Abstract
OBJECTIVE
Macrophage recruitment to adipose tissue is a reproducible feature of obesity. However, the events that result in chemokine production and macrophage recruitment to adipose tissue during states of energetic excess are not clear. Sirtuin 1 (SirT1) is an essential nutrient-sensing histone deacetylase, which is increased by caloric restriction and reduced by overfeeding. We discovered that SirT1 depletion causes anorexia by stimulating production of inflammatory factors in white adipose tissue and thus posit that decreases in SirT1 link overnutrition and adipose tissue inflammation.
RESEARCH DESIGN AND METHODS
We used antisense oligonucleotides to reduce SirT1 to levels similar to those seen during overnutrition and studied SirT1-overexpressing transgenic mice and fat-specific SirT1 knockout animals. Finally, we analyzed subcutaneous adipose tissue biopsies from two independent cohorts of human subjects.
RESULTS
We found that inducible or genetic reduction of SirT1 in vivo causes macrophage recruitment to adipose tissue, whereas overexpression of SirT1 prevents adipose tissue macrophage accumulation caused by chronic high-fat feeding. We also found that SirT1 expression in human subcutaneous fat is inversely related to adipose tissue macrophage infiltration.
CONCLUSIONS
Reduction of adipose tissue SirT1 expression, which leads to histone hyperacetylation and ectopic inflammatory gene expression, is identified as a key regulatory component of macrophage influx into adipose tissue during overnutrition in rodents and humans. Our results suggest that SirT1 regulates adipose tissue inflammation by controlling the gain of proinflammatory transcription in response to inducers such as fatty acids, hypoxia, and endoplasmic reticulum stress.
Obesity and type 2 diabetes mellitus (T2DM) are associated with low-grade, chronic inflammation characterized by elevations in tissue and plasma cytokines and infiltration of adipose tissue by cells classically associated with immune activation—principally macrophages (1,2). Nuclear factor (NF)-κB and mitogen-activated protein kinase signaling are involved in propagating obesity-associated adipose tissue inflammation (3–5), but the mechanism for activation of these pathways is enigmatic. Several potential inducers have been identified, including adipocyte endoplasmic reticulum (ER) stress (6), adipose tissue microhypoxia and hypoxia-inducible factor (HIF)-1α induction (7,8), adipocyte necrosis (2), and fatty acid stimulation of toll-like receptor (TLR) 4 (9), but little is known about how the response of inflammatory sensors or gain of inflammatory amplifiers may be modulated by nutrient-sensitive proteins.
Sirtuin 1 (SirT1), the mammalian homolog of yeast silent information-regulator 2 (Sir2), is an NAD+-dependent histone deacetylase and an important coordinator of the mammalian metabolic response to fasting and caloric restriction (10–12). During periods of nutrient deprivation, elevated levels of SirT1 in the liver increase hepatic glucose production (10) and induce the expression of oxidative machinery (13). Accordingly, experimental knockdown of SirT1 reduces fasting plasma glucose levels and increases whole-body insulin sensitivity by reducing hepatic glucose production (11,14). In adipose tissue, SirT1 enhances metabolic efficiency by promoting adiponectin production (15). Conversely, adipocyte SirT1 expression is suppressed by high-fat feeding in rodents (16), and its levels are also markedly reduced in the adipose tissue of obese humans and genetically obese rodents (16,17).
In addition to exerting effects on metabolic pathways, SirT1 may repress inflammatory signaling (18,19). siRNA-mediated knockdown of SirT1 in 3T3-L1 adipocytes in vitro increases cytokine mRNA expression when cells are stimulated with tumor necrosis factor (TNF)-α (20). In vivo, overexpression of SirT1 and Dnajc12 reduces hepatic expression of TNF-α and interleukin (IL)-6 after chronic high-fat feeding (21), whereas liver-specific deletion of SirT1 increases hepatic NF-κB pathway activity (13). It is likely that SirT1 may play a similar role in other tissues as well, although the physiologic implications are unclear and remain unexplored in vivo. Two studies have attempted to examine the impact of adipocyte and macrophage SirT1 on inflammation in white adipose tissue (WAT) (20,22). However, these studies used polyphenols to modify SirT1 function in vivo, which are compounds that have been since shown not to be direct activators of SirT1 (20,23–25). Therefore, additional studies are needed to determine the role of SirT1 in these tissues in vivo.
Several mechanisms may account for the effects of SirT1 on inflammation in vitro: SirT1 can induce transcriptional silencing by deacetylating histone 3 at lysine 9 (H3K9) and histone 4 at lysine 16 (H4K16), recruiting H1 and TLE1, and enhancing methyltransferase activity at H3K9 (19,26,27). Additionally, SirT1 may deacetylate NF-κB p65 (18), thereby reducing its transcriptional activity. Finally, SirT1 may inhibit inflammation by increasing expression of the anti-inflammatory adipokine, adiponectin (28). However, it is unclear which of these mechanisms operate in vivo, and in which tissues they are most relevant.
