Abstract
Pathogenic bacteria have increasingly been resisting to antimicrobial therapy. Recently, resistance problem has been relatively much worsened in Gram-negative bacilli. Acinetobacter spp. are typical nosocomial pathogens causing infections and high mortality, almost exclusively in compromised hospital patients. Acinetobacter spp. are intrinsically less susceptible to antibiotics than Enterobacteriaceae, and have propensity to acquire resistance. A surveillance study in Korea in 2009 showed that resistance rates of Acinetobacter spp. were very high: to fluoroquinolone 67%, to amikacin 48%, to ceftazidime 66% and to imipenem 51%. Carbapenem resistance was mostly due to OXA type carbapenemase production in A. baumannii isolates, whereas it was due to metallo-β-lactamase production in non-baumannii Acinetobacter isolates. Colistin-resistant isolates were rare but started to be isolated in Korea. Currently, the infection caused by multidrug-resistant A. baumannii is among the most difficult ones to treat. Analysis at tertiary care hospital in 2010 showed that among the 1,085 isolates of Acinetobacter spp., 14.9% and 41.8% were resistant to seven, and to all eight antimicrobial agents tested, respectively. It is known to be difficult to prevent Acinetobacter spp. infection in hospitalized patients, because the organisms are ubiquitous in hospital environment. Efforts to control resistant bacteria in Korea by hospitals, relevant scientific societies and government agencies have only partially been successful. We need concerted multidisciplinary efforts to preserve the efficacy of currently available antimicrobial agents, by following the principles of antimicrobial stewardship.
Keywords: Acinetobacter baumannii, multidrug resistance, OXA type carbapenemase, metallo-β-lactamase
INTRODUCTION
Antimicrobial agents (antibiotics) are extremely important drugs to fight against bacterial infections.1 When antibiotics were first introduced in the 1940s, they were considered as "miracle drugs": highly fatal pneumococcal pneumonia and infant meningitis patients could be saved and syphilis became easily controllable infection with penicillin G, and tuberculosis could be cured with streptomycin. With the use of antibiotics, major surgery became a relatively safe procedure, and cancer patients were protected during the critical period of decreased leucocytes due to chemotherapy.
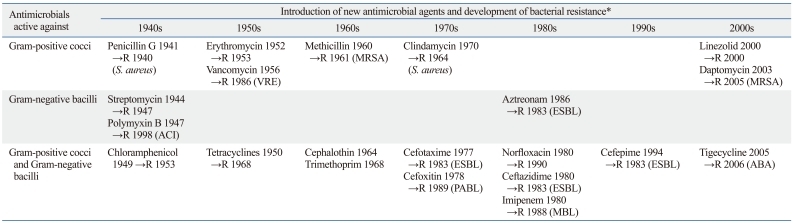
However, bacteria have increasingly been resisting to antimicrobial therapy. In general, new resistances have been detected soon after introduction of new antimicrobial agents (Table 1). To cope with resistant bacteria, new antimicrobial agents had to be developed, but it became increasingly difficult to find new ones.2 Now, we know that emergence of antibiotic resistance is an inevitable consequence when we use antibiotics. Therefore, we must seek to decrease its impact and prolong the effectiveness of currently available antimicrobial agents.3
Table 1.
The Approximate Year of Introduction of Antimicrobial Agents and Consequent Emergence of Resistant Bacteria
ABA, A. baumannii; ACI, Acinetobacter spp.; ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase; MRSA, methicillin-resistant S. aureus; PABL, plasmid-mediated AmpC β-lactamase; VRE, vancomycin-resistant enterococci.
→R, approximate year of first detection of resistance to the relevant antibiotic.
In 1998, Yonsei Medical Journal published a special issue on antibiotic resistance (Vol. 39, No. 6), because we considered that antibiotic resistance reached a very serious state in Korea. At that time, the most concerned resistances were those of nosocomial pathogens: extended-spectrum β-lactamase (ESBL)- and plasmid-mediated AmpC β-lactamase (PABL)-producing Enterobacteriaceae, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus faecium (VRE). Despite efforts to control resistant bacteria, however, Gram-positive nosocomial pathogens, MRSA and vancomycin-resistant E. faecium became more prevalent in many countries,4 and resistance problem has relatively worsened in Gram-negative bacilli.5,6
Acinetobacter baumannii causes various nosocomial infections with high mortality, and the infection caused by multidrug-resistant A. baumannii is currently among the most difficult ones to treat.7 A few extensive reviews have been published on Acinetobacter bacteriology and infection.8-10 In most Asian countries, including Korea, Acinetobacter infections are relatively more prevalent and the organisms are more often resistant.11 Therefore, it seems to be an appropriate time to review the current status of resistance in general and that of the most feared Acinetobacter spp. in Korea in particular to help understand and alleviate this serious problem.
TAXONOMY AND PATHOGENICITY OF ACINETOBACTER SPP.
The genus Acinetobacter comprises Gram-negative, aerobic, glucose-nonfermenting, nonfastidious, nonmotile, catalase-positive, and oxidase-negative bacteria. The taxonomy of Acinetobacter has been confused for a long time. Before the designation of genus Acinetobacter in 1954, the organisms had been known as at least 15 different genera.10 Besides 23 validly published Acinetobacter species (http://www.bacterio.cict.fr), there are at least nine unnamed species: genomic species 3, 6, 13TU, 13BJ, 14BJ, 15BJ, 15TU, 16 and 17. Names of new species, A. pittii and A. nosocomialis, were recently proposed for genomic sp. 3 and 13TU, respectively.12
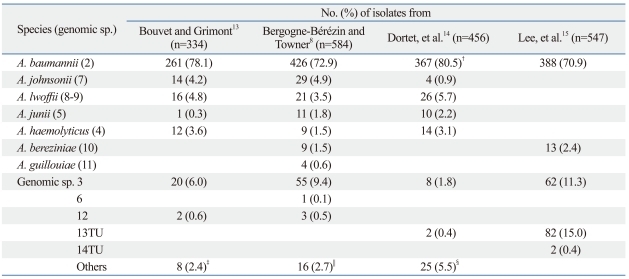
Species identification of Acinetobacter spp., based on abbreviated phenotypic tests, is mostly difficult, resulting in overestimation of prevalence of A. baumannii. By original phenotypic tests, the proportion of A. baumannii was 78.1% among the Acinetobacter isolates mostly from France,13 whereas that from Germany was 72.9% (Table 2).8 Using phenotypic and molecular methods, 80.5% of French isolates were identified as A. baumannii.14 Based on sequencing of 16-23S rRNA intergenic spacer region,15 70.9% of Acinetobacter spp. isolates in a Korean study were identified as A. baumannii. Of note was the fact that the proportion of A. baumannii was 95.4% among the isolates from tracheal aspirates, whereas it was only 59.1% among those from blood and central venous line.13
Table 2.
Prevalent Acinetobacter spp. in Clinical Specimens*
*Method of identification: references 13 and 8, phenotypic; 14, phenotypic and molecular; 15, molecular.
