Abstract
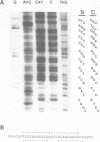
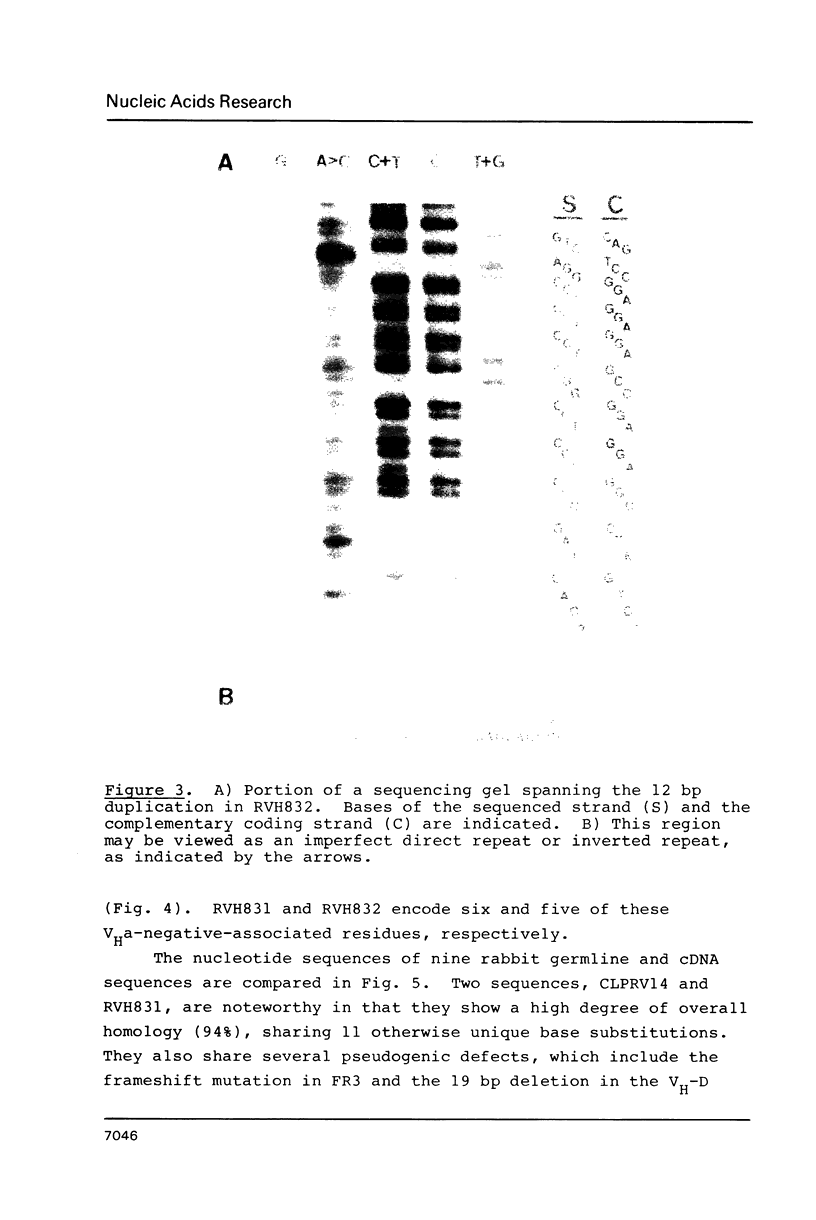
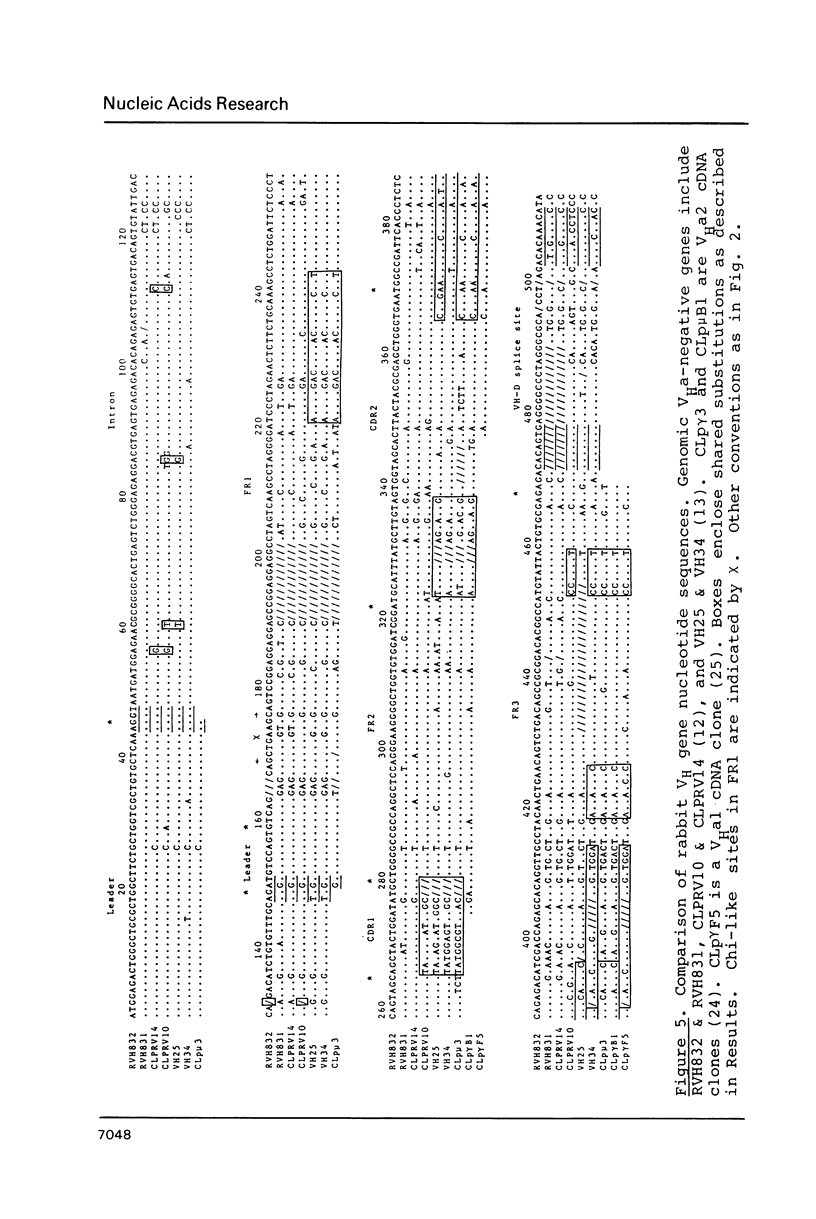
Two rabbit VHa-negative genes, RVH831 and RVH832, were isolated from a single genomic fragment selected by hybridization with the mouse VHIII gene S107V1. RVH831 is a pseudogene with a frameshift mutation in FR3 and a 19 bp deletion within the VH-D splice site. In contrast, RVH832 has an open reading frame and an intact VH-D splice site and thus may be functional. However, RVH832 displays a unique 4 codon duplication/insertion in FR1 that may be the result of an unequal exchange event between two ancestral VH genes. Sequence comparisons between these and other rabbit VH genes reveal patterns of shared blocks of nucleotide substitutions, suggestive of gene conversion. A high overall homology (greater than or equal to 73%) between the compared VH nucleotide sequences suggests that rabbit VH genes may not be organized in clearly divergent families or subgroups.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernstein K. E., Alexander C. B., Mage R. G. Germline VH genes in an a3 rabbit not typical of any one VHa allotype. J Immunol. 1985 May;134(5):3480–3488. [PubMed] [Google Scholar]
- Bernstein K. E., Alexander C. B., Mage R. G. Nucleotide sequence of a rabbit IgG heavy chain from the recombinant F-I haplotype. Immunogenetics. 1983;18(4):387–397. doi: 10.1007/BF00372471. [DOI] [PubMed] [Google Scholar]
- Bernstein K. E., Alexander C. B., Reddy E. P., Mage R. G. Complete sequence of a cloned cDNA encoding rabbit secreted mu-chain of VHa2 allotype: comparisons with VHa1 and membrane mu sequences. J Immunol. 1984 Jan;132(1):490–495. [PubMed] [Google Scholar]