Thus, while it has been established that SirT1 represses NF-κB and that robust decreases in adipose tissue SirT1 expression are observed during obesity, a direct relationship between SirT1 and adipose tissue macrophage recruitment has not been explored. Here, we recapitulated obesity-associated decreases in SirT1 expression or prevented them using a SirT1 overexpression transgene. We found that SirT1 knockdown led to adipose tissue inflammation that closely recapitulates the inflammation seen in obese humans and rodents. Moreover, these changes were prevented by the overexpression of SirT1, suggesting that SirT1 is a key upstream regulator of adipose tissue macrophage content in rodent and human obesity and overnutrition.
RESEARCH DESIGN AND METHODS
Male Sprague Dawley rats (Charles River) or C57BL/6 J mice (The Jackson Laboratory) were given ad libitum access to food and water under a standard 12-h light/dark cycle. MyD88/TRIF−/−, MyD88/IRF3−/−, Caspase-1−/−, and op/op mice were provided by Ruslan Medzhitov (Yale University). SirtT1 fl/fl mice were crossed to aP2-Cre. SirT1 transgenic mice (SirBACO) (15) were fed a high-fat diet (D12492, Research Diets) for 6 weeks. Rodents were fed regular chow (Harlan 2018S), high-fat chow (Harlan TD93075), high-fructose chow (Harlan TD89247), or a combination of high-fat/high-fructose chow as indicated. Antisense oligonucleotides (ASOs) (14) were dosed twice per week at 1.25 mg (for mice) or 37.5 mg/kg (for rats). The T2DM model is described in Erion et al. (14). All experiments were conducted in accordance with Yale University’s Institutional Animal Care and Use Committee policy.
Antibodies.
The following antibodies were used: SirT1 (Upstate Biotechnology); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling); p65 (Genway).
Cytokine measurements.
Obesity array (Phoenix Pharmaceuticals) and 7-plex electrochemiluminescent assay (Meso Scale Discovery) were performed on pooled plasma (n = 7–8/group) per the manufacturers’ instructions. Cytokines were verified in duplicate using commercially available kits (TNF-α OptiEIA, BD Biosciences; rat IL-10, R&D Systems). Membranes spotted with antibodies against 34 soluble cytokines (RayBiotech) were incubated with plasma and then developed by chemiluminescence and quantified by densitometry using ImageJ.
Quantitative RT-PCR.
RNA was extracted using the Qiagen RNeasy kit. Transcript abundance was assessed by real-time PCR on a 7500 Fast Real-Time PCR System (Applied Biosystems) and analyzed by ΔΔCt method (primers available by request). NF-κB quantitative PCR arrays and reagents were purchased from SABiosciences.
Fluorescence-activated cell sorting.
Stromal vascular fractions from bilateral epididymal fat pads (5 mice/group) were digested as previously described (1) using LiberaseTM (Roche). Cells were stained with F4/80-APC and CD11b-PE (eBioscience), run on a FACSCalibur flow cytometer (BD Biosciences), and analyzed using FlowJo (Treestar).
Human studies.
For adolescents, two gram samples of subcutaneous adipose tissue were obtained inferior to the umbilicus after local administration of 0.25% lidocaine with epinephrine, washed in PBS, and frozen in liquid nitrogen. Blood samples were obtained following an overnight fast (29). Homeostasis model assessment of insulin resistance (HOMA-IR) = [fasting glucose (mmol/L) × fasting insulin (mU/L)]/405 (30). Whole-body composition was measured by dual-energy X-ray absorptiometry with a Hologic scanner. Magnetic resonance imaging studies were performed on a GE or Siemens Sonata 1.5 Tesla system (31,32). The nature and potential risks of the study were explained to all subjects before obtaining their written informed consent. The study was approved by the Yale University Human Investigation Committee. The adult subjects were studied as described in Sears et al. (33).
Statistics.
All values are expressed as the mean ± SEM. The significance between the mean values was evaluated by two-tailed unpaired Student t test or ANOVA.
RESULTS
Physiological reduction of SirT1 causes anorexia-driven weight loss and TNF-α elevation.
In order to mimic the reductions of SirT1 expression that occur during obesity, we treated rodents with an ASO against SirT1 to acutely reduce expression in the liver and adipose tissue (Supplementary Fig. 1) (14,34). Unexpectedly, we observed anorexia-driven weight loss (Fig. 1A–C) that persisted in fasted normal chow–fed_(Supplementary Fig. 2B), high-fructose diet–fed (Supplementary Fig. 2D), and high-fat diet–fed animals (Supplementary Fig. 2E), and a reduction in adipose tissue mass that was present even in pair-fed animals (data not shown). Infusing plasma from SirT1 ASO–treated rats reduced food intake in ad libitum fed recipients, suggesting that SirT1 ASO treatment induced production of a circulating anorexigenic factor(s) (Supplementary Fig. 2A) that was independent of vagal inputs (Supplementary Fig. 2C). This effect was not caused by generalized toxicity, since there were no elevations of plasma ALT/AST (14), histolological liver abnormalities, or decreases in skeletal muscle mass (Supplementary Fig. 3A–F). Neither was the effect the result of an antiviral response to the ASO itself, since it was present in two mouse strains that are deficient in nucleic acid sensing pathways (Supplementary Fig. 4).
