Abstract
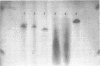
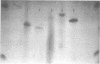
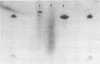
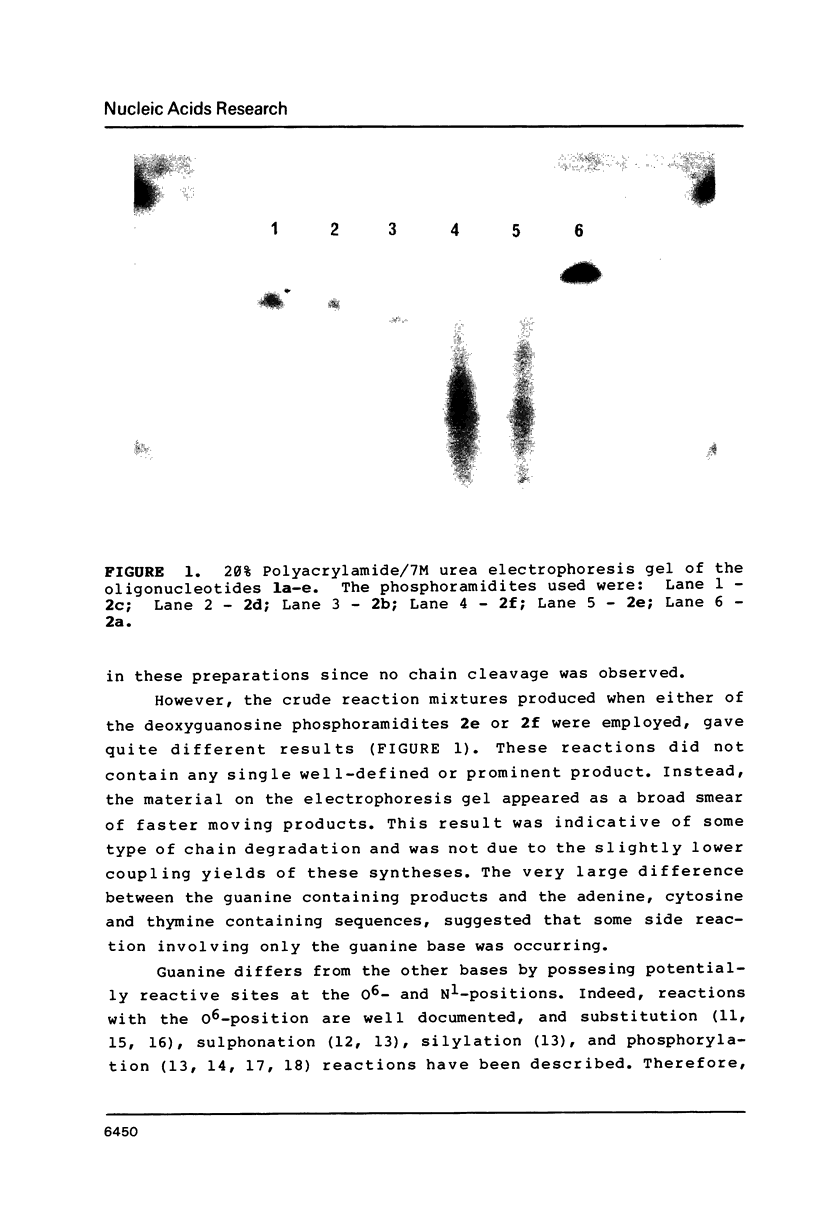
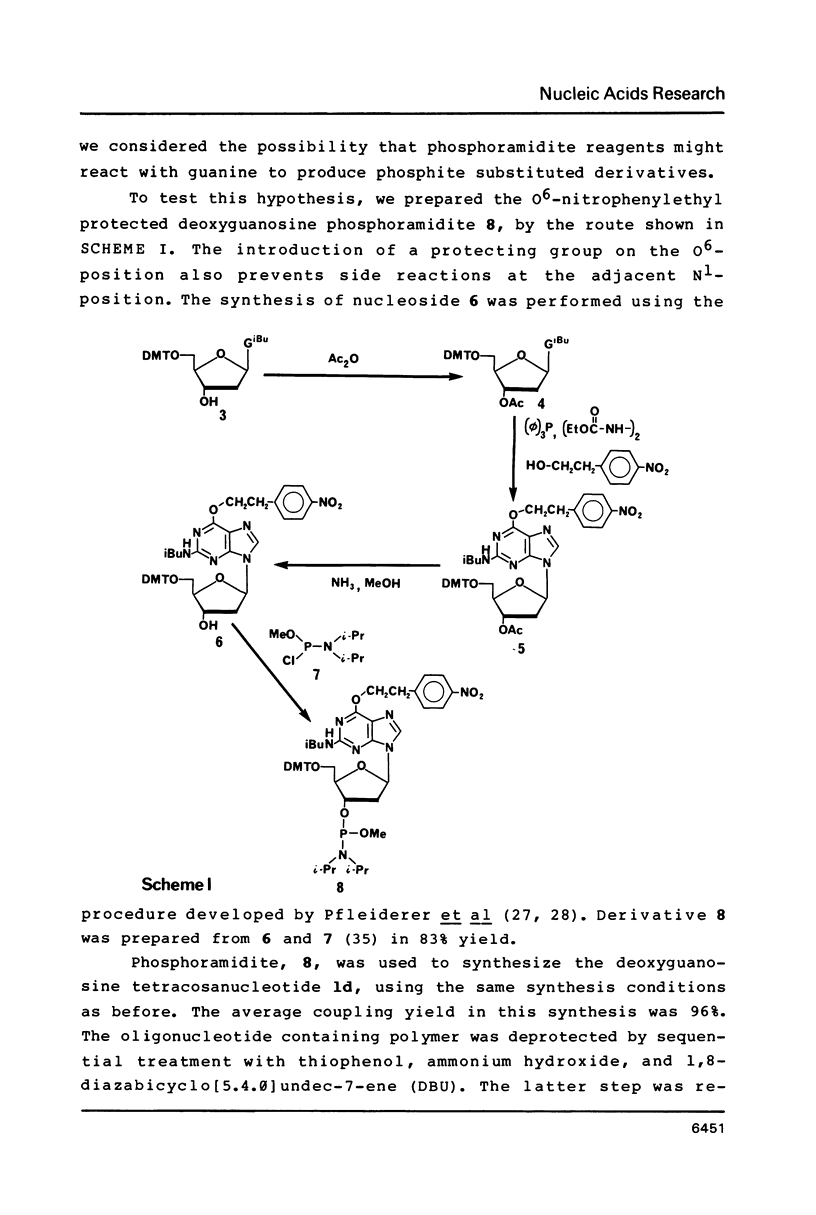
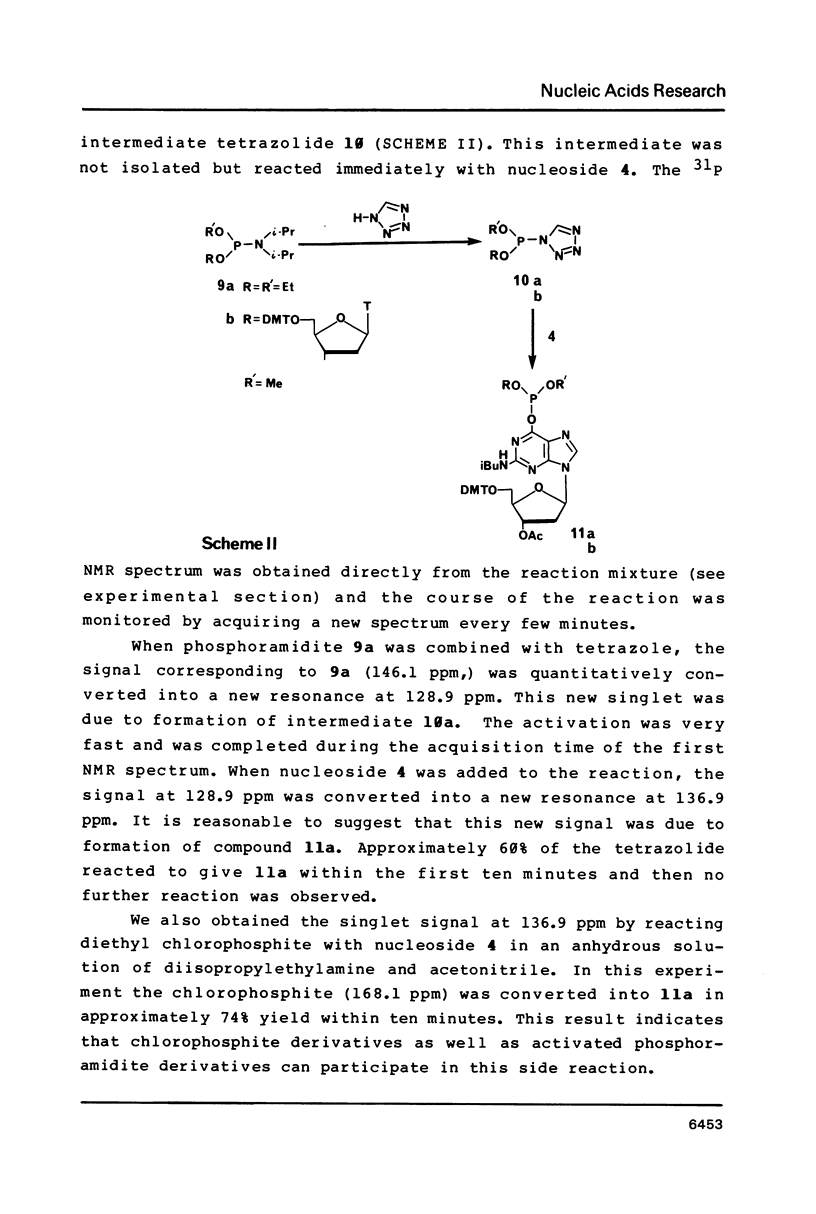
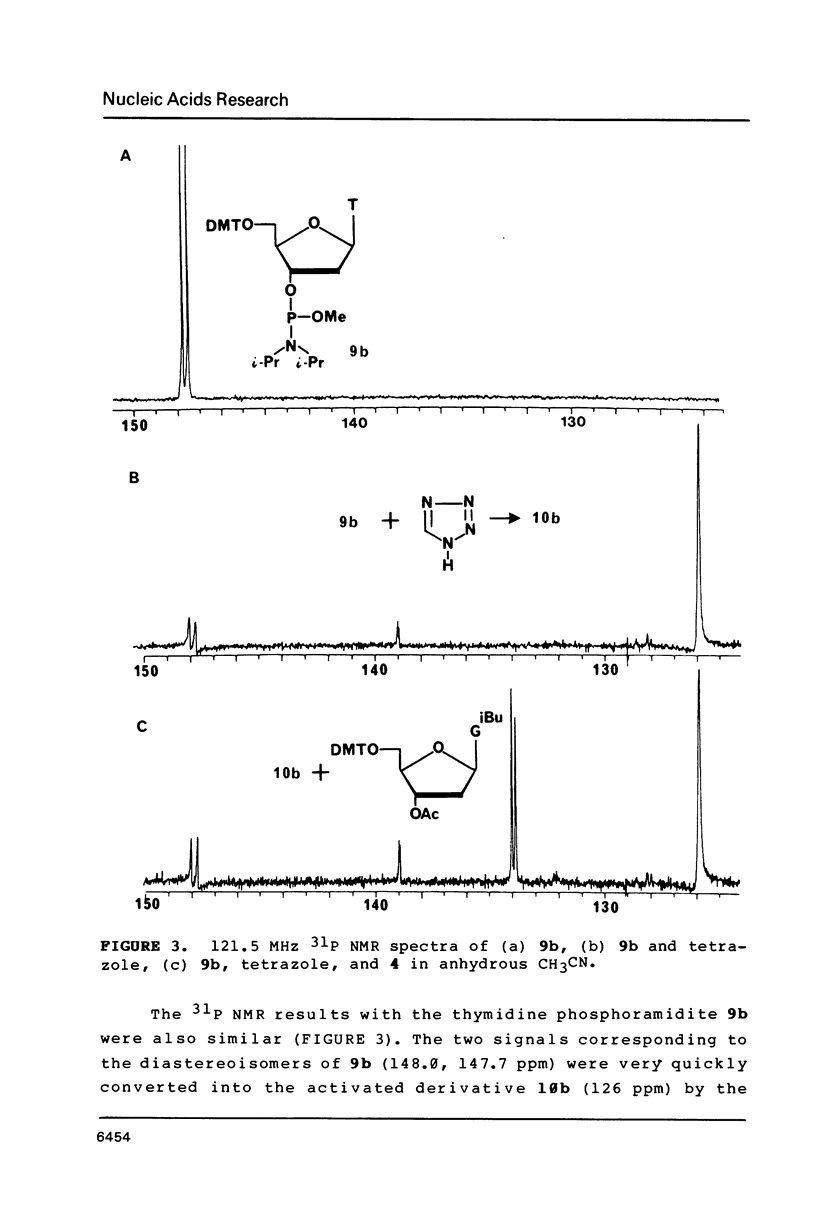
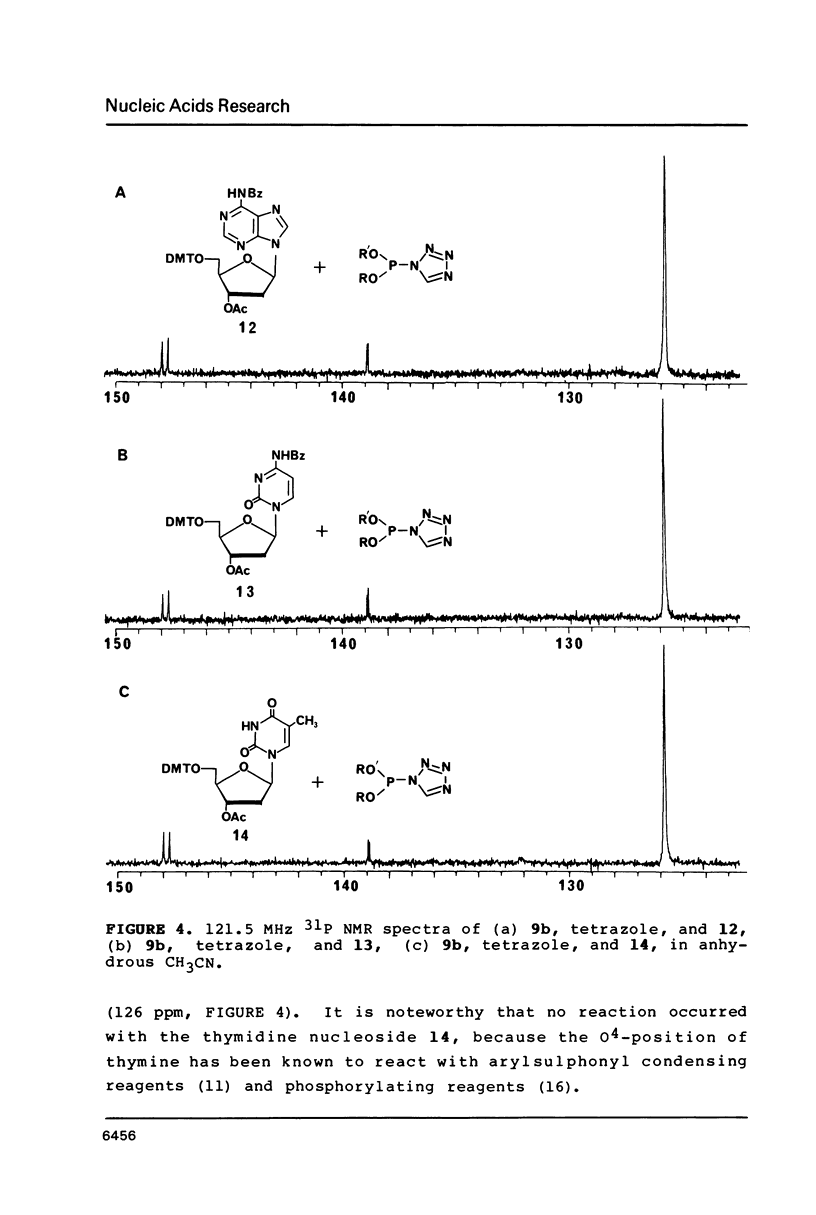
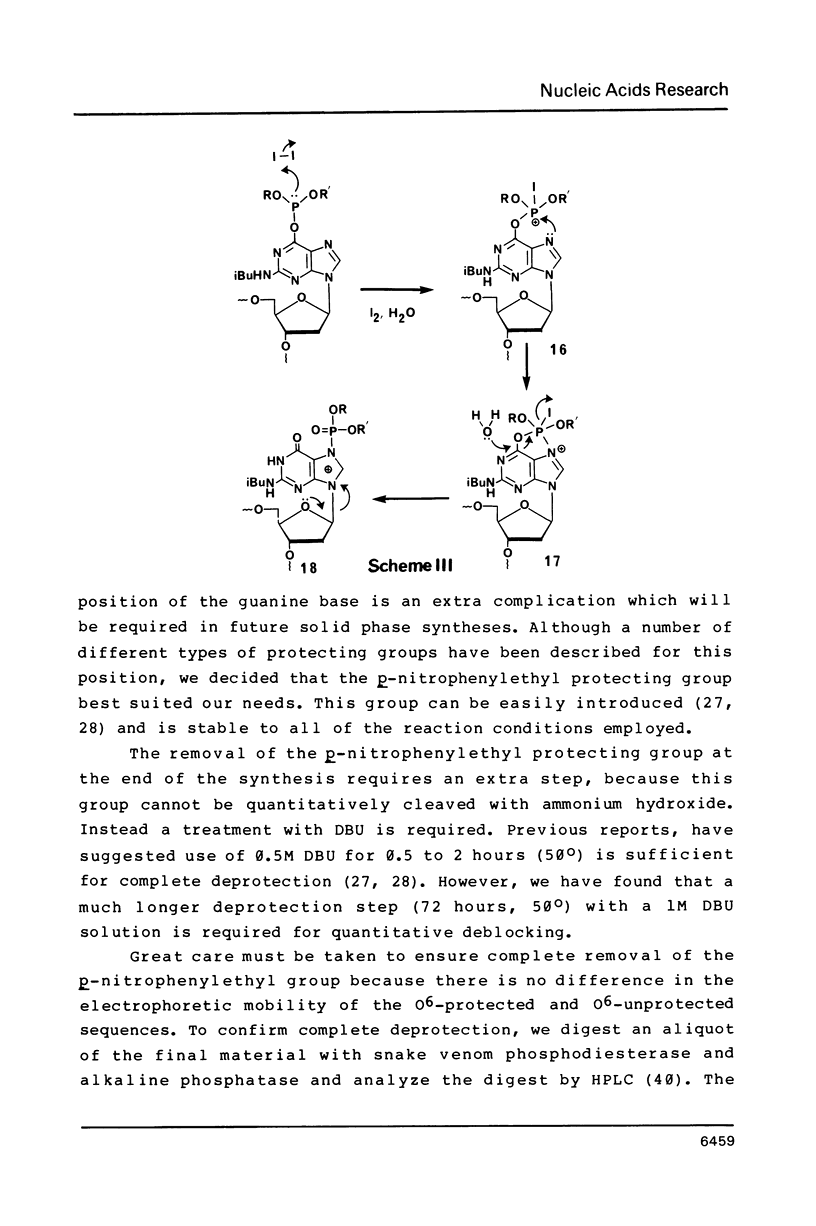
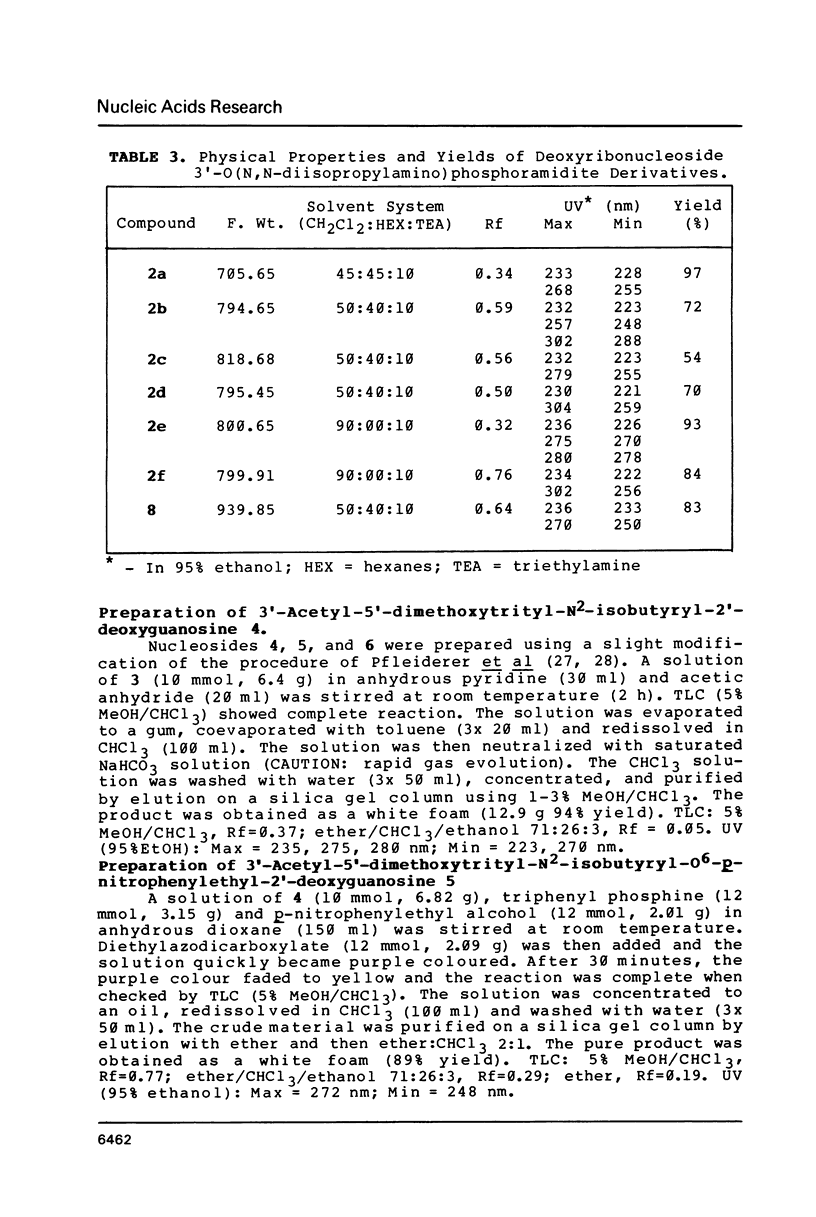
Nucleoside 3'-phosphoramidite and chlorophosphite reagents have been found to react with the lactam