†353 isolates were identified as A. calcoaceticus-A. baumannii by phenotypic tests, all others were identified by molecular method.
‡Genomic sp. (GS) 10 (n=1), GS 11 (n=1), unidentified (n=6).
§A. ursingii (n=10), A. schindleri (n=5), A. radioresistens (n=3), GS 15TU (n=4), GS 17 (n=1), GS 16BJ (n=1), and GS 10 (n=1).
∥Unidentified.
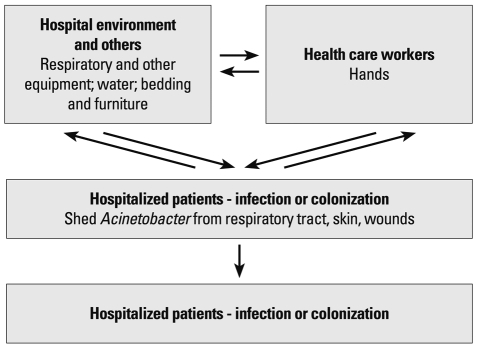
It was shown experimentally that the majority of A. baumannii strains survive longer than Escherichia coli on dry surfaces, and some strains survive for more than 4 months.16 In another study, A. baumannii survived for over 20 days on glass surfaces at room temperature.17 In contrast to P. aeruginosa, however, Acinetobacter spp. survived on both moist and dry surfaces.18 These characteristics are beneficial for the organism to survive in hospital environments and cause infection. Acinetobacter spp. are typical nosocomial pathogens causing infections almost exclusively in compromised patients. Even the Acinetobacter infections in soldiers were considered to be acquired during admission to medical facilities, rather than from the environment at the time of wounding.19 Acinetobacter spp. are more commonly isolated than S. aureus and Pseudomonas spp. from inanimate surfaces, and hands of staff in the ICU.18 It is difficult to determine significance of recovery of Acinetobacter spp. from clinical materials, because of their frequent colonization rather than infection (Fig. 1).
Fig. 1.
Major reservoirs, sources, and transmission patterns for Acineto-bacter infections in hospitalized patients.
Although it is somewhat difficult to make a direct comparison due to difference in the methods used, it is apparent that proportion of Acinetobacter spp. was much higher in Korea and Taiwan than in the U.S. For example in a U.S. study in 2006-2007, the proportion of A. baumannii was 2.7% and the rank order was 9 among the isolates from healthcare-associated infections.20 In a Korean study in 2009, the proportion was 6.6% and rank order was 7.21 An analysis at a Taiwanese hospital in 1996 to 2003 showed that the proportions of both A. baumannii and E. coli were 8% among all nosocomial blood stream isolates.22
In a Korean study involving 28 OXA carbapenemase-producing A. baumannii bloodstream infections, the 30 day mortality was 53.6% which was independently associated only with a high Pitt bacteremia score by multivariate analysis.23 Outbreaks of multidrug resistance (MDR) A. baumannii occurred in 2007 at a Korean hospital, and involved 17 patients with 13 deaths either from septic shock or from a combination of underlying diseases.24
TRENDS IN ANTIMICROBIAL RESISTANT BACTERIA IN GENERAL
Certain bacterial species, which cause community-acquired infections more often, have been increasingly resistant to certain antibiotics, i.e., pneumococci to penicillin G, and E. coli and Klebsiella pneumoniae to 3rd generation cephalosporins. However, the most feared resistant bacteria are those typically causing nosocomial infections. The Korean nationwide surveillance on antimicrobial resistance (KONSAR) studies in 1997 to 2009 showed that approximately 70% of all S. aureus were resistant to methicillin, and VRE had increased significantly from 4% to 29%.21 In the U.S., proportion of MRSA was initially much lower than that in Asian countries. But, despite intensive efforts to control the spread, the rates in 2005 were 59.2% among isolates from non-ICU inpatients, and 55% among those from ICU patients.25 Vancomycin-resistant Enterococcus was first detected in Europe and became prevalent in the U.S. In 2006 and 2007, the proportion of vancomycin-resistance was 78.9% among blood stream isolates of E. faecium.20
As for the resistance of Gram-negative bacilli, ESBL- or PABL-producing E. coli and K. pneumoniae have emerged.26-28 In Korea, these enzyme-producing organisms have increasingly been detected since the late 1990s.29 Carbapenems remained active against most of these organisms, but increased use of this class of antimicrobials to treat infection of 3rd generation cephalosporin-resistant Gram-negative bacilli resulted in emergence of carbapenem-resistant P. aeruginosa and Acinetobacter spp.27 The Korean surveillance study21 showed that, during 1997 to 2009, imipenem-resistant P. aeruginosa and Acinetobacter spp. increased from 17% to 26%, and from 1% to 51%, respectively. K. pneumoniae isolates with a new class A carbapenemase, KPC-2,30 and a new class B metallo-β-lactamase (MBL), NDM-1,31 emerged in the U.S. and in India, respectively. Enterobacteriaceae with the last two enzymes started to spread to other countries, including Korea.
ANTIMICROBIAL RESISTANCE IN ACINETOBACTER SPP.
Seven mechanisms of antibiotic resistance are known in Gram-negative bacteria: loss of porins, production of β-lacta-mases, increased expression of efflux pumps, presence of antibiotic-modifying enzymes, target site mutations, ribosomal mutations or modifications, metabolic bypass mechanisms, and a mutation in the lipopolysaccharide.32
Acinetobacter spp. are intrinsically less susceptible to antimicrobial agents than the species of Enterobacteriaceae. Outer membrane permeability of A. baumannii is less than 5% compared with other Gram-negative bacilli, because of small number and size of porins.33 Porins are pore forming proteins on the outer membrane (OMP) of bacteria. Three OMPs (33-36 kDa, 29 kDa, and 43 kDa) have been reported to be missing in the imipenem-resistant strains of A. baumannii. Decreased expression of OmpW was reported in a colistin-resistant A. baumannii mutant.
All bacteria have efflux systems. The multidrug efflux pumps actively export multiple, structurally-distinct classes of antimicrobials out of the bacterial cell. The most common antimicrobials expelled by the efflux pumps are macrolides, tetracyclines and quinolones.33 Overexpression of efflux pump further increases resistance level. Among the six families of multidrug efflux systems, major facilitator superfamily (MFS) and resistance-nodulation-division (RND) family are often associated with antimicrobial resistance in A. baumannii. Tet(A) and Tet(B) pumps belong to the MFS, and confers resistance to tetracycline, and both tetracycline and minocycline, respectively. AdeM pump is a member of the multidrug and toxic compound extrusion (MATE) family, and confers resistance to norfloxacin, ofloxacin, ciprofloxacin, and gentamicin.33 AdeABC is a three-component efflux pump, where AdeA is the membrane fusion protein, AdeB is the multidrug transporter, and AdeC is the OMP. AdeABC pump belongs to the RND family and confers resistance to aminoglycosides, β-lactams, chloramphenicol, erythromycin and tetracyclines, and reduced susceptibility to fluoroquinolones.