FIG. 1.
Knockdown of SirT1 mimicking that seen in obesity causes anorexia-driven weight loss and TNF-α elevation. A–C: Body weight (***P < 0.001) (n = 8–12/group) (A), anorexia (n = 12/group) (B), and loss of epididymal fat mass (n = 3–4/group) (C) of ad libitum fed rats treated with ASO biweekly for 1 month. D: Results from parallel measurement of plasma appetite regulatory hormones in animals treated with control or SirT1 ASO showing elevated TNF-α and possibly IL-6 in the SirT1 group (n = 7–8/group, pooled). E: Plasma TNF-α levels (n = 5/group). F: Adipose tissue levels of TNF-α (n = 3–4/group). G: SirT1 protein expression in WAT from animals treated with control ASO and fed normal chow, animals treated with SirT1 ASO and fed normal chow, and mildly diabetic animals fed high-fat/high-fructose chow. H: Quantification of representative data presented in F (n = 6/group). I: Plasma TNF-α levels in normal chow–fed control ASO–treated rats, normal chow–fed SirT1 ASO–treated rats, and high-fat diet–fed, control ASO–treated rats by electrochemiluminescence (n = 4–5/group). HFD, high-fat diet.
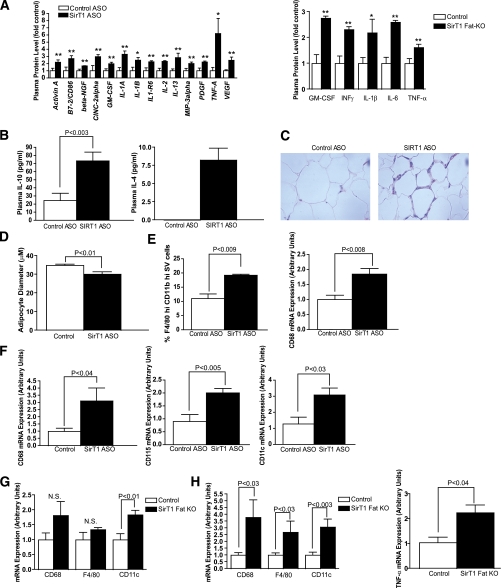
Surprisingly, among 44 previously described positive and negative regulators of appetite measured, all were reduced in SirT1 ASO plasma (consistent with an overall decrease in plasma protein content), with the exception of IL-6, which was unchanged, and TNF-α, a potent negative regulator of food intake (35), which was increased (Fig. 1D and E). We also found significant increases in TNF-α in WAT of SirT1 ASO–treated animals (Fig. 1F) but not in the liver (Supplementary Fig. 3G).
To understand how well our model approximated true physiology, we measured SirT1 protein expression in WAT of 1) normal chow–fed control ASO–treated, 2) normal chow–fed SirT1 ASO–treated, and 3) high-fat diet–, high-fructose diet–fed control ASO–treated rats (a type 2 diabetes model) (14,36). SirT1 protein levels were similarly reduced in the normal chow–fed, SirT1 ASO–treated animals, and the control ASO–treated T2DM model animals (Fig. 1G and H), demonstrating that SirT1 ASO treatment recapitulates obesity-driven decreases in WAT SirT1 expression. Additionally, we found similar increases in plasma TNF-α between SirT1 ASO–treated rats and a high-fat diet–fed group treated with control ASO compared with controls (Fig. 1I).
SirT1 knockdown induces inflammation and macrophage accumulation in WAT.
We observed significant increases in several cytokines including IL-1β, TNF-α, IL-13, IL-10, and IL-4 in plasma of SirT1 ASO–treated animals relative to controls (Fig. 2A and B). In addition, differential intensity gel electrophoresis followed by mass spectrometry revealed increases in acute phase proteins including kininogen-1 (major acute phase protein 9; 19.9 fold increase vs. control ASO–treated animals) and serum amyloid P component (2.3 fold increase from control). Cytokines were similarly elevated in plasma from fat-specific SirT1 knockout mice as early as 4 weeks of age (Supplementary Fig. 1E, Fig. 2A), although body weight did not differ from wild-type mice. Since TNF-α–neutralizing antibody only partially reduced SirT1 knockdown-induced hypophagia (Supplementary Fig. 2G), we hypothesized that some of these cytokines might also contribute to the anorexia seen in SirT1 ASO–treated animals. In support of this hypothesis, we discovered that caspase-1 knockout mice do not experience weight loss in response to SirT1 ASO treatment, suggesting that IL-1α, IL-1β, and/or IL-18 are important for anorexia in the setting of reduced SirT1 expression (Supplementary Fig. 4I).
FIG. 2.
