Abstract
Graft-versus-host disease (GVHD) is a life-threatening complication of human allogeneic haematopoietic stem cell transplantation. Non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice injected with human peripheral blood mononuclear cells (PBMC) engraft at high levels and develop a robust xenogeneic (xeno)-GVHD, which reproduces many aspects of the clinical disease. Here we show that enriched and purified human CD4 T cells engraft readily in NSG mice and mediate xeno-GVHD, although with slower kinetics compared to injection of whole PBMC. Moreover, purified human CD4 T cells engraft but do not induce a GVHD in NSG mice that lack murine MHC class II (NSG-H2-Ab1 tm1Gru, NSG-Ab°), demonstrating the importance of murine major histocompatibility complex (MHC) class II in the CD4-mediated xeno-response. Injection of purified human CD4 T cells from a DR4-negative donor into a newly developed NSG mouse strain that expresses human leucocyte antigen D-related 4 (HLA-DR4) but not murine class II (NSG-Ab° DR4) induces an allogeneic GVHD characterized by weight loss, fur loss, infiltration of human cells in skin, lung and liver and a high level of mortality. The ability of human CD4 T cells to mediate an allo-GVHD in NSG-Ab° DR4 mice suggests that this model will be useful to investigate acute allo-GVHD pathogenesis and to evaluate human specific therapies.
Keywords: graft-versus-host disease, IL-2R ‘common’ gamma chain, SCID, T cell
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is an important therapeutic option for the treatment of human malignancies and genetic disorders, including neuroblastoma, lymphoma, leukaemia, multiple myeloma, solid tumours and sickle cell disease [1–3]. However, the beneficial effects of allo-HSCT are limited by the development of graft-versus-host disease (GVHD), which is mediated by donor immune cells responding to host antigens and occurs even in the presence of immune suppression [4–6]. The GVHD response in humans is mediated by both innate and adaptive immune cells, but mature donor T cells residing within the transplanted tissues are considered the primary effector populations [7]. Ironically, inclusion of T cells with allo-HSCT promotes overall engraftment and reconstitution of protective immunity and, in cases of malignancy, provides a beneficial anti-tumour response [8].
GVHD is initiated by the cytoreductive conditioning regimens required for allo-HSCT, including radiation therapy and chemotherapy [9]. These regimens stimulate the release of proinflammatory cytokines such as interleukin (IL)-1 and tumour necrosis factor (TNF) and induce damage in the gastrointestinal tract, resulting in exposure to microbial products such as lipopolysaccharide (LPS) [10–13]. The induced inflammatory response triggers increased expression of major histocompatibility complex (MHC), co-stimulatory molecules, adhesion molecules and chemokines that promote the activation of donor immune cells [6]. As a component of GVHD, donor T cells recognize host antigens through both direct and indirect antigen presentation mechanisms, and the secondary signals provided by the initial inflammation augment their expansion and differentiation [4,5]. The activated host-reactive donor T cells then traffic to specific sites, including gut, skin, liver and lung, and mediate damage indirectly by the release of cytokines and directly by cytolysis through release of cytotoxic granules and CD95–CD95L (FAS–FASL) pathways [14]. Much of our knowledge on the complex disease course and immunobiology of GVHD is based on studies conducted with non-human cells in animal models, and questions remain regarding the relevance of these findings to the human disease [15].
The use of humanized models to study the ability of human T cells to mediate xenogeneic GVHD (xeno-GVHD) is an exciting approach to investigate the disease process [16]. Early attempts to study xeno-GVHD used CB17-scid mice or non-obese diabetic (NOD)-scid mice as recipients of human peripheral blood mononuclear cells (PBMC), but were unsuccessful due to suboptimal and highly variable levels of human cell engraftment [17–23]. Numerous strategies have been employed to increase engraftment of human PBMC in immunodeficient mice and trigger the development of xeno-GVHD, including elimination of host natural killer (NK) cells by antibody depletion and genetic mutation, irradiation preconditioning and the depletion of macrophages by injection of chlodronate-containing liposomes [24–34]. While these strategies increased the engraftment of human PBMC, the induction of xeno-GVHD was variable and often required the injection of large numbers of human PBMC (30–50 × 106).
The major advancement that dramatically enhanced the engraftment of human PBMC was the development of scid, Rag1null or Rag2null mouse stocks bearing mutations in the IL-2 receptor common γ-chain (IL-2rγ chain) [32,35–39]. The IL-2rγ chain is critical for high-affinity signalling through the IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 receptors, and deficiencies in this gene result in significant defects in both adaptive and innate immunity, including the complete elimination of mature NK cells [35,40]. We have recently developed a robust model of xeno-GVHD using the NOD-scid IL2rγnull (NSG) mouse stock [35–37]. In this system, injection of as few as 5 × 106 human PBMC into lightly irradiated (2 Gy) NSG mice supports high levels of engraftment and results in 100% lethality within 30 days [36]. Moreover, this model of xeno-GVHD has been used to evaluate therapeutic strategies to prevent disease [36,41–43], demonstrating its potential utility for the study of GVHD and for the targeted identification of the underlying mechanisms.
Here we test the ability of human CD4 T cells to induce GVHD. We first show that both enriched and purified human CD4 T cells engraft in NSG mice and mediate a xeno-GVHD. We next extend these data to describe a novel model that now permits study of allo-GVHD mediated by human CD4+ T cells in a new stock of NSG mice. For these studies, we developed a NSG mouse stock that lacks murine MHC class II but expresses HLA-DRB1-0401 [44] (NSG-Ab° DR4 mice). Injection of purified human DR4-negative CD4+ T cells into NSG-Ab° DR4 transgenic mice results in the development of an allo-GVHD in the absence of a xeno-GVHD. These findings indicate that the NSG mouse models can be used to dissect the immunobiology of both xeno- and allo-GVHD and will allow the in vivo testing of novel therapeutic agents targeting human CD4 T cells.
Materials and methods
Animals
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD-scid IL2rγnull, NSG), NOD.Cg-PrkdcscidIl2rgtm1WjlH2-Ab1tm1Gru/Sz (NOD-scid IL2rγnull Ab°, NSG-Ab°) and NOD.Cg-PrkdcscidIl2rgtm1WjlH2-Ab1tm1Gru/Tg(HLA-DRB1)31Dmz/Sz[NOD-scid IL2rγnull Ab° Tg(HLA-DR4), NSG-Ab° DR4] mice were obtained from colonies developed and maintained by L.D.S. at The Jackson Laboratory (Bar Harbor, ME, USA). NOD (and NSG) mice are deficient in the I-E alpha chain but do express transcripts for the I-E beta chain. NOD mice hemizygous for the HLA-DR4 (DRB1*0401) transgene were obtained originally from Dr Linda Wicker at Merck Research Laboratories. The DR4 transgene was constructed as a chimeric molecule composed of DR4α1 and β1 domains and I-Eα2 and β2 domains. The DR4 transgene was crossed onto the NOD.Cg-PrkdcscidIl2rgtm1WjlH2-Ab1tm1Gru/Sz background and the NOD-scid IL2rgnull Ab° Tg(HLA-DR4 stock) is maintained by continuous back-cross of the DR4 transgene in the hemizygous state [45].