A. baumannii carries intrinsic blaAmpC genes encoding Acinetobacter-derived cephalosporinases which confer natural resistance to cefoxitin. as are many other species of Gram-negative bacilli.34 Inducible AmpC expression does not occur in A. baumannii, unlike that of AmpC enzymes found in other Gram-negative bacilli. Presence of upstream ISAba1 is involved in the overexpression of this gene, resulting in resistance to 3rd generation cephalosporins.35,36 A. baumannii also naturally carries intrinsic blaOXA-51-like genes, and presence of upstream ISAba1 renders the organism resistant to carbapenems. ISAba1, ISAba2, ISAba3, and ISAba4 could increase expression of blaOXA-51-like genes. Increased expression of blaOXA-23 by the upstream ISAba10 was reported in a group of A. baumannii isolates.37
Multi-drug resistance
The most serious current problem in the treatment of Acinetobacter infection is acquired MDR, thus leaving few antimicrobial agents to use. MDR could be due to bacterial possession of a resistant determinant which confers resistance to more than one class of antimicrobial agents. MDR pump is one of the examples. MDR is also due to possession of multiple resistance determinants.32 Acinetobacter is an organism that appears to have a propensity to extremely rapidly develop antibiotic resistance.8 Fournier, et al.38 detected 45 acquired resistance genes in a MDR A. baumannii strain AYE.
Different definitions of the terms MDR and pandrug-resistant (PDR) A. baumannii have been used in the literature causing confusion to microbiologist as well as to clinicians.39 In considerable proportion of the studies, MDR was defined as the resistance to representative antimicrobial agents of at least three different classes. The most commonly included antimicrobials were aminoglycosides, antipseudomonal penicillins, carbapenems, cephalosporins, and quinolones. Colistin, ampicillin-sulbactam or tetracyclines (doxycycline or minocycline) were occasionally included. The suggested definition of PDR in A. baumannii included resistance to sulbactam, minocycline or doxycycline, and tigecycline in addition to all the above mentioned antimicrobials. This definition is similar to that of extreme drug resistance (XDR) proposed by Paterson and Doi40 in that it included resistance to tigecycline and polymyxins. Sulbactam is a β-lactamase inhibitor with no significant antimicrobial activity, but peculiarly it has bactericidal activity against A. baumannii. However, in 2009, 57% of Acinetobacter spp. isolates were found to be resistant to ampicillin-sulbactam.21
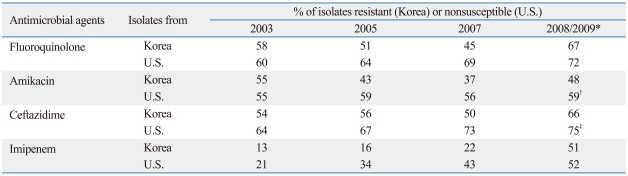
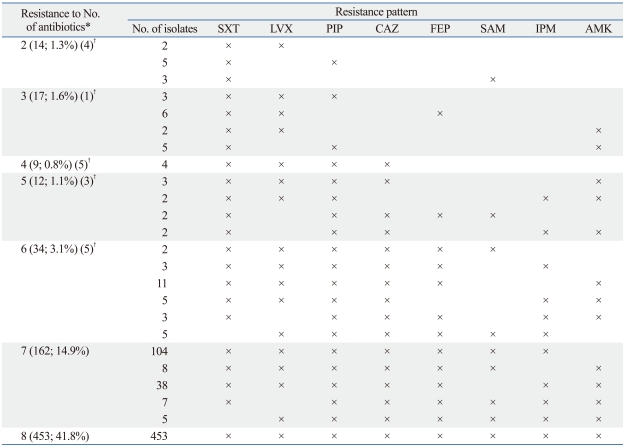
In a Surveillance Network (TSN) study in the U.S.41 a large number of A. baumannii isolates were tested. The resistance trend was largely similar to that in Korea,42 although rates of nonsusceptibility were reported in the U.S. study, while rates of resistance were reported in the Korean study (Table 3). Table 4 shows MDR patterns of Acinetobacter isolates from a Korean tertiary care hospital in 2009. Among the 1,085 isolates, 308 (28.4%) were resistant to none of the eight antimicrobials, whereas 14.9% and 41.8% were resistant to seven and to all eight antimicrobial agents, respectively. In the study, colistin susceptibility was not tested because resistant isolates to this antimicrobial agent was extremely rare.
Table 3.
Resistance Rates of Acinetobacter Isolates from Korea and from the U.S.
*Year of isolation: the U.S. in 2008 and Korea in 2009.
†Amikacin or gentamicin.
‡Ceftazidime or cefepime.
Table 4.
Multiresistance Patterns of 1,085 Acinetobacter Isolates from a Korean Tertiary Care Hospital in 2009
SXT, trimethoprim-sulfamethoxazole; LVX, levofloxacin; PIP, piperacillin; CAZ, ceftazidime; FEP, cefepime; SAM, ampicillin-sulbactam; IPM, imipenem; AMK, amikacin.
*No. and % of isolates are shown in parenthesis. Among the isolates, 308 (28.4%) were resistant to none of the antibiotics, and 76 (7.0%) were resistant to one of the eight antibiotics (36 to PIP, 15 to SXT, 12 to CAZ, 7 to AMK, 3 to. LVX, 2 to FEP, 1 to SAM).
†Total number of isolates omitted for the patterns with only one isolate each.
ESBLs
There are only few studies on ESBLs in A. baumannii. Spread of A. baumannii strains with TEM-92 in Italy, with SHV-12 in China, and with TEM-116 and SHV-12 in the Netherlands has been reported.10 CTX-M type ESBL are prevalent among Enterobacteriaceae, but a small number of Acinetobacter isolates with CTX-M-2, CTX-M-43, and CTX-M-15 have been described from Japan, Bolivia, and India, respectively.10 Vietnam extended-spectrum β-lactamase (VEB-1) was first detected in an E. coli isolate from a Vietnamese child. Dissemination of VEB-1-producing A. baumannii was reported from France,43 and then from Belgium and Argentina.10
Pseudomonas extended resistant (PER) type ESBL, initially detected in P. aeruginosa from France in 1991,44 has been detected in Acinetobacter isolates from many countries.10 In Korea, PER-1 was detected in 54.6% of 97 consecutive Acinetobacter isolates.45 All of the isolates except one of each genomic species 3 and unidentifiable Acinetobacter sp. were A. baumannii. The isolates were mostly from sputum specimens from ICU patients, and all of them were resistant to ceftazidime, cefotaxime, cefepime, and aztreonam. In another study, PER-1 was detected in 78.6% of 42 outbreak-associated isolates from a Korean hospital in 2007.24
MBLs
MBLs are molecular class B and functional group 3 β-lacta-mases which have the capability of hydrolyzing all β-lactams except the monobactam, aztreonam.46 Carbapenem-hydrolyzing activity of MBL is very potent. After introduction of imipenem into clinical practice, IMP-type and VIM-type MBL-producing Gram-negative bacilli emerged in Japan and Italy, respectively.10 In Korea, the first MBL detected was VIM-2 in P. aeruginosa in 1995,47 and then VIM-2 and IMP-1 were detected in Acinetobacter spp. in 1998.48 Of several MBLs, only IMP, VIM, and SIM types have been detected in Acinetobacter spp.10 In a surveillance study in 2003-2004, MBLs were detected in 135 of 545 (24.8%) imipenem-resistant Acinetobacter spp. isolates. The proportions of IMP-1, VIM-2, and SIM-1 were 61%, 33%, and 6%, respectively.49 A study in 2006 showed that, among 31 carbapenem-resistant Acinetobacter spp., IMP-1 was detected in 15 (48.4%) isolates, whereas VIM-2 was detected in only one isolate,50 possibly because the isolates tested were small in number and were from only three hospitals.