SirT1 knockdown/deletion causes adipose tissue inflammation and macrophage infiltration. A: Cytokine array performed on plasma from normal chow–fed control ASO– and SirT1 ASO–treated rats (n = 4/group). *P < 0.05, **P < 0.01 (left). Cytokine array performed on plasma from chow-fed wild-type and fat-specific SirT1 knockout mice (n = 4/group). *P < 0.08, **P < 0.03 (right). B: Plasma IL-10 levels collected at the onset of hypophagia (1 week) (n = 10/group) and plasma IL-4 after 1 month of ASO treatment. C: Representative hematoxylin and eosin staining from epididymal WAT of individual rats fed normal chow and treated with control ASO or SirT1 ASO (representative of 5 rats/group). D: Reduced adipocyte diameter in SirT1 ASO–treated rats. E: Adipose tissue macrophage content, as assessed by FACS, and adipose tissue CD68 mRNA expression in mice (n = 5/group). F: Macrophage/monocyte (CD68, CD115) and macrophage/dendritic cell (CD11c) marker mRNA expression in rat adipose tissue (n = 4–7). G: Macrophage-related mRNA abundance in adipose tissue from SirT1 fat-specific knockout mice fed a chow diet (8–12 weeks of age, n = 4–6/group). H: Macrophage marker/cytokine mRNA abundance in adipose tissue from SirT1 fat-specific knockout mice fed a high-fat diet for 1 month (n = 3–7/group). GM-CSF, granulocyte-macrophage colony-stimulating factor; KO, knockout; N.S., nonsignificant. (A high-quality digital representation of this figure is available in the online issue.)
Production of cytokines during an inflammatory response is generally accompanied by recruitment of cells of the immune system to the site of injury or infection. Indeed, staining of WAT from rats treated with SirT1 ASO by hematoxylin and eosin revealed a cellular infiltrate that was absent in control ASO–treated rats (Fig. 2C). Using fluorescence-activated cell sorting (FACS) analysis to identify these cells, we found that fat pads of animals treated with SirT1 ASO contained a significantly increased percentage of macrophages (F4/80-hi, CD11b-hi cells), consistent with an increase in CD68 transcript (Fig. 2E). Similarly, fat-specific SirT1 knockout mice had increased WAT expression of CD68, F4/80 (Emr1), CD11c, and TNF-α (Fig. 2G and H). Also consistent with the FACS analysis performed in mice, we found that expression of various myeloid markers including CD68, CD115 (CSF1-R), CD11c (ITGAX), TLR1, and TLR9 were significantly elevated in the adipose tissue of rats treated with SirT1 ASO (Fig. 2F and 3E). The cytokines IL-1β and IL-10 were also significantly increased in adipose tissue of SirT1 ASO–treated rats (Fig. 3E).
FIG. 3.
SirT1 knockdown in WAT results in NF-κB nuclear localization and gene expression through reduction of H3K9 deacetylation. A and B: Representative (n = 5/group) images showing that SirT1 knockdown stimulates nuclear translocation of NF-κB in rat WAT. Adipocytes are stained for caveolin (green) and NF-κB p65 nuclear localization sequence (red). Nuclei are stained with DAPI (blue). Inset box is expanded to individual channels in B.1-B.3 to demonstrate NF-κB and DAPI colocalization. Some NF-κB–positive nuclei (arrows) are in adipocytes. Because the nuclear localization sequence is masked in the cytosol, nonnuclear staining is nonspecific (i.e., erythrocyte or other autofluorescence). C: SirT1 knockdown increases abundance of phosphorylated (Ser536) and total p65 in rat WAT nuclear lysates. Line separates noncontiguous lanes from the same blot. D: Chow-fed fat-specific SirT1 knockout mice have increased phospho-p65 in WAT ∼12 h after stimulation with LPS (50 μg i.p.). E: SirT1 knockdown increases adipose tissue cytokine, complement, and TLR mRNA expression (n = 5–7/group). *P < 0.05, **P < 0.01. F: SirT1 knockdown increases H3K9 acetylation. (A high-quality digital representation of this figure is available in the online issue.)
One unresolved question in the field of obesity-associated adipose tissue inflammation is which chemokine(s) is responsible for macrophage recruitment to WAT (37,38). We observed increased complement component 3 (C3) transcript levels in WAT from SirT1 ASO–treated animals (Fig. 3E), but no change in monocyte chemoattractant protein-1 at the level of mRNA or protein (data not shown). Thus, we hypothesize that C3 could mediate monocyte/macrophage attraction to adipose tissue in the setting of SirT1 ASO treatment, as it may in obesity. Recent data have also suggested that free fatty acids associated with lipolysis and weight loss can also recruit macrophages to WAT (39). However, weight loss was not required for macrophage recruitment in our model. As mentioned, neither caspase-1 knockout mice treated with SirT1 ASO nor fat-specific SirT1 knockout mice lost weight, although they did exhibit signs of adipose tissue inflammation. A second SirT1 ASO sequence also produced WAT inflammation despite less efficient knockdown and no weight loss (Supplementary Fig. 8).
SirT1 knockdown in WAT results in NF-κB nuclear translocation and gene expression by reducing H3K9 deacetylation.