All animals were housed in a specific pathogen-free facility in micro-isolator cages, and given autoclaved food and maintained on acidified autoclaved water and sulphamethoxazole–trimethoprim-medicated water Goldline Laboratories (Fort Lauderdale, FL, USA) provided on alternate weeks. All animal use was in accordance with the guidelines of the Animal Care and Use Committee of the University of Massachusetts Medical School and The Jackson Laboratory and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Collection of human PBMC
Human PBMC were collected from healthy volunteers under signed informed consent in accordance with the Declaration of Helsinki and approval from the Institutional Review Board of the University of Massachusetts Medical School. PBMC were collected in heparin and purified by Ficoll-Hypaque density centrifugation and suspended in RPMI-1640 for injection into mice at the cell doses indicated.
HLA-DRB1-04 genotyping
HLA-DRB1-04 (HLA-DR4)-negative individuals were identified by real-time polymerase chain reaction (PCR) using a LightCycler (Roche Diagnostics, Indianapolis, IN, USA). Briefly, DNA was extracted from 1 × 106 donor PBMC using the High Pure PCR Template Preparation Kit (Roche). Genomic HLA-DRB1-04 DNA was amplified using the following forward primer: 5′-GTTTCTTGGAGCAGGTTAAACA-3′ and two reverse primers in the same reaction: 5′-CTGCACTGTGAAGCTCTCAC-3′, 5′-CTGCACTGTGAAGCTCTCCA-3′[46]. The following cycling parameters were used: after an initial melt of 2 min at 95°C, 40 cycles of 95°C, 10 s; 68°C, 10 s; 72°C, 23 s with a single acquisition per cycle at 72°C. All temperature transitions were 20°C/s. Samples were then subjected to a melting curve analysis with the following conditions: 95°C, 0 s (slope 20°/s); 65°C, 10 s (slope 20°/s) and then heated to 95°C with a slope of 0·3°/s using step acquisition. Positive and negative samples were distinguished by the presence or absence of fluorescence signal during the PCR reaction and the presence of a melting peak matching that of the positive control cell lines (∼91°C).
Fractionation of human T cells
Ficoll-Hypaque-purified human CD4 T cells were fractionated from whole PBMC using magnetic bead separation technology (Miltenyi Biotec Inc., Auburn, CA, USA). CD4 T cells were fractionated by either negative selection to recover enriched populations (E) or positive selection to recover purified populations (P). To obtain enriched populations of CD4 T cells (CD4-E), PBMC were incubated with anti-CD8 microbeads and the flow-through was collected from a LD column. This approach is successful because human T cells are the primary populations that survive and engraft in NSG mice [37]. To obtain purified populations of CD4 T cells (CD4-P), PBMC were incubated with anti-CD4 microbeads and cells retained within a LS column were recovered. The purity of the recovered populations was confirmed by flow cytometry (Fig. 1). To inject the corresponding number of CD4 cells found on average in 10 × 106 unfractionated PBMC, 4 × 106 CD4-P or 7 × 106 enriched CD4-E were injected intravenously into the indicated mice.
Fig. 1.
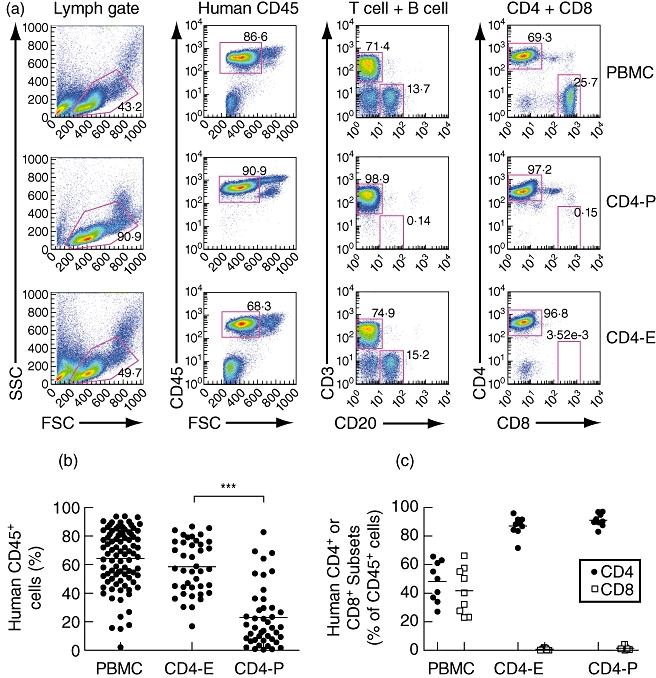
Isolation of human CD4 T cells and engraftment in the peripheral blood of non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice. Human CD4 T cells were fractionated from total peripheral blood mononuclear cells (PBMC) using either negative (E, enriched) or positive (P, purified) selection as described in Materials and methods. (a) The purity of the recovered populations was confirmed using flow cytometry and representative data are shown for both CD4-P and CD4-E compared to the starting population of PBMC. (b) Engraftment of human CD45-positive cells was evaluated at 2 weeks in recipient NSG mice injected with PBMC (10 × 106 total cells), CD4-E (7 × 106 total cells) or CD4-P cells (4 × 106 total cells). (c) The engraftment of human CD4 T cells was evaluated 2 weeks after injection. The values shown represent the percentage of CD4 or CD8 T cells within the human CD45 population. Data are representative of at least two PBMC donors, and cells from each donor were injected into multiple mice. Each symbol represents an individual mouse. ***P < 0·001.
For the allo-GVHD experiments, CD4-P cells were generated by two successive purifications, first by depletion of CD8+ T cells and then a positive selection of CD4+ T cells. This two-step approach led to reproducible recovery of highly pure CD4 T cell populations (> 99%).
Induction of xeno- and allo-GVHD by human T cells
The induction of xeno-GVHD was performed as described [36,37]. Briefly, NSG mice were irradiated with 2 Gy and injected intravenously at least 4 h later with indicated populations and doses of Ficoll-Hypaque-purified human PBMC. The induction of allo-GVHD was performed by injecting purified populations of human CD4 T cells (4 × 106 total cells) into NSG-Ab° DR4. In all experiments, mice were weighed two to three times weekly and monitored for the appearance of xeno-GVHD-like symptoms, including weight loss, hunched posture, ruffled fur, reduced mobility and tachypnoea. Mice were euthanized after loss of > 20% body weight or the loss of 1 g/day over 2 days; this time-point was recorded as the survival time. We have shown previously that ∼15% of the mice irradiated with 2 Gy do not survive the irradiation-only treatment, with death occurring within 10 days [36]. Therefore, irradiated mice that died within 10 days after injection of human PBMC were excluded from these studies.