In the majority of earlier Korean studies, MBL-producing Acinetobacter spp. had been reported as A. baumannii, based on abbreviated phenotypic tests, which are now known to be mostly unreliable. Genetic identification of 58 Acinetobacter spp. isolates showed that 40 were A. baumannii, 9 were genomic sp. 13TU, 5 were phenon 6/ct 13TU, and 4 were genomic sp. 3.51 However, among the 13 MBL-producing isolates, 5 each were genomic sp. 13TU and phenon 6/ct 13TU, and 2 were genomic sp. 3, and only one was A. baumannii. In another study, it was shown that, among the imipenem-resistant isolates, all 14 isolates of Acinetobacter genomic sp. 13TU carried blaVIM-2, whereas 13 A. baumannii carried blaOXA-23 or blaOXA-51.52 In a Korean surveillance study, all 28 MBL-producing isolates were non-baumannii Acinetobacter.53 Likewise, in a Taiwanese study, among 75 MDR Acinetobacter isolates, all three VIM-11- or IMP-1-producing isolates were genomic sp. 13TU.36 These results indicate necessity of caution in reporting phenotypically-identified species of MBL-producing Acinetobacter spp.
OXA carbapenemases
OXA-type β-lactamases are molecular class D enzymes.46 OXA type enzymes continued to increase and became outnumbered TEM types, i.e., 204 vs. 182 as of April 2011 (www.lahey.org). OXA-type β-lactamases include narrow spectrum, extended-spectrum, and carbapenem-hydrolyzing ones. Of the OXA type β-lactamases, those with carbapenemase activity are the most concerned. The first OXA-type carbapenemase, OXA-23, was detected in 1985 from an A. baumannii strain from Scotland. The blaOXA-23 gene was located on transferable plasmid. Four main groups of OXA carbapenemases include OXA-23-like, OXA-40-like, OXA-51-like, and OXA-58 enzymes.54
An outbreak of A. baumannii with OXA-23 was first reported at a Korean hospital in 2003 involving 36 patients.55 At another hospital in 2006-2007, all 49 isolates of A. baumannii with OXA-23 were found to have identical or closely related PFGE patterns, indicating that rapid increase of this determinant was due to clonal spread.56 In a Korean surveillance study in 2000-2001, only 27 of 267 (10.1%) imipenem-nonsusceptible Acinetobacter spp. isolates had MBLs.57 In another study in 2005,53 vast majority of imipenem resistance in Acinetobacter spp. isolates were due to OXA carbapenemase production: among the 144 imipenem-resistant isolates only 19.4% had MBLs, whereas 74.3% had OXA carbapenemases.
Prevalent types of OXA carbapenemase varied significantly depending on reports in Korea. In a surveillance study in 2005,53 among the 105 imipenem-resistant isolates of A. baumannii, 47 had blaOXA-23-like and 56 had upstream ISAba1-associated blaOXA-51-like genes. In another study in 2007,58 among 178 isolates of A. baumannii, isolates with blaOXA-23-like genes were more prevalent (80%) than those with ISAba1-asssociated blaOXA-51-like genes (12%). It is of an interest to note in this study that 12 isolates had a novel blaOXA-182 which is related to blaOXA-143, first reported in Brazil in 2004.59 At a Taiwanese regional hospital, among imipenem-resistant A. baumannii isolates, blaOXA-23-like gene was detected in only 2 of 97 isolates in 2005 and 2006, but the gene was detected in 24 of 38 isolates in 2007.60 Presence of an identical PFGE type in 18 of the 38 isolates indicated that the rapid increase was due to outbreaks. In a study, among 544 Acinetobacter isolates collected from 10 Asia Pacific countries in 2006-2007,61 230 (42.3%) were nonsusceptible to carbapenems and OXA-23 was detected in 134 of 156 A. baumannii isolates from China, Hong Kong, India, Korea, Singapore, and Thailand. A. baumannii isolates from China, Indonesia, Taiwan and Thailand carried other OXA carbapenemase: OXA-24/40 (n=5), OXA-58 (n=2), OXA-23 plus OXA-58 (n=11), OXA-24 plus OXA-58 (n=1), and OXA-23 plus OXA-24/40 plus OXA-58 (n=3). PFGE showed clonal dissemination of OXA carbapenemase-producing A. baumannii isolates within medical centers among different countries.
Aminoglycoside resistance
Aminoglycosides have been important antibiotics for treatment of serious bacterial infections, especially those with aerobic Gram-negative bacteria. However, bacteria became increasingly resistant to aminoglycosides by acquiring plasmid-borne genes encoding aminoglycoside-modifying enzymes, N-acetyltransferases (AAC), O-nucleotidyltransferases (ANT), or O-phosphotransferases (APH). Many of the genes encoding aminoglycoside-modifying enzymes are associated with transposons, which aid in the rapid dissemination of the resistance gene even between different species.62 Moreover, transposons may concomitantly carry other resistance determinants which contribute to multiresistance. In a study using 75 isolates of Acinetobacter spp., majority of which were recovered from injured military patients who returned from Iraq/Kuwait, 89% were resistant to at least three classes of antibiotics, and 15% were resistant to all nine antibiotics tested.63 Resistance rates to amikacin and tobramycin were 53% and 67%, respectively. The majority of aminoglycoside-modifying enzyme genes detected by PCR were aacC1 (56%), aadB (48%), and aphA6 (71%). aphA6 confers resistance to amikacin, gentamicin, kanamycin, and neomycin.