We observed an increase in NF-κB nuclear localization in WAT of SirT1 ASO–treated rats compared with controls (Fig. 3A and B), consistent with an increase in nuclear phosphorylated and total p65 seen on Western blot (Fig. 3C). Phosphorylated p65 was also increased in WAT of lipopolysaccharide-treated fat-specific SirT1 knockout mice (Fig. 3D). When we compared WAT from SirT1 ASO–treated rats to control ASO–treated rats, we observed a global increase in H3K9 acetylation (Fig. 3F), a transcriptionally activating histone modification previously shown to be reduced by SirT1 and SirT6 (26,40). We did not, however, observe alterations in p65 acetylation or p53 acetylation (Supplementary Fig. 5A and B). Thus, we believe that the increases we observe in inflammatory markers is the result of H3K9 hyperacetylation that leads to ectopic expression of normally silenced genes in adipose tissue where SirT1 has been knocked down. This conclusion agrees with a recent report in which SirT1 was shown to accumulate at the TNF-α and IL-1β promoters to terminate NF-κB–dependent signaling via histone deacetylation (27). In addition, we observed a large reduction in adiponectin expression (∼90% decrease in mRNA) and plasma concentrations with SirT1 knockdown (14). Since accumulating evidence suggests that adiponectin can suppress transcription of NF-κB–regulated targets (28), this reduction might also contribute to the initiation and/or propagation of adipose tissue inflammation.
To assess the contribution of various cells to the phenotype observed in WAT with SirT1 knockdown, we separated the adipocyte and stromal vascular fractions in rats treated with SirT1 or control ASO. We observed SirT1 knockdown (∼90% relative to control) in both the adipocyte and stromal vascular fractions from animals treated with SirT1 ASO (Supplementary Fig. 6A and B) but were unable to generate reliable measurements of cytokines in each fraction. To address this problem indirectly, we harvested peritoneal macrophages from rats treated with control or SirT1 ASO. SirT1 ASO treatment significantly reduced SirT1 mRNA while elevating TNF-α expression in these macrophages, suggesting that macrophages may contribute to the systemic and local inflammation caused by SirT1 depletion (Supplementary Fig. 6C and D). Similarly, we found that peritoneal macrophages harvested from high-fat diet–fed mice had reduced SirT1 expression relative to controls (data not shown), suggesting that SirT1 expression in both macrophages and adipocytes is decreased in obesity as well as ASO treatment and may contribute to the associated inflammation.
When we compared the adipose tissue of wild-type and macrophage-deficient mice op/op, we discovered that many NF-κB–associated genes were increased in op/op mice during SirT1 knockdown (Supplementary Fig. 6E and F). Thus, although SirT1 knockdown can cause inflammation in macrophages, this is not required for the adipose tissue inflammation we observe in wild-type animals.
Overexpression of SirT1 prevents high-fat diet–induced increases in adipose tissue inflammation and macrophage infiltration.
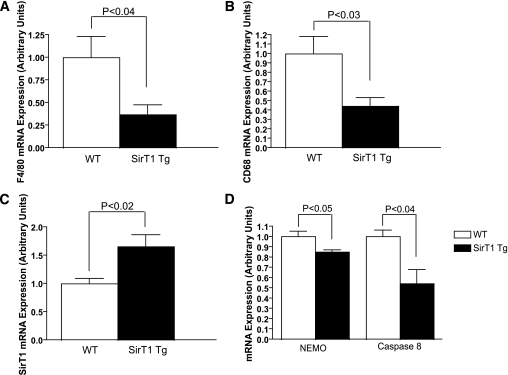
If decreases in SirT1 expression during obesity are responsible for the low-grade inflammation seen in the adipose tissue of obese rodents and humans, preventing such decreases would be expected to reduce obesity-associated inflammation. To assess this possibility, we examined SirT1-overexpressing mice (which have two- to threefold normal levels of SirT1 in most tissues at baseline) (15) (Fig. 4C) made obese through 6 weeks of high-fat diet. These animals have been previously shown to have higher plasma concentrations of adiponectin and are partially protected from diet-induced insulin resistance (15). Supporting our aforementioned results, we found a >50% reduction of CD68, F4/80, and other macrophage-related transcripts in WAT from obese SirT1-overexpressing transgenic animals relative to obese wild-type mice (Fig. 4A and B, Supplementary Fig.7), as well as reduced expression of NEMO (IKBKG), a scaffold required for NF-κB activation, and caspase-8, an apoptotic effector downstream of TNF-α signaling (Fig. 4D). These data demonstrate that preventing the normal decrease in SirT1 caused by high-fat feeding is sufficient to prevent the majority of obesity-associated adipose tissue inflammation.
FIG. 4.
Overexpression of SirT1 reduces high-fat diet–induced increases in WAT macrophage content. A and B: Reduced F4/80 (A) and CD68 (B) expression in WAT of high-fat diet–fed SirT1-overexpressing mice by qPCR. C: Verification of SirT1 overexpression in WAT (n = 5/group). D: NEMO and CASP8 expression, also in WAT (n = 3/group).