- Blankenstein T., Zoebelein G., Krawinkel U. Analysis of immunoglobulin heavy chain V-region genes belonging to the V NP-gene family. Nucleic Acids Res. 1984 Sep 11;12(17):6887–6900. doi: 10.1093/nar/12.17.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Egel R. Intergenic conversion and reiterated genes. Nature. 1981 Mar 19;290(5803):191–192. doi: 10.1038/290191a0. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Gilman-Sachs A., Horng W. J., Dray S. Immunoregulation of rabbit immunoglobulin allotypes through an allotype-idiotype network. Surv Immunol Res. 1983;2(4):351–359. doi: 10.1007/BF02918452. [DOI] [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A. P., Thunberg A. L., Kindt T. J. Homogeneous rabbit immunoglobulin lacking group a allotypes: amino acid sequence analysis of the heavy chain. Biochemistry. 1978 Apr 4;17(7):1337–1344. doi: 10.1021/bi00600a031. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Tyler B., Bernard O., Gough N., Gerondakis S., Adams J. M., Cory S. Organization of genes and spacers within the mouse immunoglobulin VH locus. J Mol Appl Genet. 1981;1(3):245–261. [PubMed] [Google Scholar]
- Kenter A. L., Birshtein B. K. Chi, a promoter of generalized recombination in lambda phage, is present in immunoglobulin genes. Nature. 1981 Oct 1;293(5831):402–404. doi: 10.1038/293402a0. [DOI] [PubMed] [Google Scholar]
- Kim B. S., Dray S. Expression of the a, x, and y variable region genes of heavy chains among IgG, IgM, and IgA molecules of normal and a locus allotype-suppressed rabbits. J Immunol. 1973 Sep;111(3):750–760. [PubMed] [Google Scholar]
- Kim B. S., Dray S. Identification and genetic control of allotypic specificities on two variable region subgroups of rabbit immunoglobulin heavy chains. Eur J Immunol. 1972 Dec;2(6):509–514. doi: 10.1002/eji.1830020608. [DOI] [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage R. G., Bernstein K. E., McCartney-Francis N., Alexander C. B., Young-Cooper G. O., Padlan E. A., Cohen G. H. The structural and genetic basis for expression of normal and latent VHa allotypes of the rabbit. Mol Immunol. 