Amikacin is insensitive to modification by the majority of the plasmid-encoded enzymes that confer resistance to kanamycin, gentamicin and tobramycin. Resistance rates of E. coli to gentamicin and tobramycin at a Korean hospital in 2010 were 27% and 10%, respectively, whereas that to amikacin was only 1%. In contrast, resistance rates of Acinetobacter spp. to these three aminoglycosides were over 53% (Antimicrobial Resistance Newsletter, Ser. No. 72; www.whonetkorea.org). armA is a plasmid-borne gene encoding 16S rRNA methylase, and this enzyme modifies the target of aminoglycosides and renders the bacteria highly resistant to all aminoglycosides. armA gene was detected in 14 of 33 consecutive Acinetobacter spp. isolates from a Korean tertiary care hospital in 2005.64 All of the armA-positive isolates were highly resistant (MIC >1,024 µg/mL) to amikacin, gentamicin, netilmicin, and tobramycin. In an investigation of highly aminoglycoside-resistant A. baumannii isolates associated with outbreak in 2007, armA together with blaOXA-23 and blaPER-1 genes were detected in 23 of 42 MDR A. baumannii isolates.24 Again, this is an example of A. baumannii isolates carrying multiple resistance determinants.
Fluoroquinolone resistance
The fluoroquinolones are widely used broad-spectrum antimicrobial agents. This class antimicrobials inhibit bacterial DNA gyrase and DNA topoisomerase IV, which are the enzymes required for bacterial DNA replication. The primary mechanism for quinolone resistance is alterations in the target enzymes, GyrA and ParC.65 In a Korean surveillance study in 2009, 67% of the Acinetobacter spp. isolates were resistant to fluoroquinolone,21 whereas, 52.4% of A. baumannii were nonsusceptible to ciprofloxacin in a U.S surveillance study in 2000.66 In a Korean study, the MIC of ciprofloxacin was ≥64 µg/mL for 31 of 35 imipenem-resistant and MDR A. baumannii isolates, and the isolates had mutations in both gyrA and parC.67
Plasmid-mediated quinolone resistance genes have been detected in many Enterobacteriaceae spp.,68 but qnrA was first reported in an environmental isolate of A. baumannii from Algeria in 2008.69 qnr genes do not render a wild-type organism fluoroquinolone nonsusceptible, but the low-level resistance conferred by this mechanism enhances selection of fluoroquinolone-resistant Enterobacteriaceae mutants.70
Tetracycline and tigecycline resistance
Resistance to tetracyclines and their derivatives can be mediated by efflux or ribosomal protection. tet(A) to tet(E) genes encoding tetracycline specific efflux pumps are often detected in Gram-negative bacilli, however, the tet(A) and tet(B) determinants have thus far been detected in A. baumannii.10 Tet(A) confers resistance to tetracycline but not minocycline, an agent with greater activity against A. baumannii. Tigecycline is a new glycylcycline and is a derivative of minocycline. Tigecycline appears as a promising therapeutic option for MDR A. baumannii. In a study using global collection of Acinetobacter spp. isolates, the MIC range of tigecycline was ≤0.008-8 µg/mL and MIC creep was reported between 2004 and 2007.71 Apart from tetracycline-specific efflux pumps, this class of antimicrobial is also susceptible to efflux by the multidrug efflux systems, such as AdeABC, and AdeIJK pumps. Tigecycline is also extruded by these three-component RND efflux systems.72
In a Korean study, all 43 imipenem-resistant A. baumannii isolates were susceptible to colistin, while 44% of them were not susceptible to tigecycline.73 In another study, 14 of 145 (9.7%) colistin-resistant Acinetobacter spp. isolates were not susceptible to tigecycline.74 In a Spanish study, the MIC ranges of tigecycline and minocycline were 0.03-8 µg/mL and ≤0.06-32 µg/mL, respectively, for 150 isolates of A. baumannii which included 61 colistin-resistant ones.75 It was reported that two patients developed A. baumannii bloodstream infection while receiving tigecycline. The MICs of tigecycline for the two isolates were 4 and 16 µg/mL, respectively.76
Polymyxin resistance
Polymyxins (colistin and polymyxin B) are polypeptide antimicrobial agents active against almost all Gram-negative infecbacilli. These antimicrobial agents bind to the cell membrane of Gram-negative bacilli, and make it more permeable, leading to bacterial death. Colistin was discovered in the 1940s, but it has not been used systemically because of nephrotoxicity and neurotoxicity. However, it has recently been increasingly used to treat MDR Acinetobacter infections.
Colistin-resistant Acinetobacter spp. isolates have been rare in Korea. In a study, none of the 139 imipenem-nonsusceptible A. baumannii isolates recovered from four hospitals in 2006 showed resistance to colistin.77 However, 30.6% of 214 A. baumannii isolates recovered between 2002 and 2006 at two hospitals showed resistance to colistin, although only 11.7% were resistant to meropenem,78 suggesting possible further spreading of colistin resistant isolates in Korea in the near future.
The mechanism of polymyxin resistance in Enterobacteriaceae involves modifications of lipid A, which reduce binding to polymyxins. A study suggested that the mechanism of polymyxin resistance in A. baumannii is associated with mutations in pmrA and pmrB. PmrAB and PhoPQ are involved in sensing environmental pH, and Fe3+ and Mg2+ levels, leading to altered expression of a set of genes involved in lipid A modification.79 However, another study showed that in vitro selected colistin-resistant variant from A. baumannii type strain ATCC 19606 did not have mutations in either pmrA or pmrB.80 Instead, the variant had a deletion of nucleotide 90 within lpxA, which would result in premature termination of LpxA translation. lpxA is predicted to encode the UDP-N-acetylglucosamine acyltransferase that catalyzes the first step in the biosynthesis of lipid A, and therefore, lipopolysaccharide. Loss of lipid A leading to colistin resistance was also observed in a colistin-resistant clinical A. baumannii isolate.
CONTROL OF ANTIMICROBIAL RESISTANT BACTERIA
Antimicrobial resistant bacteria emerge as a consequence of antimicrobial use whether it is appropriate or not (Table 1). Resistance to broad spectrum antimicrobial agents is more common among nosocomial pathogens, and lax in control measures enhances spread of resistant bacteria and increase infection. We now know that, however hard we try, bacteria will always overcome whatever we do to them.81 Moreover, as it has been increasingly difficult to develop new antimicrobials active against multi-resistant Gram-negative bacilli, preserving the efficacy of currently available ones has become very important.82
Antimicrobial stewardship is a method recommended to achieve the control of resistance.83 In 2001, WHO published the global strategy for containment of antimicrobial resistance (WHO/CDS/CSR/DRS/2001.2). As antimicrobial resistance is a multifaceted problem,84 various interventions are required in bundle to prevent its emergence and spread. The strategies include (1) reduce reservoirs of resistant bacteria, and execute infection-control measures to prevent transmission of resistant bacteria, (2) improve diagnostics to identify the etiology of infections and help direct therapy, (3) develop new antibiotics and vaccines, and improve the use of currently available vaccines, and (4) educate physicians and patients for the importance of reducing resistance. Most of these goals are considered to be difficult to achieve, since whole problem of resistance is intertwined with moral, social, political, and commercial issues.85
The KONSAR program has been in operation since 1988, and the Antimicrobial Resistance Newsletter (www.whonetkorea.org) has been published. The program was initially initiated with supports from WHO. Later, the Korean Nosocomial Infection Surveillance (KONIS) System was established by the Korean Society for Nosocomial Infection Control (KOSNIC) together with the government agencies. The main target of this surveillance is nosocomial urinary tract infection, blood stream infection, and pneumonia in ICU patients.86 KOSNIC also has been performing government supported researches, and workshops, related to nosocomial infection control. High prevalence of resistant Acinetobacter spp. is often due to clonal spread.24,55,56,87 The primary goals for the control of MDR Acinetobacter spp. infection are to recognize its presence in a hospital, and control the spread.9 To reduce reservoirs, the U.S. governmental agencies have mandated the MRSA screening programs, however, several specialists in infection control have questioned its appropriateness.88 It is a question concerning the need for active surveillance for much less virulent Acinetobacter spp.
