Abstract
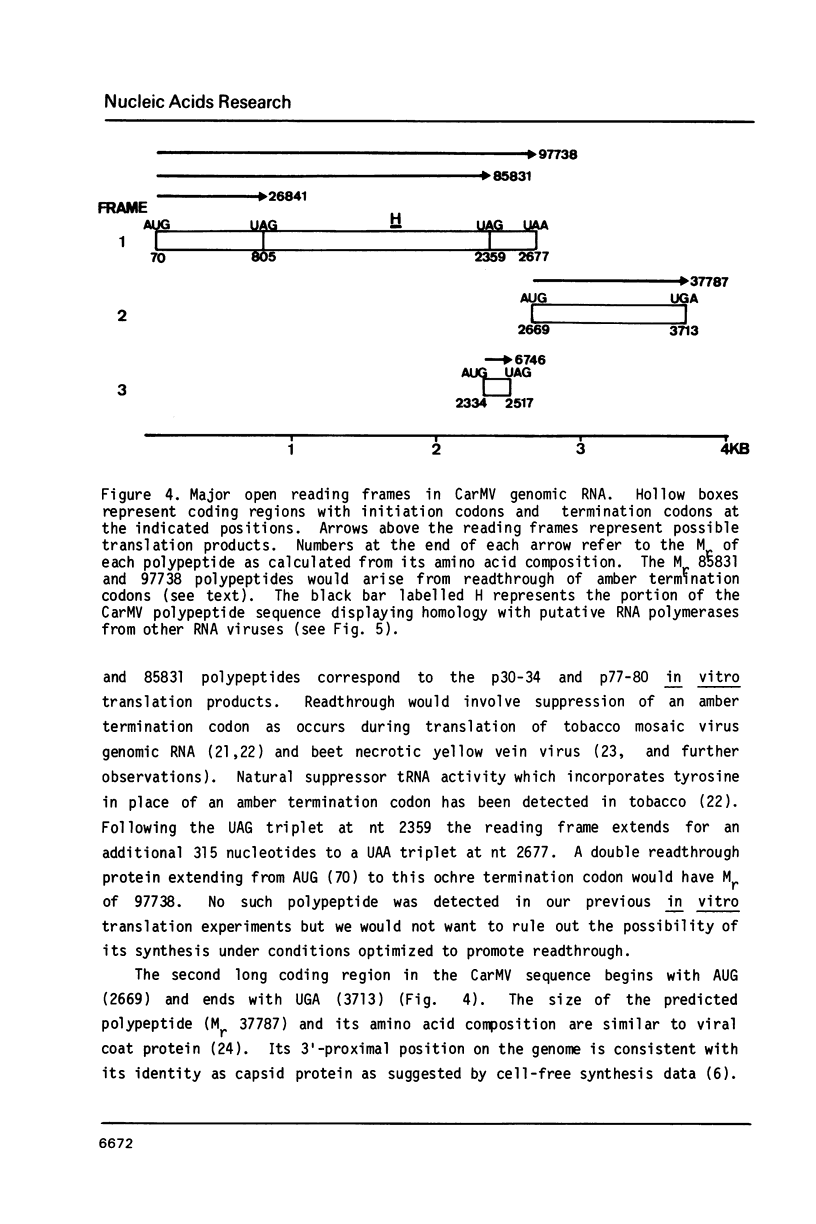
The complete nucleotide sequence of carnation mottle genomic RNA (4003 nucleotides) is presented. The sequence was determined for cloned cDNA copies of viral RNA containing over 99% of the sequence and was completed by direct sequence analysis of RNA and cDNA transcripts. The sequence contains two long open reading frames which together can account for observed translation products. One translation product would arise by suppression of an amber termination codon and the sequence raises the possibility that a second suppression event could also occur. Sequence homology exists between a portion of the carnation mottle virus sequence and that of putative RNA polymerases from other RNA viruses.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Ghosh A., Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985 Mar 20;182(2):183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide-sequence heterogeneity and sequence rearrangements in influenza virus cDNA. Gene. 1981 Nov;15(2-3):207–214. doi: 10.1016/0378-1119(81)90130-x. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Harbison S. A., Wilson T. M., Davies J. W. An encapsidated, subgenomic messenger RNA encodes the coat protein of carnation mottle virus. Biosci Rep. 1984 Nov;4(11):949–956. doi: 10.1007/BF01116893. [DOI] [PubMed] [Google Scholar]
- Harrison S. C. Virus structure: high-resolution perspectives. Adv Virus Res. 1983;28:175–240. doi: 10.1016/s0065-3527(08)60724-1. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper P., Harrison S. C., Sauer R. T. Structure of tomato bushy stunt virus. V. Coat protein sequence determination and its structural implications. J Mol Biol. 1984 Aug 25;177(4):701–713. doi: 10.1016/0022-2836(84)90045-7. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M., Waterworth H. E. Comparison of molecular weights of single-stranded viral RNAs by two empirical methods. Virology. 1973 Jan;51(1):183–190. doi: 10.1016/0042-6822(73)90378-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Richards K., Guilley H., Jonard G., Hirth L. Nucleotide sequence at the 5' extremity of tobacco-mosaic-virus RNA. 1. The noncoding region (nucleotides 1-68). Eur J Biochem. 1978 Mar 15;84(2):513–519. doi: 10.1111/j.1432-1033.1978.tb12194.x. [DOI] [PubMed] [Google Scholar]
- Richards K., Guilley H., Jonard G., Keith G. Leader sequence of 71 nucleotides devoid of G in tobacco mosaic virus RNA. Nature. 1977 Jun 9;267(5611):548–550. doi: 10.1038/267548a0. [DOI] [PubMed] [Google Scholar]
- Salomon R., Bar-Joseph M., Soreq H., Gozes I., Littauer U. Z. Translation in vitro of carnation mottle virus RNA. Regulatory function of the 3'-region. Virology. 1978 Oct 15;90(2):288–298. doi: 10.1016/0042-6822(78)90313-6. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tremaine J. H., Goldsack D. E. The structure of regular viruses in relation to their subunit amino acid composition. Virology. 1968 Jun;35(2):227–237. doi: 10.1016/0042-6822(68)90263-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Tavernier J., Derynck R., Devos R., Fiers W. Molecular mechanisms of nucleotide-sequence rearrangements in cDNA clones of human fibroblast interferon mRNA. Gene. 1981 Nov;15(2-3):215–223. doi: 10.1016/0378-1119(81)90131-1. [DOI] [PubMed] [Google Scholar]