Antibodies and flow cytometry
Tissues including peripheral blood and spleen were collected at specified times and were processed for flow cytometry analysis. Single-cell suspensions were prepared from spleen. Red blood cells were lysed using a solution of hypotonic ammonium chloride [0·83%, supplemented with 1% KHCO3 and 1 mm ethylenediamine tetraacetic acid (EDTA)] and leucocytes were washed with fluorescence activated cell sorter (FACS) buffer comprised of phosphate-buffered saline (PBS) supplemented with 2% Fetalclone serum (HyClone, Rockford, IL, USA) and 0·02% sodium azide (Sigma, St. Louis, MO, USA) prior to incubation with antibodies. Typically, 1 × 106 cells from spleen preparations or 100 µl of peripheral blood were used for flow cytometry. Cell suspensions were incubated first with a monoclonal antibody (mAb) specific for CD16/32 (2·4G2) to block Fc binding and then with the indicated mAbs, including mAb directed against murine CD45 (30-F11) and human CD45 (HI30), CD3 (UCHT1), CD4 (RPA-T4), HLA-DR (TU36), CCR5 (2D7/CCR5) and CD45RO (UCHL1). After labelling, spleen and peripheral blood samples were washed in FACS buffer and fixed using 1% paraformaldehyde (Electron Microscopy Sciences, PA, USA). Samples containing residual red blood cells were treated with BD FACS Lysing Solution (BD Biosciences, San Jose, CA, USA) prior to fixation. Samples were analysed using either a LSRII (BD) or a FACSCaliber (BD Biosciences) and analysis performed with FlowJo software (Tree-Star, Ashland, OR, USA).
In-vitro proliferation of human CD4 T cells
To prepare stimulator cells, mouse splenocytes were recovered from the indicated mouse strains and cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin G, 100 mg/ml streptomycin sulphate and 2 mm l-glutamine and in the presence of LPS (15 µg/ml), as described previously [47]. After 3 days cells were collected, rinsed, irradiated with 30 Gy and frozen at −80°C until used. For effector cells, freshly isolated human CD4 T cells were labelled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen). CFSE-labelled CD4+ effector cells (1 × 106) and murine stimulator cells (4 × 105) were co-cultured in 96-well plates for 7 days at 37°C in a humidified atmosphere containing 95% air 5% CO2, after which time they were harvested and analysed by flow cytometry for dilution of CFSE labelling. Each sample was cultured in triplicate.
In-vitro cytokine production assay
Cytokine production was quantified using the BD Cytofix/Cytoperm Kit Plus GolgiStop (BD Biosciences), according to the manufacturer's instructions. Splenocytes were recovered from mice injected with CD4-P cells and exhibiting symptoms of GVHD. Red blood cells were lysed and 1 × 106 cells were then left unstimulated or stimulated with phorbol myristate acetate (PMA) (0·5 µg/ml) and ionomycin (0·5 µg/ml) in the presence of GolgiStop™ (0·1 µg/ml) for 4 h at 37°C in 5% CO2. Cells were then fixed and permeabilized using Cytofix/Cytoperm solution and stained with mAb to interferon (IFN)-γ (clone 4S.B3, eBioscience) and TNF (clone MAb11; eBioscience).
Haematological analyses
Blood samples were collected in 1·5 ml Microvette haematological tubes coated with EDTA (Sarstedt, Newton, NC, USA) and haematological analyses including haematocrit (HCT), red blood cell (RBC), platelet (PLT) and haemoglobin (HGB) values, were recorded on a Heska CBC-Diff Veterinary Hematology analyser (Loveland, CO, USA).
Histological analyses
For histological examination, tissue samples including lung, skin, liver, small intestine and spleen were recovered, immersed overnight in 10% neutral buffered formalin and embedded in paraffin. Bones were decalcified after fixation. Sections (5 µm) were cut and stained with haematoxylin and eosin. Immunohistochemical staining was performed with mAbs specific for human CD45 (clone 2B11+PD7/26; Dako, Glostrup, Denmark) using a DakoCytomation EnVisionDual Link system implemented on a Dako Autostainer Universal Staining System (Dako). The results of the immunohistochemistry staining are summarized graphically on a scale of 0–3. The scoring was performed blind using the following criteria scale: 0 = no infiltrate; 1 = infiltrate accounted for < 10% total cells with infiltrate only detectable peripherally, 2 = infiltrate accounted for 10–50% total cells and infiltrate was spread uniformly throughout the section, and 3 = infiltrate accounted for > 50% of cellular content and was characterized by a dense stain throughout the section.
Statistical analysis
Survival data were analysed by Kaplan–Meier life method tables using log-rank analysis performed with Graphpad Prism software (Graphpad Software, San Diego, CA, USA). All other analysis involved comparison of means using the independent-samples t-test. Values of P ≤ 0·05 were considered statistically significant. Standard error of the mean is shown for averages of engraftment data.
Results
Human CD4 T cells engraft and survive in NOD-scid IL2rγnull (NSG) mice
We have recently developed a model of human GVHD using NSG mice [36,37]. However, the ability of human CD4 T cells to mediate disease in the absence of CD8 T cells has not been described. To determine whether human CD4 cells mediate GVHD, CD4 T cells were fractionated from human PBMC using either positive or negative magnetic selection. Positive selection allowed the recovery of CD4 T cell populations (CD4-P) at greater than 95% purity (Fig. 1a and data not shown). Negative selection or enrichment of CD4 T cells was performed by depletion of CD8+ cells (CD4-E). The efficiency of CD8 depletion is shown in Fig. 1a, with greater than 95% of remaining CD3+ cells expressing CD4. To inject the corresponding number of CD4 cells found on average in 10 × 106 unfractionated PBMC, 4 × 106 CD4-P or 7 × 106-enriched CD4-E were injected intravenously into irradiated NSG mice.
Engraftment levels in the peripheral blood were evaluated 2 weeks after injection and expressed as the percentages of human CD45+ cells present (Fig. 1b). Both CD4-P and CD4-E cells were present at high levels in the peripheral blood at 2 weeks, but CD4-E cells were detected at a significantly higher frequency, suggesting that a non-T cell component augments the engraftment of CD4 T cells. Moreover, CD4-E engraftment levels were comparable to the CD4 T cell levels attained using whole PBMC. The purity of the injected CD4 populations was confirmed in the blood of recipient mice at 2 weeks, with the majority of human cells detected being CD4+ (Fig. 1c). The engraftment level with CD4-P cells was increased when co-injected with CD3 T cell depleted PBMC, suggesting that the presence of anti-CD4 beads on the CD4 T cells did not decrease survival in vivo and that there is a non-T cell component that facilitates human CD4+ cell engraftment (Supplementary Fig. S1).
Human CD4 T cells mediate xeno-GVHD in NSG mice
We next tested the ability of human CD4 cells to mediate a xeno-GVHD in NSG mice. NSG mice injected with unfractionated human PBMC displayed weight loss as soon as 7 days post-injection (Fig. 2a). Mice that received CD4-E or CD4-P cells also lost weight compared to non-engrafted mice, but with slower kinetics compared to mice engrafted with whole PBMC. The survival of mice injected with either PBMC, CD4-E cells or CD4-P cells was reduced significantly compared to non-engrafted recipients, but the death of mice injected with total PBMC occurred earlier than that observed following injection of CD4 T cells alone (<20 days, Fig. 2b). The induction of xeno-GVHD in mice receiving CD4 T cells was confirmed by haematological analysis at late-stage disease, as described [36]. A decrease in platelet counts was observed in animals receiving whole human PBMC, CD4-E cells or CD4-P cells (Fig. 2c). In general, blood analyses at the end of the experiment showed that CD4-E- and PBMC-injected mice have similar parameters with respect to HCT, RBC and HGB values, whereas peripheral blood values in CD4-P-injected animals were not statistically different from those in irradiated-only animals (Supplementary Fig. S2). All organs recovered at the final stage of disease, including blood, spleen, skin, lung, liver and bone marrow (Figs 2d and 3a,b), showed a high level of human CD45+ cell engraftment and infiltration in various tissues following injection of human PBMC, CD4-E cells or CD4-P cells. Moreover, the high purity of the injected CD4 T cells was maintained in the blood (Fig. 2e) and spleen (Fig. 2f) of mice that developed xeno-GVHD. Together these results indicate that both enriched and purified human CD4 T cells can independently mediate xeno-GVHD in NSG mice.