Surprisingly, WAT of SirT1 transgenic animals fed regular chow exhibited elevated expression of macrophage markers and inflammatory genes (Supplementary Fig. 7A). Thus, both SirT1 deficiency and excess in adipose tissue leads to inflammatory pathology. While surprising, this observation is reminiscent of results observed with NF-κB pathway disruption; not only does NF-κB activation lead to inflammation but in many cell types, inhibition of NF-κB also causes severe tissue destruction and inflammation (41,42). Thus, we hypothesize that SirT1 inhibition of the NF-κB pathway could lead to inflammation in the basal state and reduced inflammation during times of tissue stress.
SirT1 level is inversely related to BMI and adipose tissue macrophage infiltration in human subjects.
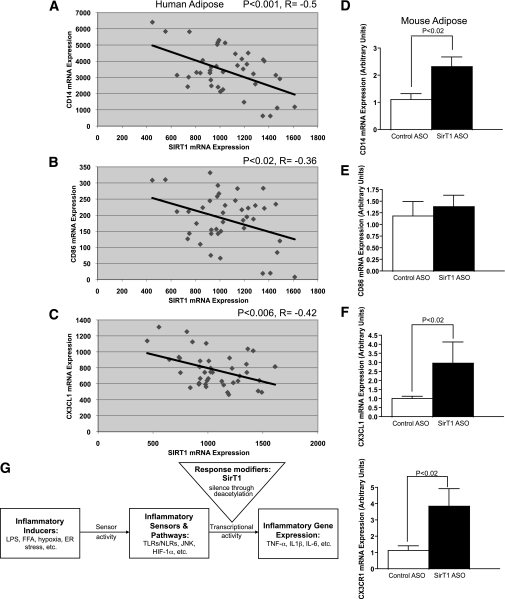
To determine whether our finding that SirT1 knockdown led to adipose tissue inflammation and macrophage infiltration in rodents translates to humans, we examined SirT1 levels and macrophage content in subcutaneous adipose tissue of nonobese, moderately obese, and severely obese adolescents (Fig. 5A, Supplementary Table 1). Consistent with numerous existing reports, obese subjects had substantially increased HOMA-IR (a commonly used indicator of whole-body insulin resistance) (30) (Fig. 5D). Strikingly, in agreement with our observations in rodents, moderate and severe obesity were associated with significant reductions in SirT1 mRNA expression (Fig. 5B) and increased adipose tissue macrophage content (Fig. 5C). Using a second cohort of mixed lean and obese adults, we found that SirT1 mRNA expression was inversely correlated with the established human monocyte/macrophage markers CD14 and CD86, and the monocyte chemokine CX3CL1 (Fig. 6A–C), confirming that SirT1 expression is negatively correlated with adipose tissue macrophage content in lean and obese human subjects. Likewise, in rodents, CX3CL1, CX3CR1, and CD14 were all increased with SirT1 knockdown (Fig. 6D–F). Thus, in rodents, obese human adolescents, and lean or obese human adults, SirT1 expression is directly related to adipose tissue macrophage content. These data support a mechanistic relationship between adipose tissue SirT1 dysregulation and inflammation.
FIG. 5.
SirT1 transcript level is inversely correlated with both BMI and adipose tissue macrophage content in humans. A: Control, obese, and severely obese subjects were stratified by BMI. Obese and severely obese subjects have decreased SirT1 expression (B) and increased macrophage content (C) in subcutaneous adipose tissue. Macrophages (CD68+ cells) within an entire section were counted by two independent observers using a light microscope. % macrophages = number of macrophages / number of adipocytes × 100. Obese and severely obese subjects have elevated HOMA-IR (D). **P < 0.01, *P < 0.05, #P < 0.10. Note: 18 of the 45 subjects examined are also being studied for the contributions of a gene polymorphism to SirT1 gene expression.
FIG. 6.
SirT1 transcript level is inversely correlated with macrophage markers in human adults. A: Correlation between SirT1 mRNA expression and CD14 (a macrophage/monocyte marker) in adipose tissue of a mixed-weight population of humans. B: Correlation between SirT1 and CD86 mRNA (another monocyte/macrophage marker) in WAT from the same group of subjects. C: Correlation between SirT1 and CX3CL1 (a monocyte chemoattractant) mRNA in WAT from the same group of subjects. D–F: SirT1 knockdown increases expression of CX3CL1, CX3CR1, and CD14 in rodent WAT. G: Schematic model of SirT1 regulation of inflammation. SirT1 deacetylation of inflammatory gene promoters causes decreased cytokine production in response to stimulation of inflammatory sensors by fatty acids, hypoxia, and ER stress. In turn, decreased SirT1 expression in obesity sensitizes these networks to activation by stressors. FFA, free fatty acid; NLR, NOD-like receptor.
DISCUSSION
Building on well-established observations that SirT1 levels and activity are reduced during states of overnutrition, we recreated these SirT1 reductions in nonobese animals. We find that reducing SirT1 expression in lean animals is sufficient to cause adipose tissue NF-κB activation, tissue and plasma cytokine elevations, and macrophage infiltration similar to that seen in obesity. This suggests that SirT1 may be causally implicated in obesity-associated adipose tissue macrophage infiltration. In further support of this hypothesis, we found that SirT1 expression in subcutaneous adipose tissue of human subjects was inversely correlated with macrophage content. Conversely, overexpression of SirT1 substantially reduced macrophage content in mice made obese by high-fat feeding.