The prevalence of hospital-acquired infections could be as high as 25% in an ICU.89 In 2008-2009, nosocomial infection rates in ICU patients per 1,000 patient days were 4.80 for urinary tract infection, 3.27 for central line-associated blood stream infection, and 1.86 for ventilator-associated pneumonia.86 Some U.S. studies showed that control efforts achieved significant decrease in nosocomial infection rates particularly in the ICUs. This has led to "zero risk concept". Nevertheless, the goal has been considered to be difficult to achieve in high risk patients with high severity score, long hospital or ICU stay.89 The problem with nosocomial infection is that only approximately one third of hospital-acquired infections are preventable.32 However, the police investigation of a major outbreak of MDR A. baumannii in Japan reflects public expectation of complete prevention of nosocomial infections.90
Rapid microbiological diagnosis can improve appropriate antibiotic use and patient recovery. Conventional bacterial examination is typically slow. Automation and use of molecular methods may improve the efficiency of diagnostic bacteriology in the future.
It is difficult to develop new antimicrobial agents in general,2,32 particularly those active against multiresistant Gram-negative bacilli.85 Use of vaccines against S. pneumoniae and Haemophilus significantly prevented the infections, resulting in reduction of antimicrobial use, but it may not be possible to develop vaccines against every nosocomial bacterial pathogens.
The treatment guideline for community-acquired pneumonia91 is an example of guidelines for various infections, provided by relevant Korean scientific societies. Also, the majority of large hospitals have guidelines for antibiotic prescription to reduce inappropriate use of broad spectrum antibiotics in particular. The Korean Health Insurance Review and Assessment Service reviews appropriateness of antibiotics use in hospitals, and Korean government has ambitious programs to reduce inappropriate use of antibiotics. Some successful example has been reported.92 Nevertheless, education of physicians and patients can not always achieve the goal. In the U.S., despite the presence of guidelines, it has been difficult to achieve the goal of appropriate antimicrobial prescription. Physicians are primarily concerned with recovery of individual patients for whom they are responsible,93 and it is also known that patient demands are the major reason to prescribe probably unnecessary antimicrobial agents. Even at public hospitals in Singapore, where high quality medical care is provided, carbapenem usage was not always appropriate.94 High rates of inappropriate carbapenem use in the ICU were considered to be due to the understanding that increased mortality is associated with initial inactive antibiotic prescription in critically ill patients. Overall, the evidence that better prescribing can reduce resistance rates is not obvious.85 Doubtful effectiveness of antimicrobial cycling83 can be evident by the prevalence of nosocomial pathogens possessing multiple resistance determinants. In general, cost vs. benefit is a great obstacle to execute the antimicrobial stewardship program.
In summary, Acinetobacter spp. have become particularly problematic nosocomial pathogens in Korea, partly due to clonal spread. It is difficult to prevent Acinetobacter spp. infection in hospitalized patient, because the organisms are ubiquitous in hospital environment. Recent clinical isolates of Acinetobacter spp. in Korea were often found to be multiresistant to carbapenems, fluoroquinolones, and aminoglycosides. Carbapenem resistance was mostly due to OXA type carbapenemase production in A. baumannii isolates, whereas it was due to MBL production in non-baumannii Acinetobacter isolates. Colistin-resistant isolates were rare, but started to be isolated. Since our past experience shows that emergence and spread of resistant bacteria are inevitable, we need concerted multidisciplinary efforts to preserve the activity of currently available antimicrobial agents by following the principles of antimicrobial stewardship.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Salyers AA, Whitt DD. Revenge of the microbes: how bacterial resistance is undermining the antibiotic miracle. Washington, DC: ASM Press; 2005. [Google Scholar]
- 2.Brötz-Oesterhelt H, Sass P. Postgenomic strategies in antibacterial drug discovery. Future Microbiol. 2010;5:1553–1579. doi: 10.2217/fmb.10.119. [DOI] [PubMed] [Google Scholar]
- 3.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(Suppl 1):S4–S16. doi: 10.1016/S0163-4453(09)60003-7. [DOI] [PubMed] [Google Scholar]
- 4.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchaim D, Zaidenstein R, Lazarovitch T, Karpuch Y, Ziv T, Weinberger M. Epidemiology of bacteremia episodes in a single center: increase in Gram-negative isolates, antibiotics resistance, and patient age. Eur J Clin Microbiol Infect Dis. 2008;27:1045–1051. doi: 10.1007/s10096-008-0545-z. [DOI] [PubMed] [Google Scholar]
- 6.Ho J, Tambyah PA, Paterson DL. Multiresistant Gram-negative infections: a global perspective. Curr Opin Infect Dis. 2010;23:546–553. doi: 10.1097/QCO.0b013e32833f0d3e. [DOI] [PubMed] [Google Scholar]
- 7.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 8.Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 10.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37:291–295. doi: 10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Nemec A, Krizova L, Maixnerova M, van der Reijden TJ, Deschaght P, Passet V, et al. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU) Res Microbiol. 2011;162:393–404. doi: 10.1016/j.resmic.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Bouvet PJ, Grimont PA. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987;138:569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- 14.Dortet L, Legrand P, Soussy CJ, Cattoir V. Bacterial identification, clinical significance, and antimicrobial susceptibilities of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J Clin Microbiol. 2006;44:4471–4478. doi: 10.1128/JCM.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Lee J, Jeong SH, Lee J, Bae IK, Lee K. Carbapenem-non-susceptible Acinetobacter baumannii of sequence type 92 or its single-locus variants with a G428T substitution in zone 2 of the rpoB gene. J Antimicrob Chemother. 2011;66:66–72. doi: 10.1093/jac/dkq402. [DOI] [PubMed] [Google Scholar]