Fig. 2.
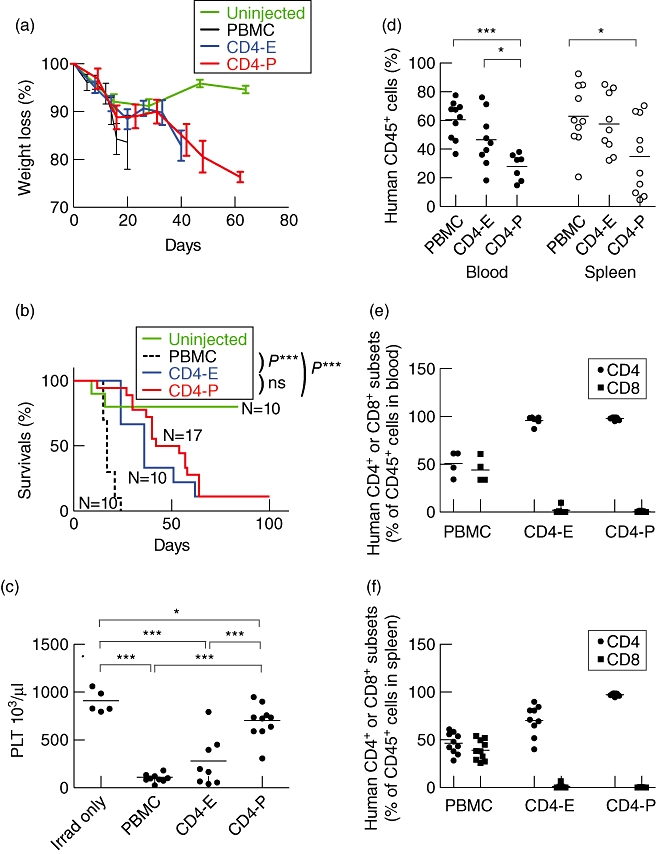
Human CD4 T cells mediate xenograft-versus-host disease (xeno-GVHD) in non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice. Total human peripheral blood mononuclear cells (PBMC), CD4-E or CD4-P cell subsets were injected into irradiated NSG mice and monitored for the development of xeno-GVHD, including (a) Weight loss and (b) survival of recipient mice. (c) Platelet counts (PLT) were determined for mice that received no human lymphocytes (irradiated-only, measured at 5 months) or injected with whole PBMC (10 × 106 total cells), CD4-E (7 × 106 total cells) or CD4-P cells (4 × 106 total cells). (d) Engraftment of human CD45+ cells was determined by flow cytometry analysis in the peripheral blood and spleen at the time of removal from the experiment. The values shown in (e) and (f) represent the percentage of CD4 or CD8 T cells within the human CD45+ population present in the blood and spleen, respectively. Data are representative of at least two different PBMC donors. Each symbol represents an individual mouse. *P < 0·05; ***P < 0·001.
Fig. 3.
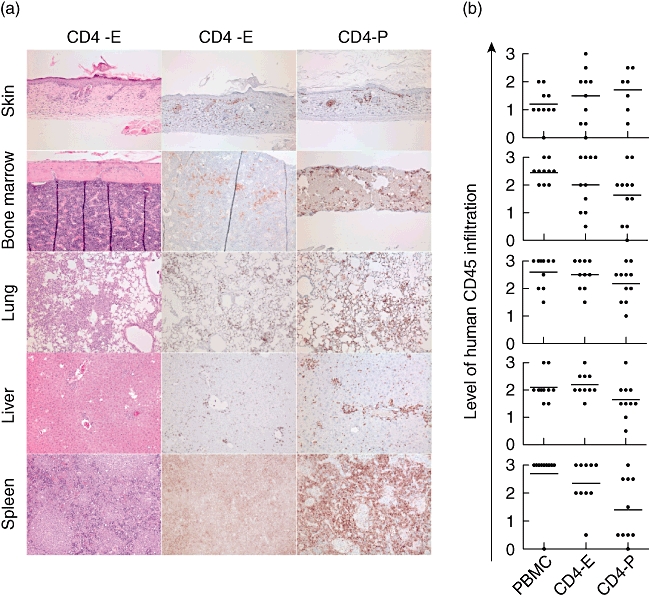
Mononuclear cell infiltration of tissues during xenograft-versus-host disease (xeno-GVHD) in non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice. Total human peripheral blood mononuclear cells (PBMC) or CD4-E or CD4-P T cell subsets were injected into NSG mice. (a) At the end of the experiment, human cell infiltrates were examined in skin, lung, liver, spleen and bone marrow of NSG mice injected with CD4 T cells by immunohistochemistry staining using anti-human CD45 monoclonal antibody (mAb). Representative haematoxylin and eosin staining is shown in tissues from mice injected with CD4-E cells. (b) The results of the immunohistochemistry staining are summarized graphically on a scale of 0–3 scored as described in Material and methods. Each symbol represents an individual mouse.
Development of novel NSG stocks to study allo-GVHD mediated by human CD4 T cells
Using NSG mice that lack either murine MHC class I (NSG-β2mnull) or class II (NSG-Ab°), we have shown previously that the murine MHC is a major target of the xeno-GVHD response [36]. Recently, we have developed a NSG-Ab° mouse stock that transgenically expresses a chimeric human HLA-DRB1*0401 (DR4) molecule (NSG-Ab° DR4). This stock would be predicted to show reduced xeno-GVHD mediated by human CD4 T cells but permit the development of allospecific GVHD responses mediated by CD4 T cells from a DR4-negative donor.
To confirm that human CD4 T cells engraft and survive in NSG mice that lack murine MHC class II, CD4-P cells derived from HLA-DR4-negative donors were injected into NSG-Ab° and NSG-Ab° DR4 mice. For these experiments, human CD4 T cells were purified by positive selection to permit examination of an allospecific GVHD response resulting from direct antigen presentation. Two weeks after injection, CD4-P cells were detected in NSG-Ab° and NSG-Ab° DR4 mice and engraftment levels were comparable in the blood of the two strains (Fig. 4a). The engraftment of CD4-P cells in the blood of irradiated NSG mice is also shown here to compare overall engraftment. As shown in Fig. 4a, overall engraftment of CD4-P cells was significantly higher at 2 weeks in NSG mice (22·9 ± 3·2%) compared to NSG-Ab° DR4 mice (12·5 ± 2·4%). In all experiments there were some NSG-Ab° (5%) and NSG-Ab° DR4 (40%) mice that did not engraft with detectable levels (>1%) of CD45+ cells and were excluded from further analysis. These results indicate that human CD4 T cells engraft and expand in NSG mice lacking murine class II with or without co-expression of human HLA-DR4.