In addition to general markers of macrophages, we found that CD11c transcripts were significantly increased in WAT of SirT1 ASO–treated rats. Although CD11c is generally considered a marker of dendritic cells, recent work has suggested that a subset of adipose tissue macrophages expressing CD11c are disproportionately increased during obesity and are the major contributors to adipose tissue inflammation (43,44). Thus, SirT1 knockdown did not simply induce adipose tissue inflammation, but specifically led to the recruitment of a macrophage population phenotypically similar to that seen during obesity.
A large body of literature suggests that infiltrating monocytes, perhaps recruited to dispose of damaged adipocytes, differentiate by default into “M1” macrophages and propagate adipose tissue inflammation through the production of inflammatory cytokines (2). However, we observed increases in plasma IL-10, IL-4, and IL-13, all cytokines that are associated with “M2” anti-inflammatory macrophage polarization (45). This is consistent with data showing that although “M1” macrophages are disproportionately increased, “M2” macrophages (CD11c-) are also dramatically increased in obese WAT (44). In addition, we found that macrophage-deficient mice had exacerbated NF-κB–associated gene expression when treated with SirT1 ASO. This underscores the need to better appreciate the heterogeneous roles of infiltrating macrophages, some of which, through the production anti-inflammatory cytokines, may actually temper, rather than perpetuate, adipocyte inflammation.
As discussed, adipose tissue macrophages are thought to be major producers of obesity-associated proinflammatory cytokines and are hypothesized to worsen the already compromised adipose tissue function through pathological induction of insulin resistance and lipolysis. In our model, however, inflammatory changes were not accompanied by a deterioration of metabolic parameters in either normal or diabetic rats. Rather, SirT1 ASO led to a substantial improvement in hyperglycemia in the T2DM rat model that was attributable to a reduction in gluconeogenesis caused by SirT1 knockdown in the liver (14,36,46). This effect was observed in even pair-fed animals (14), ruling out reduced body weight as a trivial explanation. However, we observed adipose tissue atrophy in SirT1 knockdown animals, in accord with the potent lipolytic and antiadipogenic effects of increased local inflammatory cytokines. Over time, it is possible that this phenomenon could lead to impaired insulin responsiveness in liver and skeletal muscle via ectopic lipid accumulation (46,47). Moreover, although it is clear that genetic reduction of SirT1 in fat recapitulates the inflammation seen during acute knockdown, it is not clear whether this inflammation will produce similar metabolic effects over the long term. Acute and chronic inflammation can create apparently opposite results as negative regulators desensitize inflammatory signals over time—lipolysis and anorexia are well-recognized features of acute endotoxemia, for example, yet chronic endoxemia was reported to cause obesity (48). In support of this possibility, elevated plasma glucose and insulin levels were observed in high-fat diet–fed fat-specific SirT1 knockout mice (Supplementary Fig. 1F and G).
Interestingly, although we observed inflammation in WAT of SirT1 ASO–treated animals, we did not observe gross evidence of inflammation in the liver. This initially surprised us, in light of work by Purushotham et al. (13) demonstrating hepatic inflammation following deletion of SirT1 in the liver. However, our model, in contrast to that of Purushotham et al., uses an acute physiological reduction rather than complete embryonic deletion of SirT1. Thus, moderate reduction of hepatic SirT1 with the ASO may not be sufficient in vivo to induce overt hepatic inflammation.
Sirtuins have been shown capable of suppressing NF-κB–dependent transcription in vitro by deacetylating p65/RelA, p53, and/or histones 3/4 (18,26,49). In congruence with these reports, we observed a significant increase in H3K9 acetylation in WAT of SirT1 ASO–treated animals relative to controls. Whether the chromatin modifications induced by SirT1 knockdown are sufficient to recruit polymerases and initiate transcription is unclear. We present a hypothetical model (Fig. 6G) in which SirT1 modulates the sensitivity (“gain”) of inflammatory pathways, thereby making them more prone to or prolonging their activation by previously implicated stimuli (e.g., TLR4 activation, ER stress). It is also possible that SirT1 knockdown induces inflammation indirectly by activating HIF-1α, ER stress, or other proposed pathways.
In vitro, SirT1 is reported to suppress peroxisome proliferator–activated receptor γ (PPARγ) (50). However, SirT1 gain-of-function in mice leads to hyperadiponectinemia and greater obesity on the ob/ob background (15), suggestive of increased PPARγ activity. According to our data, a major function of SirT1 in whole adipose tissue in vivo is to suppress inflammation, which will in turn disinhibit PPARγ—an effect that would not be observed in cell culture.