- 16.Wendt C, Dietze B, Dietz E, Rüden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getchell-White SI, Donowitz LG, Gröschel DH. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989;10:402–407. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- 19.Petersen K, Cannegieter SC, van der Reijden TJ, van Strijen B, You DM, Babel BS, et al. Diversity and clinical impact of Acinetobacter baumannii colonization and infection at a military medical center. J Clin Microbiol. 2011;49:159–166. doi: 10.1128/JCM.00766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, et al. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009. Yonsei Med J. 2011;52:793–802. doi: 10.3349/ymj.2011.52.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CJ, Lee HC, Lee NY, Shih HI, Ko NY, Wang LR, et al. Predominance of Gram-negative bacilli and increasing antimicrobial resistance in nosocomial bloodstream infections at a university hospital in southern Taiwan, 1996-2003. J Microbiol Immunol Infect. 2006;39:135–143. [PubMed] [Google Scholar]
- 23.Song JY, Cheong HJ, Choi WS, Heo JY, Noh JY, Kim WJ. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol. 2011;60:605–611. doi: 10.1099/jmm.0.029439-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Heo ST, Jin JS, Choi CH, Lee YC, Jeong YG, et al. Characterization of Acinetobacter baumannii carrying blaOXA-23, blaPER-1 and armA in a Korean hospital. Clin Microbiol Infect. 2008;14:716–718. doi: 10.1111/j.1469-0691.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 25.Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 27.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauernfeind A, Chong Y, Schweighart S. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989;17:316–321. doi: 10.1007/BF01650718. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Kwon Y, Pai H, Kim JW, Cho DT. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36:1446–1449. doi: 10.1128/jcm.36.5.1446-1449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vila J, Martí S, Sánchez-Céspedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 34.Hujer KM, Hamza NS, Hujer AM, Perez F, Helfand MS, Bethel CR, et al. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 β-lactamase: defining a unique family of class C enzymes. Antimicrob Agents Chemother. 2005;49:2941–2948. doi: 10.1128/AAC.49.7.2941-2948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corvec S, Caroff N, Espaze E, Giraudeau C, Drugeon H, Reynaud A. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J Antimicrob Chemother. 2003;52:629–635. doi: 10.1093/jac/dkg407. [DOI] [PubMed] [Google Scholar]
- 36.Lin YC, Hsia KC, Chen YC, Sheng WH, Chang SC, Liao MH, et al. Genetic basis of multidrug resistance in Acinetobacter clinical isolates in Taiwan. Antimicrob Agents Chemother. 2010;54:2078–2084. doi: 10.1128/AAC.01398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Kim CK, Lee H, Jeong SH, Yong D, Lee K. A novel insertion sequence, ISAba10, inserted into ISAba1 adjacent to the blaOXA-23 gene and disrupting the outer membrane protein gene carO in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:361–363. doi: 10.1128/AAC.01672-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55:1619–1629. doi: 10.1099/jmm.0.46747-0. [DOI] [PubMed] [Google Scholar]
- 40.Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45:1179–1181. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- 41.Mera RM, Miller LA, Amrine-Madsen H, Sahm DF. Acinetobacter baumannii 2002-2008: increase of carbapenem-associated multiclass resistance in the United States. Microb Drug Resist. 2010;16:209–215. doi: 10.1089/mdr.2010.0052. [DOI] [PubMed] [Google Scholar]
- 42.Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, et al. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010;51:901–911. doi: 10.3349/ymj.2010.51.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J Clin Microbiol. 2003;41:3542–3547. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordmann P, Ronco E, Naas T, Duport C, Michel-Briand Y, Labia R. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:962–969. doi: 10.1128/aac.37.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yong D, Shin JH, Kim S, Lim Y, Yum JH, Lee K, et al. High prevalence of PER-1 extended-spectrum β-lactamase-producing Acinetobacter spp. in Korea. Antimicrob Agents Chemother. 2003;47:1749–1751. doi: 10.1128/AAC.47.5.1749-1751.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K, Lim JB, Yum JH, Yong D, Chong Y, Kim JM, et al. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob Agents Chemother. 2002;46:1053–1058. doi: 10.1128/AAC.46.4.1053-1058.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yum JH, Yi K, Lee H, Yong D, Lee K, Kim JM, et al. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J Antimicrob Chemother. 2002;49:837–840. doi: 10.1093/jac/dkf043. [DOI] [PubMed] [Google Scholar]
- 49.Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, et al. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005;49:4485–4491. doi: 10.1128/AAC.49.11.4485-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung JY, Kwon KC, Park JW, Kim YS, Kim JM, Shin KS, et al. Dissemination of IMP-1 and OXA type β-lactamase in carbapenem-resistant Acinetobacter baumannii. Korean J Lab Med. 2008;28:16–23. doi: 10.3343/kjlm.2008.28.1.16. [DOI] [PubMed] [Google Scholar]
- 51.Lim YM, Shin KS, Kim J. Distinct antimicrobial resistance patterns and antimicrobial resistance-harboring genes according to genomic species of Acinetobacter isolates. J Clin Microbiol. 2007;45:902–905. doi: 10.1128/JCM.01573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JH, Choi CH, Kang HY, Lee JY, Kim J, Lee YC, et al. Differences in phenotypic and genotypic traits against antimicrobial agents between Acinetobacter baumannii and Acinetobacter genomic species 13TU. J Antimicrob Chemother. 2007;59:633–639. doi: 10.1093/jac/dkm007. [DOI] [PubMed] [Google Scholar]
- 53.Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009;33:520–524. doi: 10.1016/j.ijantimicag.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Brown S, Amyes S. OXA β-lactamases in Acinetobacter: the story so far. J Antimicrob Chemother. 2006;57:1–3. doi: 10.1093/jac/dki425. [DOI] [PubMed] [Google Scholar]
- 55.Jeon BC, Jeong SH, Bae IK, Kwon SB, Lee K, Young D, et al. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 β-lactamase in Korea. J Clin Microbiol. 2005;43:2241–2245. doi: 10.1128/JCM.43.5.2241-2245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang HY, Lee HJ, Suh JT, Lee KM. Outbreaks of imipenem resistant Acinetobacter baumannii producing OXA-23 β-lactamase in a tertiary care hospital in Korea. Yonsei Med J. 2009;50:764–770. doi: 10.3349/ymj.2009.50.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y Korean Nationwide Surveillance of Antimicrobial Resistance Group. VIM- and IMP-type metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003;9:868–871. doi: 10.3201/eid0907.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim CK, Lee Y, Lee H, Woo GJ, Song W, Kim MN, et al. Prevalence and diversity of carbapenemases among imipenem-nonsusceptible Acinetobacter isolates in Korea: emergence of a novel OXA-182. Diagn Microbiol Infect Dis. 2010;68:432–438. doi: 10.1016/j.diagmicrobio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:5035–5038. doi: 10.1128/AAC.00856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin MF, Kuo HY, Yeh HW, Yang CM, Sung CH, Tu CC, et al. Emergence and dissemination of blaOXA-23-carrying imipenem-resistant Acinetobacter sp in a regional hospital in Taiwan. J Microbiol Immunol Infect. 2011;44:39–44. doi: 10.1016/j.jmii.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY Surveillance Program. J Antimicrob Chemother. 2009;63:55–59. doi: 10.1093/jac/dkn434. [DOI] [PubMed] [Google Scholar]
