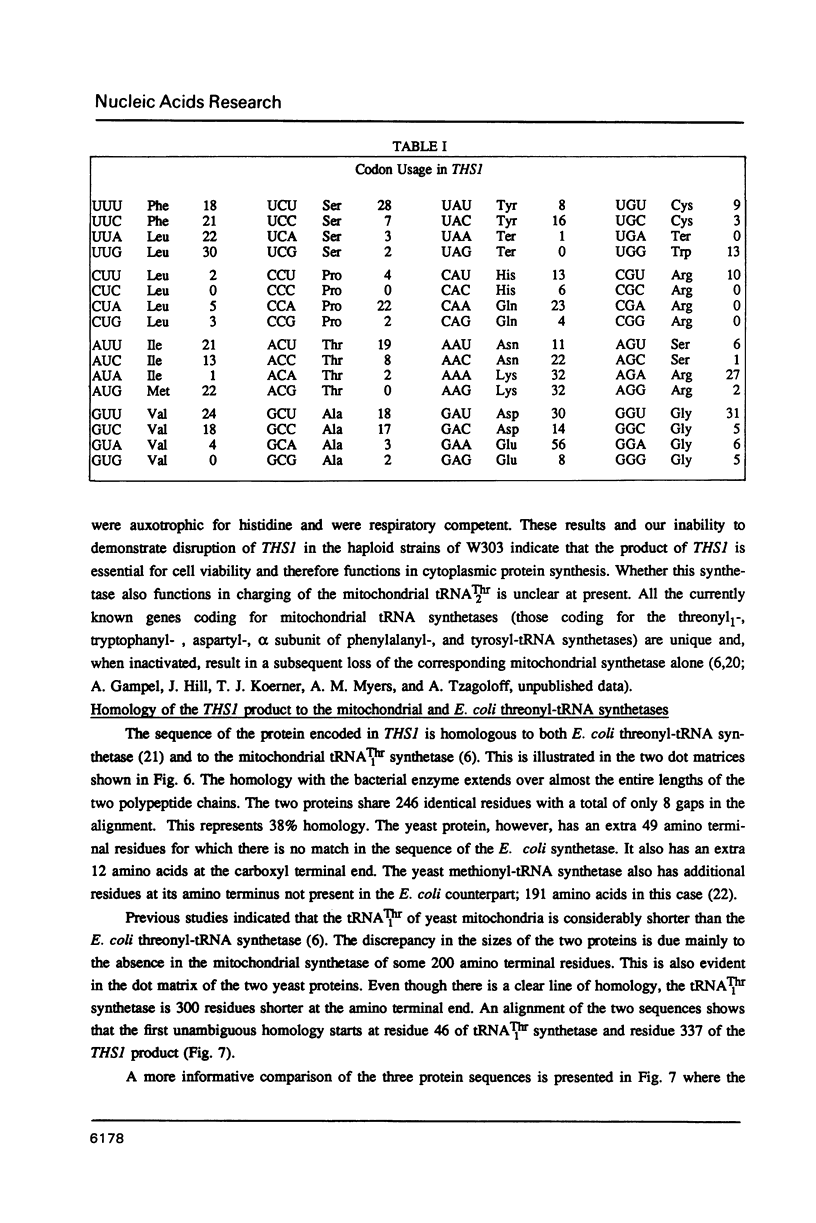
A fragment of DNA from the yeast nuclear gene MST1 that codes for the mitochondrial tRNAThr1 synthetase was used as a probe to screen for other yeast threonyl-tRNA synthetase genes. At low stringency, the MST1 probe hybridizes strongly to a 6.6 kb EcoRI fragment of yeast genomic DNA with the homologous gene and in addition hybridizes more weakly to a smaller 3.6 kb EcoRI fragment with a second threonyl-tRNA synthetase gene (THS1). To clone THS1, a library was constructed by ligation to pUC18 of size selected (3-4.5 kb) EcoRI fragments of genomic DNA. Several clones containing the 3.6 kb EcoRI fragment were isolated. A 2,202 nucleotide long open reading frame corresponding to THS1 has been identified in the cloned fragment of DNA. The predicted protein encoded by THS1 is 38% identical to the E. coli threonyl-tRNA synthetase over the latter's length (642 amino acids) and is 42% identical to the predicted MST1 product over its 462 residues. In situ disruption of the chromosomal copy of THS1 is lethal to the cell, indicating that this gene codes for the cytoplasmic threonyl-tRNA synthetase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann J. S., Johnson P. F., Abelson J. Cloning of yeast transfer RNA genes in Escherichia coli. Science. 1977 Apr 8;196(4286):205–208. doi: 10.1126/science.322282. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Specific interaction of anticodon loop residues with yeast phenylalanyl-tRNA synthetase. Biochemistry. 1982 Aug 17;21(17):3921–3926. doi: 10.1021/bi00260a003. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Mutants of yeast with temperature-sensitive isoleucyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1968 Feb;59(2):422–428. doi: 10.1073/pnas.59.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: two separate genes coding for threonyl-tRNA in the mitochondrial DNA of Saccharomyces cerevisiae. Mol Gen Genet. 1979 Jan 31;169(2):183–188. doi: 10.1007/BF00271669. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaux J. F., Fayat G., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Structural and transcriptional evidence for related thrS and infC expression. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6152–6156. doi: 10.1073/pnas.80.20.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Hartwell L. H. A mutant of yeast with a defective methionyl-tRNA synthetase. Genetics. 1969 Mar;61(3):557–566. doi: 10.1093/genetics/61.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P., Ludmerer S. W. Identification of a glutaminyl-tRNA synthetase mutation Saccharomyces cerevisiae. J Bacteriol. 1984 May;158(2):530–534. doi: 10.1128/jb.158.2.530-534.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G., Poralla K. Genetics of borrelidin resistant mutants of Saccharomyces cerivisiae and properties of their threonyl-tRNA-synthetase. Mol Gen Genet. 1976 Aug 10;147(1):39–43. doi: 10.1007/BF00337933. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Ramos F., Wiame J. M. Synthesis and activation of asparagine in asparagine auxotrophs of Saccharomyces cerevisiae. Eur J Biochem. 1979 Mar;94(2):409–417. doi: 10.1111/j.1432-1033.1979.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Recognition of tRNAs by aminoacyl-tRNA synthetases: Escherichia coli tRNAMet and E. coli methionyl-tRNA synthetase. Fed Proc. 1984 Dec;43(15):2977–2980. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walter P., Gangloff J., Bonnet J., Boulanger Y., Ebel J. P., Fasiolo F. Primary structure of the Saccharomyces cerevisiae gene for methionyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1983 May;80(9):2437–2441. doi: 10.1073/pnas.80.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]