Fig. 4.
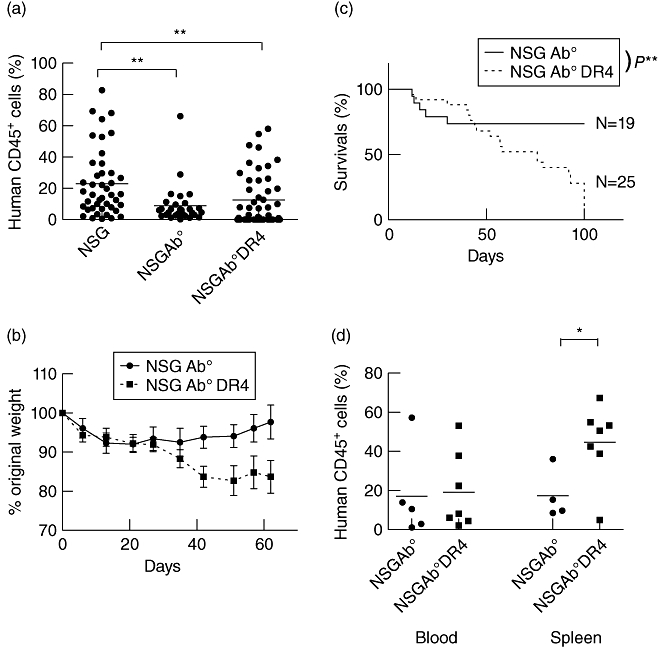
Human CD4 T cells induce allograft-versus-host disease (allo-GVHD) in non-obese diabetic (NOD)-scid IL2rγnull (NSG)-Ab° DR4 mice. CD4-P cells (4 × 106 total cells) from a DR4-negative human peripheral blood mononuclear cells (PBMC) donor were injected into NSG, NSG-Ab° or NSG-Ab° DR4 mice, and the recipient mice were monitored for the development of GVHD. (a) Human CD45 cell engraftment was evaluated at two weeks in recipient mice in the peripheral blood. Data are representative of eight (PBMC) and four (CD4-P) different PBMC donors. Each symbol represents an individual mouse. Only animals demonstrating human CD45 engraftment at 2 weeks of >1% were monitored and included in the study. (b) Weight loss shown is an average of two independent experiments (i.e. two different PBMC donors) with at least eight mice from each group and (c) survival of recipient mice. Data are representative of six independent experiments (five different PBMC donors) with n = 19 and 25 mice per group. (d) Percentage of human CD45+ cells in the blood and spleen at the end of the experiment. Each symbol represents an individual mouse. *P < 0·05; **P < 0·01.
Human CD4 T cells mediate an allo-GVHD in NSG-Ab° mice expressing human HLA class II
NSG-Ab° and NSG-Ab° DR4 transgenic mice injected with DR4-negative CD4-P cells were monitored for the development of xeno- and allo-GVHD, respectively. NSG-Ab° mice lack expression of murine MHC class II and did not develop symptoms of xeno-GVHD such as hair loss and weight loss (Fig. 4b). Most NSG-Ab° mice injected with CD4-P survived to the end of the study (Fig. 4c, 100 days), in contrast to NSG mice expressing both MHC class I and II (Fig. 2b). These data suggest that expression of murine MHC II is required for the efficient induction of xeno-GVHD by human CD4-P in NSG mice.
In contrast to NSG-Ab° mice, NSG-Ab° DR4 mice injected with CD4-P cells from DR4-negative donors developed symptoms of GVHD, as indicated by weight (Fig. 4b) and hair loss initially on the back and the face and around the eyes and nose, as early as 40 days after injection. The NSG-Ab° DR4 mice that received CD4-P cells had a significantly reduced survival compared to NSG-Ab° mice (Fig. 4c). The weight loss and reduced survival indicate that NSG-Ab° DR4 mice developed allo-GVHD. The allo-GVHD in NSG-Ab° DR4 mice (MST = 66·5 days, Fig. 4c) appeared to develop with slower kinetics as compared to xeno-GVHD in NSG mice (MST = 40 days, Fig. 2b) injected with CD4-P cells, although this was not significant (P = 0·19). Human CD4 T cells were detected in the peripheral blood and spleen at the final stages of disease in both NSG-Ab° and NSG-Ab° DR4 mice, confirming that CD4-P cells engrafted and survived in the absence of murine MHC class II (Fig. 4d). This in-vivo response was confirmed in vitro by demonstrating that human CD4 T cells proliferated to a significantly higher level in response to NSG-Ab° DR4 stimulator cells compared to the NSG-Ab° stimulator cells (Supplementary Fig. S3).Immunohistochemical staining for human CD45 demonstrated the infiltration of CD4-P cells in various organs, including skin, bone marrow, lung and liver in both NSG-Ab° and NSG-Ab° DR4 mice (Fig. 5a,b). Similar cellular infiltration was observed in tissues from NSG mice injected with CD4-P cells and undergoing a xeno-GVHD (Fig. 3). These results suggest that the presence of cellular infiltrates is not predictive for the development of allo-GVHD in this model.
Fig. 5.
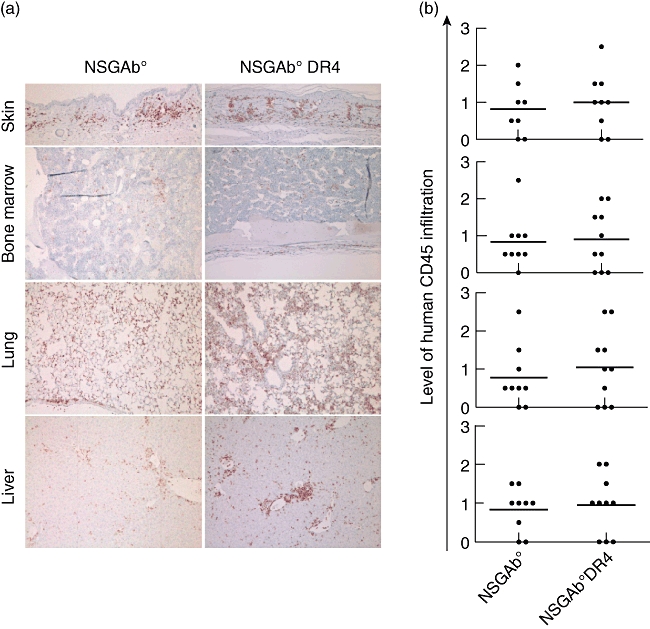
Mononuclear cell infiltration in various tissues during allograft-versus-host disease (allo-GVHD). Human CD4-P fractionated T cells were injected into non-obese diabetic (NOD)-scid IL2rγnull (NSG)-Ab° and NSG-Ab° DR4 mice. (a) At the end of the experiment, human cell infiltrates were examined in skin, bone marrow, lung and liver by haematoxylin and eosin and immunohistochemistry following staining with anti-human CD45 monoclonal antibody (mAb). (b) The results of the immunohistochemistry staining are summarized graphically on a scale of 0–3 scored as described in Material and methods. Each symbol represents an individual mouse. *P < 0·05; **P < 0·01; ***P < 0·001.
