Abstract
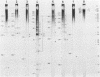
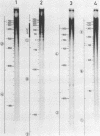
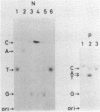
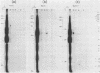
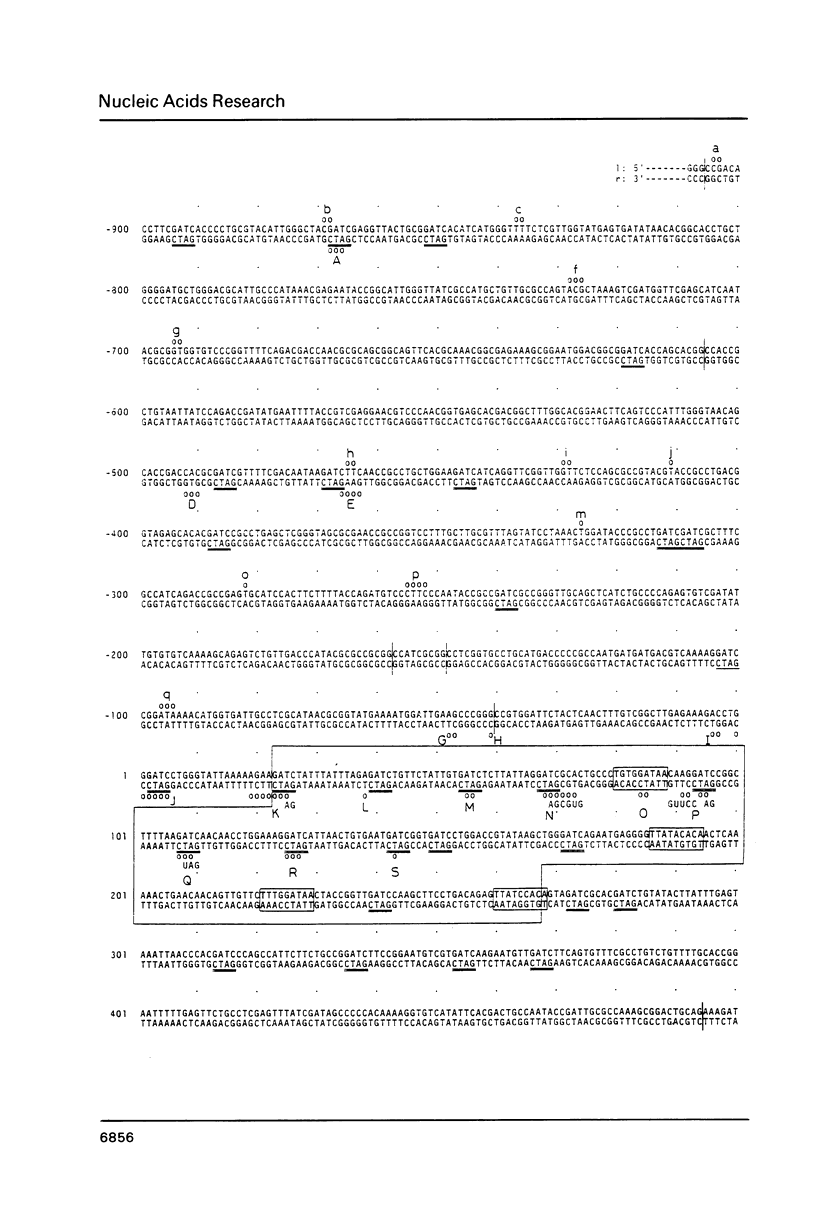
RNA-linked DNA molecules were obtained from E. coli dnaCts cells synchronously initiating a new round of chromosome replication. The deoxynucleotides at the transition from primer RNA to DNA were 32P-labeled, and their positions were located on the nucleotide sequence of 1.4 kb genomic region (position -906 to +493) including the oriC and its leftside flanking region. In the r-strand (the counterclockwise strand), many strong transition sites were mapped in the left half portion of the oriC and a few weak sites in the left outside region. In the 1-strand (the clockwise strand), no transition sites were found inside the oriC but many weak sites were found in the left outside region. The results support the initiation mechanism in which the first leading strand synthesis starts with the r-strand counterclockwise from the oriC that is followed by the 1-strand synthesis on the displaced template strand on the left of oriC. Primer RNA molecules attached to the strong r-strand transition sites were only a few residues in length. Properties of the transition sites were discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Hansen F. G. Effect of dnaA and rpoB mutations on attenuation in the trp operon of Escherichia coli. J Bacteriol. 1983 Dec;156(3):985–992. doi: 10.1128/jb.156.3.985-992.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Vovis G. F., Zinder N. D. Insertion mutant of bacteriophage f1 sensitive to EcoRI. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2699–2702. doi: 10.1073/pnas.76.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Kohara Y., Okazaki T. Initiation sites for discontinuous DNA synthesis of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Feb;78(2):903–907. doi: 10.1073/pnas.78.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
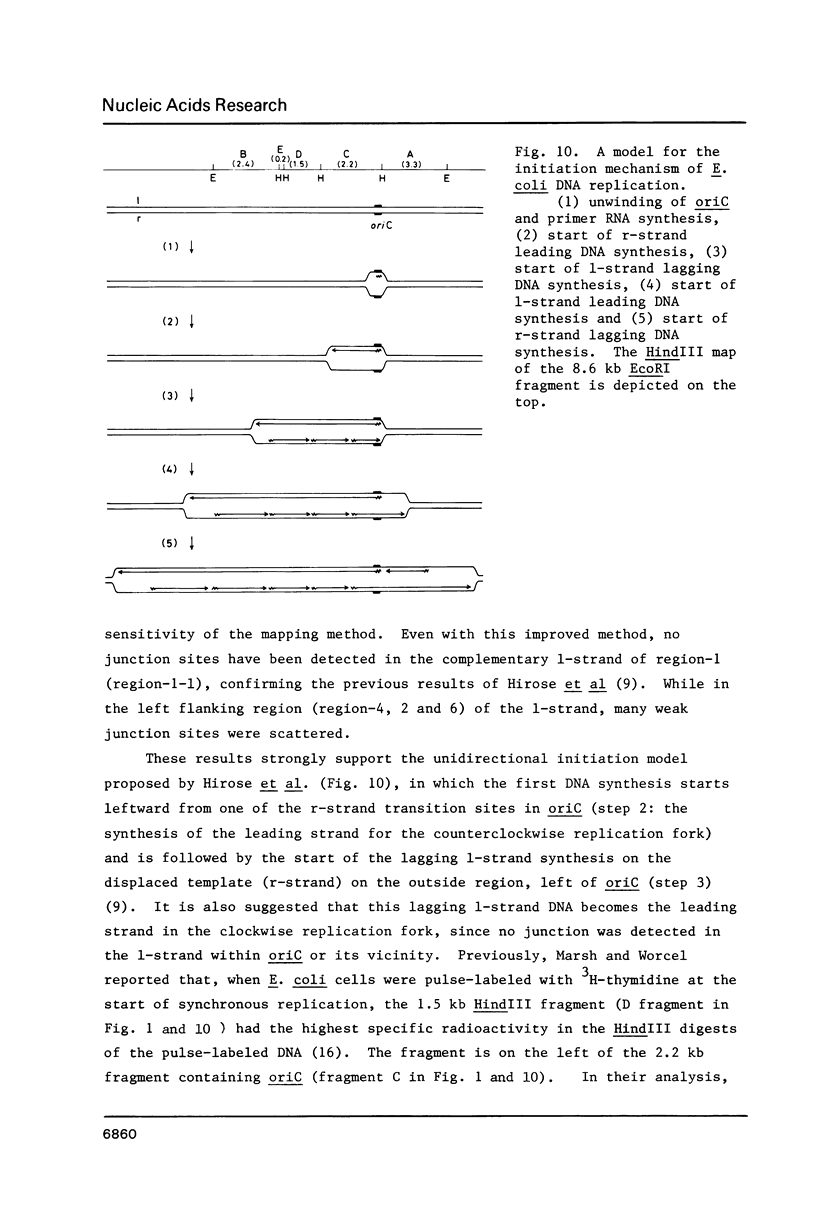