- 62.Shaw KJ, Rather PN, Hare RS, Miller GH. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H, Yong D, Yum JH, Roh KH, Lee K, Yamane K, et al. Dissemination of 16S rRNA methylase-mediated highly amikacin-resistant isolates of Klebsiella pneumoniae and Acinetobacter baumannii in Korea. Diagn Microbiol Infect Dis. 2006;56:305–312. doi: 10.1016/j.diagmicrobio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Hooper DC. Mechanisms of fluoroquinolone resistance. Drug Resist Updat. 1999;2:38–55. doi: 10.1054/drup.1998.0068. [DOI] [PubMed] [Google Scholar]
- 66.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Evangelista AT, Critchley IA, et al. Susceptibility to fluoroquinolones among commonly isolated Gram-negative bacilli in 2000: TRUST and TSN data for the United States. Tracking Resistance in the United States Today. The Surveillance Network. Int J Antimicrob Agents. 2002;19:21–31. doi: 10.1016/s0924-8579(01)00466-6. [DOI] [PubMed] [Google Scholar]
- 67.Koo SH, Kwon KC, Cho HH, Sung JY. Genetic basis of multidrug-resistant Acinetobacter baumannii clinical isolates from three university hospitals in Chungcheong Province, Korea. Korean J Lab Med. 2010;30:498–506. doi: 10.3343/kjlm.2010.30.5.498. [DOI] [PubMed] [Google Scholar]
- 68.Martínez-Martínez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 69.Touati A, Brasme L, Benallaoua S, Gharout A, Madoux J, De Champs C. First report of qnrB-producing Enterobacter cloacae and qnrA-producing Acinetobacter baumannii recovered from Algerian hospitals. Diagn Microbiol Infect Dis. 2008;60:287–290. doi: 10.1016/j.diagmicrobio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang YF, Dowzicky MJ. In vitro activity of tigecycline and comparators on Acinetobacter spp. isolates collected from patients with bacteremia and MIC change during the Tigecycline Evaluation and Surveillance Trial, 2004 to 2008. Diagn Microbiol Infect Dis. 2010;68:73–79. doi: 10.1016/j.diagmicrobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Damier-Piolle L, Magnet S, Brémont S, Lambert T, Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song JY, Kee SY, Hwang IS, Seo YB, Jeong HW, Kim WJ, et al. In vitro activities of carbapenem/sulbactam combination, colistin, colistin/rifampicin combination and tigecycline against carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;60:317–322. doi: 10.1093/jac/dkm136. [DOI] [PubMed] [Google Scholar]
- 74.Park YK, Choi JY, Song JH, Ko KS. In vitro activity of tigecycline against colistin-resistant Acinetobacter spp. isolates from Korea. Int J Antimicrob Agents. 2009;33:289–290. doi: 10.1016/j.ijantimicag.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Arroyo LA, Mateos I, González V, Aznar J. In vitro activities of tigecycline, minocycline, and colistin-tigecycline combination against multi- and pandrug-resistant clinical isolates of Acinetobacter baumannii group. Antimicrob Agents Chemother. 2009;53:1295–1296. doi: 10.1128/AAC.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peleg AY, Potoski BA, Rea R, Adams J, Sethi J, Capitano B, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–131. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- 77.Roh KH, Kim CK, Yum JH, Yong D, Jeong SH, Lim CS, et al. Carbapenem Resistance Mechanisms and Molecular Epidemiology of Acinetobacter spp. from Four Hospitals in Seoul and Gyeonggi Province in 2006. Korean J Clin Microbiol. 2010;13:27–33. [Google Scholar]
- 78.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60:1163–1167. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 79.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009;53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sykes R. The 2009 Garrod lecture: the evolution of antimicrobial resistance: a Darwinian perspective. J Antimicrob Chemother. 2010;65:1842–1852. doi: 10.1093/jac/dkq217. [DOI] [PubMed] [Google Scholar]
- 82.Talbot GH. The antibiotic development pipeline for multidrug-resistant gram-negative bacilli: current and future landscapes. Infect Control Hosp Epidemiol. 2010;31(Suppl 1):S55–S58. doi: 10.1086/655988. [DOI] [PubMed] [Google Scholar]
- 83.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fishman N. Antimicrobial stewardship. Am J Med. 2006;119:S53–S61. doi: 10.1016/j.amjmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Livermore DM. Bacterial resistance: origins, epidemiology, and impact. Clin Infect Dis. 2003;36:S11–S23. doi: 10.1086/344654. [DOI] [PubMed] [Google Scholar]
- 86.Kwak YG, Cho YK, Kim JY, Lee SO, Kim HY, Kim YK, et al. Korean Nosocomial Infections Surveillance System, Intensive Care Unit Module Report: Data Summary from July 2008 through June 2009 and Analysis of 3-Year Results. Korean J Nosocomial Infect Control. 2010;15:14–25. [Google Scholar]
- 87.Lu PL, Doumith M, Livermore DM, Chen TP, Woodford N. Diversity of carbapenem resistance mechanisms in Acinetobacter baumannii from a Taiwan hospital: spread of plasmid-borne OXA-72 carbapenemase. J Antimicrob Chemother. 2009;63:641–647. doi: 10.1093/jac/dkn553. [DOI] [PubMed] [Google Scholar]
- 88.Peterson LR, Diekema DJ. To screen or not to screen for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2010;48:683–689. doi: 10.1128/JCM.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlet J, Fabry J, Amalberti R, Degos L. The "zero risk" concept for hospital-acquired infections: a risky business! Clin Infect Dis. 2009;49:747–749. doi: 10.1086/604720. [DOI] [PubMed] [Google Scholar]
- 90.Yuji K, Oiso G, Matsumura T, Murashige N, Kami M. Police investigation into multidrug-resistant Acinetobacter baumannii outbreak in Japan. Clin Infect Dis. 2011;52:422. doi: 10.1093/cid/ciq081. [DOI] [PubMed] [Google Scholar]
- 91.Song JH, Jung KS, Kang MW, Kim DJ, Pai H, Suh GY, et al. Treatment Guidelines for Community-acquired Pneumonia in Korea: An Evidence-based Approach to Appropriate Antimicrobial Therapy. Tuberc Respir Dis. 2009;67:281–302. [Google Scholar]
- 92.Park S, Soumerai SB, Adams AS, Finkelstein JA, Jang S, Ross-Degnan D. Antibiotic use following a Korean national policy to prohibit medication dispensing by physicians. Health Policy Plan. 2005;20:302–309. doi: 10.1093/heapol/czi033. [DOI] [PubMed] [Google Scholar]
- 93.Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. 2002;17:87–94. doi: 10.1046/j.1525-1497.2002.10711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liew YX, Lee W, Kwa AL, Lye DC, Yeo CL, Hsu LY. Inappropriate carbapenem use in Singapore public hospitals: opportunities for antimicrobial stewardship. Int J Antimicrob Agents. 2011;37:87–88. doi: 10.1016/j.ijantimicag.2010.09.008. [DOI] [PubMed] [Google Scholar]