In summary, although the mechanism connecting obesity to adipose tissue inflammation has long been enigmatic, this current work demonstrates that SirT1 can sense nutrient status and directly translate this signal to disinhibition of NF-κB–driven inflammation. Moreover, our model successfully unites observations not previously appreciated to be related: elevations in adipose tissue macrophage content and cytokine production during obesity and overnutrition, evidence demonstrating that SirT1 is a negative regulator of NF-κB, and in vivo data indicating that high-fat feeding and obesity decrease SirT1 protein levels in adipocytes (20). Here we show that inhibiting SirT1 gene expression in the liver and WAT is sufficient to recapitulate the cytokine elevations seen in rodent and human overnutrition, as well as to trigger adipose tissue macrophage recruitment. Conversely, overexpression of SirT1 effectively blunts obesity-induced adipose tissue macrophage infiltration. Finally, we find that SirT1 expression in subcutaneous fat of human subjects is inversely correlated with BMI, macrophage content, and HOMA-IR. Taken together these data suggest that positive energy balance in both rodents and humans suppress expression and activity of adipose tissue SirT1, disinhibiting cytokine/chemokine production, and inducing monocyte and macrophage infiltration.
ACKNOWLEDGMENTS
This study was funded by grants from the United States Public Health Service: R01 DK40936 and U24 DK059635 (G.I.S.) and R01 HD40787, R01 HD 28016, and K24 HD01464 (S.C.); by Clinical and Translational Science Awards Grant Number UL1 RR 0249139 from the National Center for Research Resources, a component of the National Institutes of Health (NIH); by R01 EB006494 (Bioimage Suite) and Distinguished Clinical Scientist Awards from the American Diabetes Association to S.C.; and by NIH MSTP TG 2T32GM07205 (M.E.K.) and the Bumpus Foundation (M.P.G.).
S.B. is an employee of Isis Pharmaceuticals and owns stock in the company. E.S.M. is a Merck employee and owns stock in the company. No other potential conflicts of interest relevant to this article were reported.
M.P.G., M.E.K., D.M.E., P.C., J.M.O., D.D.S., and G.I.S. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. R.K., K.T.N., E.S.M., J.J.H., D.W.F., S.Y., A.S.B., and L.Q. researched data. S.B. and S.C. researched data and reviewed and edited the manuscript.
The authors thank Ruslan Medzhitov, Howard Hughes Medical Institute, Yale University School of Medicine, for generously providing MyD88/Trif, MyD88/IRF3, and Caspase-1 knockout mice and for his advice regarding experiments and in preparing this manuscript. They also thank Dominico Accili, Columbia University College of Physicians and Surgeons, for sharing the SirT1-overexpressing transgenic mice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0616/-/DC1.
See accompanying commentary, p. 3100.
REFERENCES
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 3.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 4.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 5.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008;322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 7.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–911 [DOI] [PubMed] [Google Scholar]
- 8.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 2009;29:4467–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–118 [DOI] [PubMed] [Google Scholar]
- 11.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA 2007;104:12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med 2007;13:64–71 [DOI] [PubMed] [Google Scholar]
- 13.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 2009;9:327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erion DM, Yonemitsu S, Nie Y, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci USA 2009;106:11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 2006;281:39915–39924 [DOI] [PubMed] [Google Scholar]
- 17.Costa CD, Hammes TO, Rohden F, et al. SirT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg 2009;20:633–639 [DOI] [PubMed]
- 18.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh HS, Spencer JV, Ng B, McBurney MW, Robbins PD. Sirt1 interacts with transducin-like enhancer of split-1 to inhibit nuclear factor kappaB-mediated transcription. Biochem J 2007;408:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizaki T, Milne JC, Imamura T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 2009;29:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 2008;105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshizaki T, Schenk S, Imamura T, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 2009;298:E419–E428 [DOI] [PMC free article] [PubMed]
- 23.Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem 2005;280:17038–17045 [DOI] [PubMed] [Google Scholar]
- 24.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 2005;280:17187–17195 [DOI] [PubMed] [Google Scholar]
- 25.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 2010;285:8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 2004;16:93–105 [DOI] [PubMed] [Google Scholar]
- 27.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem 2011;286:9856–9864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo N, Liu J, Chung BH, et al. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes 2010;59:791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cali’ AM, Bonadonna RC, Trombetta M, Weiss R, Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab 2008;93:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 31.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 2006;91:4287–4294 [DOI] [PubMed] [Google Scholar]
- 32.Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 2009;49:1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sears DD, Hsiao G, Hsiao A, et al. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci USA 2009;106:18745–18750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai Y, Yonemitsu S, Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 2009;9:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol 2004;25:193–200 [DOI] [PubMed] [Google Scholar]
- 36.Nie Y, Erion DM, Yuan Z, et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol 2009;11:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamane Y, Chung Chan C, Lavallee G, et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes 2009;58:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 2010;120:3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009;136:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makris C, Godfrey VL, Krähn-Senftleben G, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 2000;5:969–979 [DOI] [PubMed] [Google Scholar]
- 42.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol 2009;9:778–788 [DOI] [PubMed] [Google Scholar]
- 43.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35 [DOI] [PubMed] [Google Scholar]
- 46.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 49.Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001;107:149–159 [DOI] [PubMed] [Google Scholar]
- 50.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004;429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]