- Hirose S., Hiraga S., Okazaki T. Initiation site of deoxyribonucleotide polymerization at the replication origin of the Escherichia coli chromosome. Mol Gen Genet. 1983;189(3):422–431. doi: 10.1007/BF00325904. [DOI] [PubMed] [Google Scholar]
- Hughes P., Squali-Houssaini F., Forterre P., Kohiyama M. In vitro replication of a dam methylated and non-methylated ori-C plasmid. J Mol Biol. 1984 Jun 15;176(1):155–159. doi: 10.1016/0022-2836(84)90386-3. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Lother H., Kölling R., Kücherer C., Schauzu M. dnaA protein-regulated transcription: effects on the in vitro replication of Escherichia coli minichromosomes. EMBO J. 1985 Feb;4(2):555–560. doi: 10.1002/j.1460-2075.1985.tb03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Messer W. Promoters in the E. coli replication origin. Nature. 1981 Nov 26;294(5839):376–378. doi: 10.1038/294376a0. [DOI] [PubMed] [Google Scholar]
- Low R. L., Arai K., Kornberg A. Conservation of the primosome in successive stages of phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1436–1440. doi: 10.1073/pnas.78.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Arai K., Okazaki T. Site selection and structure of DNA-linked RNA primers synthesized by the primosome in phage phi X174 DNA replication in vitro. J Biol Chem. 1983 Nov 10;258(21):13353–13358. [PubMed] [Google Scholar]
- Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth XVI. Analyses of RNA-linked DNA pieces in Escherichia coli with polynucleotide kinase. J Mol Biol. 1977 May 5;112(1):121–140. doi: 10.1016/s0022-2836(77)80160-5. [DOI] [PubMed] [Google Scholar]
- Oka A., Sasaki H., Sugimoto K., Takanami M. Sequence organization of replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 15;176(4):443–458. doi: 10.1016/0022-2836(84)90171-2. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Hirose S., Okazaki T., Ogawa T., Kurosawa Y. Assay of RNA-linked nascent DNA pieces with polynucleotide kinase. Biochem Biophys Res Commun. 1975 Feb 17;62(4):1018–1024. doi: 10.1016/0006-291x(75)90424-6. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Bacteriophage T7: minimal requirements for the replication of a duplex DNA molecule. Cell. 1983 Jun;33(2):315–317. doi: 10.1016/0092-8674(83)90411-7. [DOI] [PubMed] [Google Scholar]