function of guanine. This reaction caused unsatisfactory results when oligodeoxyribonucleotides containing a large number of guanine bases were prepared in an automated solid phase synthesizer. The guanine modification is unstable, and leads to depurination and chain cleavage. This side reaction can be eliminated by protecting the O6-position. A new O6-p-nitrophenylethyldeoxyguanosine phosphoramidite derivative, 8, was used to prepare sequences containing up to 24 guanine bases with greatly improved results. A hexatriacontanucleotide, d(CGCGGGGTGGAGCAGCCTGGTAGCTCGTCGGGCTCA), was also prepared using O6-protected deoxyguanosine nucleosides.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado-Urbina G., Sathe G. M., Liu W. C., Gillen M. F., Duck P. D., Bender R., Ogilvie K. K. Automated synthesis of gene fragments. Science. 1981 Oct 16;214(4518):270–274. doi: 10.1126/science.6169150. [DOI] [PubMed] [Google Scholar]
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R., Köster H. DNA chain length markers and the influence of base composition on electrophoretic mobility of oligodeoxyribonucleotides in polyacrylamide-gels. Nucleic Acids Res. 1979;6(6):2069–2087. doi: 10.1093/nar/6.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Gaffney B. L., Senior M., Riddle R. R., Jones R. A. Methylation of thymine residues during oligonucleotide synthesis. Nucleic Acids Res. 1985 Jan 25;13(2):573–584. doi: 10.1093/nar/13.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Yamane A., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XLV. Block condensation of ribooligonucleotides containing 2'-O-tetrahydrofuranyl-5'-O-dimethoxytritylnucleosides. Nucleic Acids Res. 1983 Mar 11;11(5):1325–1335. doi: 10.1093/nar/11.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. P., Chauncey M. A., Millican T. A., Bose C. C., Eaton M. A. A rapid deprotection procedure for phosphotriester DNA synthesis. Nucleic Acids Res. 1984 Sep 11;12(17):6853–6859. doi: 10.1093/nar/12.17.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Driller H. Solid-phase synthesis of the self-complementary hexamer d(c7GpCpc7GpCpc7GpC) via the O-3'-phosphoramidite of 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1985 Feb 11;13(3):911–926. doi: 10.1093/nar/13.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner B. D., Warner M. E., Karns G. A., Ku L., Brown-Shimer S., Urdea M. S. Construction and evaluation of an instrument for the automated synthesis of oligodeoxyribonucleotides. DNA. 1984 Oct;3(5):401–411. doi: 10.1089/dna.1984.3.401. [DOI] [PubMed] [Google Scholar]