We next asked whether the activation profile and the functionality of the CD4-P populations injected into NSG-Ab° DR4 and NSG-Ab° mice differ (Fig. 6). At 2 weeks after injection, human CD4 T cells in the peripheral blood of NSG-Ab° DR4 mice displayed a strongly activated phenotype, with higher frequencies of cells expressing HLA-DR (Fig. 6a), CD45RO (Fig. 6b) and CCR5 (Fig. 6c) compared to NSG-Ab° mice. By 3 weeks, the CD4 T cells in NSG-Ab° mice also started to display an activated phenotype with increased expression of HLA-DR, CD45RO and CCR5, although still reduced compared to the expression of these antigens in NSG-Ab° DR4 mice. Higher levels of HLA-DR-positive CD4 T cells were also detected in the peripheral blood (Fig. 6d) and spleens (Fig. 6e) of NSG-Ab° DR4 mice at 9 weeks after injection, a time-point at which these mice were developing disease symptoms. The activation profile of CD4 T cells in NSG-Ab° mice indicates that these cells are acquiring an activated phenotype but are not mediating disease. We next determined whether CD4 T cells maintained in NSG-Ab° mice were functional. To test this, splenocytes were recovered from NSG-Ab° DR4 mice and NSG-Ab° mice at 9 weeks after injection with DR4-negative CD4-P cells, stimulated in vitro with PMA and ionomycin and then assessed for the production of IFN-γ and TNF using a standard intracellular cytokine assay (Fig. 6f,g). CD4 T cells recovered from either strain of mice were capable of cytokine production to a similar extent, suggesting that the cells present within the NSG-Ab° mice retain functionality but are not mediating disease. Overall, these results demonstrate that the NSG-Ab° DR4 mouse can be used to simulate a human allo-GVHD following engraftment of PBMC from HLA-DR4-negative donors.
Fig. 6.
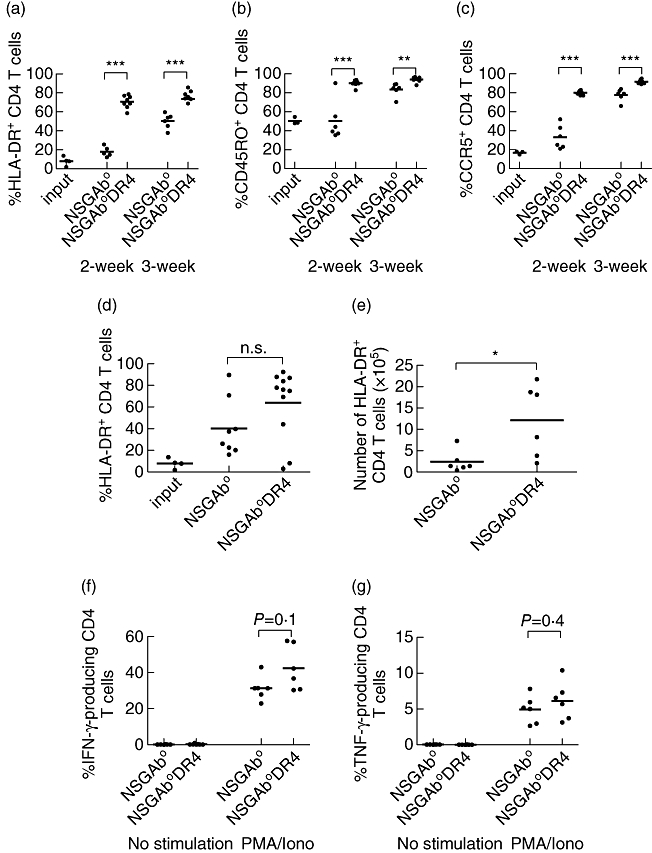
Phenotypic and functional characterization of human CD4 T cells in non-obese diabetic (NOD)-scid IL2rγnull (NSG)-Ab° and NSG-Ab° DR4 mice. CD4-P cells from HLA-DR4-negative donors were injected into NSG-Ab° or NSG-Ab° DR4 mice. The expression of human leucocyte antigen D-related (HLA-DR) (a), CD45RO (b) and CCR5 (c) was evaluated on human CD4 T cells prior to injection (input) and in the peripheral blood of recipient mice at the 2- and 3-week time-points after injection. The values represent the percentages of CD3+/CD4+ cells that expressed the indicated marker. At 9 weeks after injection, the expression of HLA-DR by human CD4 T cells was examined in the peripheral blood (d, percentage) and the spleen (e, total number of CD4 T cells). Each symbol represents an individual mouse. *P < 0·05; **P < 0·01; ***P < 0·001. The ability of human CD4 T cells from the spleens of recipient mice to produce interferon (IFN)-γ and tumour necrosis factor (TNF) was determined at 9 weeks after injection by ex-vivo stimulation with phorbol myristate acetate (PMA) and ionomycin for 5 h and visualized using a standard intracellular cytokine assay, as described in Materials and methods (f,g).
Discussion
We have previously developed a model of human GVHD in the NSG mouse and established the critical role of murine MHC class I and II in the development of xeno-GVHD [36]. Our present data indicate clearly that enriched and purified populations of human CD4 T cells will engraft in NSG mice, and mediate xeno-GVHD. Given the capacity for human CD4 T cells to mediate GVHD, we used a novel NSG mouse strain that lacks expression of murine MHC class II, but transgenically expresses a chimeric HLA-DR4 molecule (NSG-Ab° DR4) to create a model of CD4-mediated allo-GVHD. NSG-Ab° DR4 mice injected with purified CD4 T cells from HLA-DR4-negative donors developed an allo-GVHD in the absence of xeno-GVHD, displaying weight loss, pathological changes and higher levels of mortality compared to NSG mice lacking murine MHC class II. Thus, these results show that by expressing a chimeric HLA-DR4 in mice that do not express murine MHC class-II, we were able to re-establish a human CD4 T cell-mediated disease process that is driven by a human T cell receptor (TCR) recognizing the DRβ1*0401 peptide binding region. These findings suggest that this model can be used to investigate human allo-GVHD in vivo.
While NSG mice provide a sensitive and reliable model system to study the ability of human immune cells to mediate xeno- and allo-GVHD and to test novel immunoregulatory therapies, the mediators of disease have not been elucidated clearly [48]. Previous studies have suggested that human CD3+ cells are necessary for induction of disease in SCID mice and that CD8 as well as CD4 cells become activated after injection [27,32]. Moreover, the delayed onset of disease in MHC class I- or class II-deficient NSG mice also indicates an important role for both CD4 and CD8 T cells [36]. Here we show directly that human CD4 T cells engraft in NSG mice and mediate xeno-GVHD. Interestingly, the initial engraftment of enriched versus purified CD4 T cells was different in NSG mice with enriched CD4 T cells detectable at higher levels compared to purified CD4 T cells at 2 weeks post-injection. The differences observed for enriched and purified populations suggest that CD4 T cell engraftment is augmented by the presence of other human cell populations. This conclusion is consistent with the finding that purified CD4 T cells readily engraft in NSG mice bearing human allogeneic skin grafts and that these cells mediate rejection of the skin [49]. CD3-negative human cells may enhance the survival of CD4 T cell subsets by providing either human-specific cytokines or cell contact-dependent signals. These possibilities are currently being evaluated. Despite this early difference in engraftment, purified CD4 T cells induced GVHD with similar kinetics to enriched CD4 T cells, indicating that CD4 T cells can survive and function in NSG mice in the absence of other human immune cells.