- Sims J., Benz E. W., Jr Initiation of DNA replication by the Escherichia coli dnaG protein: evidence that tertiary structure is involved. Proc Natl Acad Sci U S A. 1980 Feb;77(2):900–904. doi: 10.1073/pnas.77.2.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Meijer M. Maintenance and incompatibility of plasmids carrying the replication origin of the Escherichia coli chromosome: evidence for a control region of replication between oriC and asnA. Nucleic Acids Res. 1983 Aug 25;11(16):5775–5791. doi: 10.1093/nar/11.16.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Weisbeek P. J., Meijer M. Initiation signals for complementary strand DNA synthesis in the region of the replication origin of the Escherichia coli chromosome. Nucleic Acids Res. 1984 Apr 11;12(7):3321–3332. doi: 10.1093/nar/12.7.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata S., Oka A., Sugimoto K., Takanami M., Yasuda S., Hirota Y. The 245 base-pair oriC sequence of the E. coli chromosome directs bidirectional replication at an adjacent region. Nucleic Acids Res. 1983 May 11;11(9):2617–2626. doi: 10.1093/nar/11.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Saito H., Richardson C. C. Physical mapping of primary and secondary origins of bacteriophage T7 DNA replication. Proc Natl Acad Sci U S A. 1980 May;77(5):2656–2660. doi: 10.1073/pnas.77.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Dyer T. A., Brownlee G. G. An improved direct RNA sequence method; its application to Vicia faba 5.8S ribosomal RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1259–1272. doi: 10.1093/nar/8.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Multiple initiation sites of DNA replication flanking the origin region of lambda dv genome. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7402–7406. doi: 10.1073/pnas.81.23.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]