1984 Nov;21(11):1067–1081. doi: 10.1016/0161-5890(84)90117-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D. W. Regulation of rabbit allotypes. A comparison with murine idiotype and allotype systems. Surv Immunol Res. 1983;2(4):360–366. [PubMed] [Google Scholar]
- Metzger D. W., Roux K. H. A predominant idiotype on rabbit anti-VH a1 allotype antibodies: sharing by both major and minor VH subgroups. J Immunol. 1982 Sep;129(3):1138–1142. [PubMed] [Google Scholar]
- OUDIN J. Allotypy of rabbit serum proteins. II. Relationships between various allotypes: their common antigenic specificity, their distribution in a sample population; genetic implications. J Exp Med. 1960 Jul 1;112:125–142. doi: 10.1084/jem.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Rodakis G. C., Lecanidou R., Eickbush T. H. Diversity in a chorion multigene family created by tandem duplications and a putative gene-conversion event. J Mol Evol. 1984;20(3-4):265–273. doi: 10.1007/BF02104732. [DOI] [PubMed] [Google Scholar]
- Roux K. H. A fourth heavy chain variable region subgroup, w, with 2 variants defined by an induced auto-antiserum in the rabbit. J Immunol. 1981 Aug;127(2):626–632. [PubMed] [Google Scholar]
- Roux K. H., McCormack W. T., Dhanarajan P. A reevaluation of rabbit anti-allotype antibody for the presence of cross-reactive idiotypes. I. A species-specific idiotype on rabbit anti-a1 antibody is recognized by guinea pig anti-IdX antibody. J Immunol. 1985 Sep;135(3):1961–1966. [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Unequal crossover and the evolution of multigene families. Cold Spring Harb Symp Quant Biol. 1974;38:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]