We have demonstrated previously that murine MHC molecules are potential targets for human T cells in NSG mice [36]. To show directly that murine MHC is critical to the xeno-GVH reaction in NSG mice, we injected purified CD4 T cells into NSG mice lacking murine class II (NSG-Ab°). Our results show that the purified human CD4 T cells engrafted in NSG hosts lacking murine class II, but did not cause disease in the majority of mice. The survival of human CD4 T cells in NSG mice lacking MHC class II and the absence of xeno-GVHD suggests that this mouse model will be useful to study other aspects of human CD4 T cell function, such as the rejection of allogeneic skin in the absence of the confounding effects of xeno-GVHD [49]. We are also testing whether a CD8-mediated allo-GVHD in the absence of xeno-GVHD will develop in NSG mice deficient in murine MHC class I but expressing an allogeneic human HLA class I molecule.
All previous models of GVHD in immunodeficient mice have been based on human T cells responding to murine antigens in a xenoreaction [48]. Injection of purified human CD4 T cells from HLA-DR4 negative donors into NSG-Ab° DR4 induced a GVHD response that was not observed in NSG-Ab° mice, suggesting that the human cells were responding to the allogeneic HLA-DR4 molecule. Human cells infiltrated the skin, lung and liver of NSG-Ab° DR4 mice, reminiscent of the distribution in patients with GVHD. Moreover, CD4 T cells were activated rapidly following injection into NSG-Ab° DR4 mice, up-regulating expression of HLA-DR, CCR5 and CD45RO within 2 weeks. Although the majority of CD4-injected NSG-Ab° mice did not develop GVHD symptoms, human cells were detected within the skin, lung and liver and the recovered CD4 T cells were functional, as determined by the ability to produce cytokines directly ex vivo. This finding underscores the requirement of DR4 expression for the induction of allo-GVHD and suggests that activation phenotypes of human lymphocytes and histological infiltration are not sufficient for identifying mice that will develop disease. However, the kinetics of up-regulation of activation molecules on T cells may be predictive of the mice that will eventually develop disease. In addition, the development of GVHD in a small number of NSG-Ab° mice suggests that the injected CD4 T cells may be able to indirectly present mouse antigen and induce disease at a low level [6]. We are currently evaluating potential effector mechanisms that CD4 T cells may employ to mediate disease in the NSG-Ab° DR4 mice.
The peptides presented by the HLA-DR4 molecule expressed by the NSG-Ab° DR4 transgenic mice will be of murine origin. The contribution of specific peptides in the recognition of non-self MHC (allogeneic or xenogeneic) by a TCR can vary dramatically [50]. However, recent studies have suggested that all TCR have a germline encoded affinity for MHC, both self and non-self, based on evolutionarily conserved structure of the MHC [51], and that a single TCR has the capacity to recognize a non-self MHC presenting multiple peptides [52]. Therefore, the presentation of murine peptides in the chimeric HLA-DR4 molecule should still permit allogeneic recognition in a manner similar to the presentation of human-derived peptides. Moreover, HLA-transgenic mice have been used to identify peptides that were confirmed to be recognized by human T cells [53–55], suggesting that there is a level of similarity between the peptides presented by HLA in both humans and mice. In addition, there is a potential for pairing of the endogenous murine IEβ chain with the transgenically expressed HLA-DRA1 chain in these mouse models [45,56]. However, this pairing would be a random occurrence and is impossible to predict. To address this and to improve the model, we are currently crossing the chimeric DR4 molecule onto a NSG background that completely lacks all murine class II genes [57].
In summary, we have developed two clinically relevant mouse models to study the functionality of mature human T cells. The first model using NSG-Ab° mice will permit the in-vivo study of human CD4 T cell-mediated alloimmunity, autoimmunity and viral immunity in the absence of xeno-GVHD. The second model using the NSG-Ab° DR4 mice creates a novel system to study allo-GVHD in which disease is induced as a result of human CD4 T cells recognizing allogeneic HLA class II molecules instead of murine MHC, as occurs in standard NSG mice. Both these mouse strains will be useful as preclinical models to study in-vivo mechanisms underlying human immune responses.
Acknowledgments
We thank Linda Paquin, Mike Bates, Amy Cuthbert, Celia Hartigan, Amy Sands, Lisa Burzenski, Bruce Gott, Allison Ingalls and Candy Knoll for their technical assistance. We thank Dr Dale Greiner (UMass) for critical discussions and comments on the manuscript. We also thank the participation of the Clinical Trials Unit at the University of Massachusetts Medical School. This work was supported by National Institutes of Health Grants AI083911, AI46629, DK53006, HL077642, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520, an institutional Center for AIDS Research (CFAR) grant AI042845, Cancer Center Core grant CA34196, a Beta Cell Biology Consortium grant DK72473, the Helmsley Foundation and grants from the Juvenile Diabetes Foundation, International. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosure
None of the authors have a financial interest related to the work presented in this manuscript.
Supporting information
Additional supporting information may be found in the online version of this article.
Fig. S1. Presence of anti-CD4 antibodies does not affect human CD4 T cell engraftment negatively in non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice. Human CD4 T cells were isolated from peripheral blood mononuclear cells (PBMC) using microbeads coupled to anti-CD4 or anti-CD8 antibodies. The CD4-P fraction was isolated by positive selection, and therefore these cells are coated with anti-CD4 microbeads. CD4-E cells were prepared by depleting CD8 T cells from the PBMC, resulting in a population of CD4 T cells not coated with microbeads. The CD4-E+ anti-CD4 population was obtained by combining CD4-P (microbead-coated CD4 T cells) and the column flow-through, which was further depleted of CD8 T cells resulting in a population of predominantly antigen-presenting cells (APC). The percentage of CD4 engraftment in the (a) blood, (a) spleen and (a) the number of CD4 T cells in the spleen were determined at 2 weeks post-injection. *P > 0·05; **P > 0·01; ***P > 0·001.
Fig. S2. Haematological analyses [a, haemoglobin (HGB); b, haematocrit (HCT); c, red blood cell (RBC) count] at death or the end of the observation period of non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice either irradiated only or injected with the peripheral blood mononuclear cells (PBMC), CD4-E cells or CD4-P cells. *P > 0·05; **P > 0·01; ***P > 0·001.
Fig. S3. In-vitro proliferation of human CD4 T cells. A carboxyfluorescein succinimidyl ester (CFSE) dilution assay was used to assess CD4 T cell proliferation in vitro, using splenocytes isolated from non-obese diabetic (NOD)-scid IL2rγnull (NSG), NSG-Ab° or NSG-Ab° DR4 mice as stimulator cells, as described in theMaterials and methods. The data are expressed as the % of CD4 T cells that were CFSE low. **P > 0·01.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125:S324–35. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenq RR, van den Brink MRM. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–21. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 3.Ringdén O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147:614–33. doi: 10.1111/j.1365-2141.2009.07886.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 6.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 7.Duran-Struuck R, Reddy P. Biological advances in acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplantation. 2008;85:303–8. doi: 10.1097/TP.0b013e318162d357. [DOI] [PubMed] [Google Scholar]
- 8.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–72. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 11.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 12.Hill GR, Teshima T, Gerbitz A, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–67. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–9. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Sun K, Welniak LA, Murphy WJ. Immunomodulation and pharmacological strategies in the treatment of graft-versus-host disease. Exp Opin Pharmacother. 2008;9:2305–16. doi: 10.1517/14656566.9.13.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–36. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 17.Hesselton RM, Koup RA, Cromwell MA, Graham BS, Johns M, Sullivan JL. Human peripheral blood xenografts in the SCID mouse: characterization of immunologic reconstitution. J Infect Dis. 1993;168:630–40. doi: 10.1093/infdis/168.3.630. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann-Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood. 1993;81:3440–8. [PubMed] [Google Scholar]
- 19.Martino G, Anastasi J, Feng J, et al. The fate of human peripheral blood lymphocytes after transplantation into SCID mice. Eur J Immunol. 1993;23:1023–8. doi: 10.1002/eji.1830230506. [DOI] [PubMed] [Google Scholar]
- 20.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 21.Murphy WJ, Bennett M, Anver MR, Baseler M, Longo DL. Human–mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur J Immunol. 1992;22:1421–7. doi: 10.1002/eji.1830220614. [DOI] [PubMed] [Google Scholar]
- 22.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–27. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tary-Lehmann M, Saxon A, Lehmann PV. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–33. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 24.Cao T, Leroux-Roels G. Antigen-specific T cell responses in human peripheral blood leucocyte (hu-PBL)-mouse chimera conditioned with radiation and an antibody directed against the mouse IL-2 receptor beta-chain. Clin Exp Immunol. 2000;122:117–23. doi: 10.1046/j.1365-2249.2000.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–57. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy WJ, Conlon KC, Sayers TJ, et al. Engraftment and activity of anti-CD3-activated human peripheral blood lymphocytes transferred into mice with severe combined immune deficiency. J Immunol. 1993;150:3634–42. [PubMed] [Google Scholar]
- 27.Nervi B, Rettig MP, Ritchey JK, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–38. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandhu J, Shpitz B, Gallinger S, Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994;152:3806–13. [PubMed] [Google Scholar]
- 29.Sandhu JS, Gorczynski R, Shpitz B, Gallinger S, Nguyen HP, Hozumi N. A human model of xenogeneic graft-versus-host disease in SCID mice engrafted with human peripheral blood lymphocytes. Transplantation. 1995;60:179–84. [PubMed] [Google Scholar]
- 30.Shpitz B, Chambers CA, Singhal AB, et al. High level functional engraftment of severe combined immunodeficient mice with human peripheral blood lymphocytes following pretreatment with radiation and anti-asialo GM1. J Immunol Methods. 1994;169:1–15. doi: 10.1016/0022-1759(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 31.van Rijn RS, Simonetti ER, Hagenbeek A, et al. Quantitative assessment of human T lymphocytes in RAG2(-/-)gammac(-/-) mice: the impact of ex vivo manipulation on in vivo functionality. Exp Hematol. 2007;35:117–27. doi: 10.1016/j.exphem.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 32.van Rijn RS, Simonetti ER, Hagenbeek A, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/- double-mutant mice. Blood. 2003;102:2522–31. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 33.Tournoy KG, Depraetere S, Meuleman P, Leroux-Roels G, Pauwels RA. Murine IL-2 receptor beta chain blockade improves human leukocyte engraftment in SCID mice. Eur J Immunol. 1998;28:3221–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3221::AID-IMMU3221>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Tournoy KG, Depraetere S, Pauwels RA, Leroux-Roels GG. Mouse strain and conditioning regimen determine survival and function of human leucocytes in immunodeficient mice. Clin Exp Immunol. 2000;119:231–9. doi: 10.1046/j.1365-2249.2000.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 36.King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–18. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–14. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Ito R, Katano I, Kawai K, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87:1654–8. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- 39.King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized mice for the study of type 1 diabetes and beta cell function. Ann NY Acad Sci. 2008;1150:46–53. doi: 10.1196/annals.1447.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohana-Kashtan O, Morisot S, Hildreth R, Brayton C, Levitsky HI, Civin CI. Selective reduction of graft-versus-host disease-mediating human T cells by ex vivo treatment with soluble Fas ligand. J Immunol. 2009;183:696–705. doi: 10.4049/jimmunol.0800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golovina TN, Mikheeva T, Suhoski MM, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–68. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson J, Cullup H, Lourie R, et al. Antibody to the dendritic cell surface activation antigen CD83 prevents acute graft-versus-host disease. J Exp Med. 2009;206:387–98. doi: 10.1084/jem.20070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCluskey J, Kanaan C, Diviney M. Nomenclature and serology of HLA class I and class II alleles. In: Coligan JE, Kruisbeek AM, Margulies DM, Shevach EM, Strober W, editors. Current protocols in immunology. New Jersey: John Wiley & Sons; 2002. pp. S.1–S.8. Appendix 1. [Google Scholar]
- 45.Woods A, Chen HY, Trumbauer ME, Sirotina A, Cummings R, Zaller DM. Human major histocompatibility complex class II-restricted T cell responses in transgenic mice. J Exp Med. 1994;180:173–81. doi: 10.1084/jem.180.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunce M. PCR-sequence-specific primer typing of HLA class I and class II alleles. Methods Mol Biol. 2003;210:143–71. doi: 10.1385/1-59259-291-0:143. [DOI] [PubMed] [Google Scholar]
- 47.Brehm MA, Mangada J, Markees TG, et al. Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood. 2007;109:819–26. doi: 10.1182/blood-2006-03-008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pino S, Brehm MA, Covassin-Barberis L, et al. Development of novel major histocompatibility complex class I and class II-deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease. Methods Mol Biol. 2010;602:105–17. doi: 10.1007/978-1-60761-058-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Racki WJ, Covassin L, Brehm M, et al. NOD-scid IL2rgamma(null) mouse model of human skin transplantation and allograft rejection. Transplantation. 2010;89:527–36. doi: 10.1097/TP.0b013e3181c90242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colf LA, Bankovich AJ, Hanick NA, et al. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–46. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 51.Huseby ES, White J, Crawford F, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–60. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Felix NJ, Donermeyer DL, Horvath S, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–97. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 53.Jarchum I, Baker JC, Yamada T, et al. In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes. 2007;56:2551–60. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- 54.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–65. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 55.Unger WW, Pinkse GG, Mulder-van der Kracht S, et al. Human clonal CD8 autoreactivity to an IGRP islet epitope shared between mice and men. Ann NY Acad Sci. 2007;1103:192–5. doi: 10.1196/annals.1394.024. [DOI] [PubMed] [Google Scholar]
- 56.Ito K, Bian HJ, Molina M, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med. 1996;183:2635–44. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madsen L, Labrecque N, Engberg J, et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–43. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


