Abstract
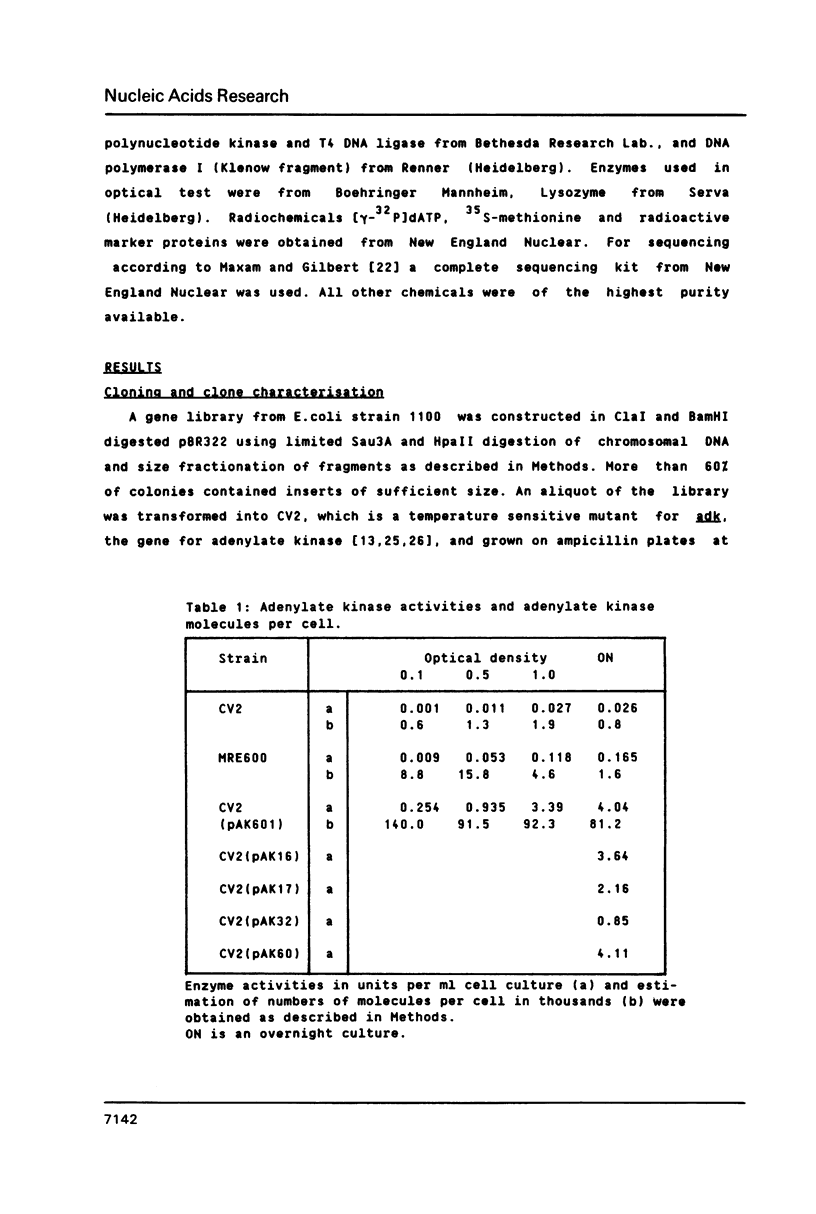
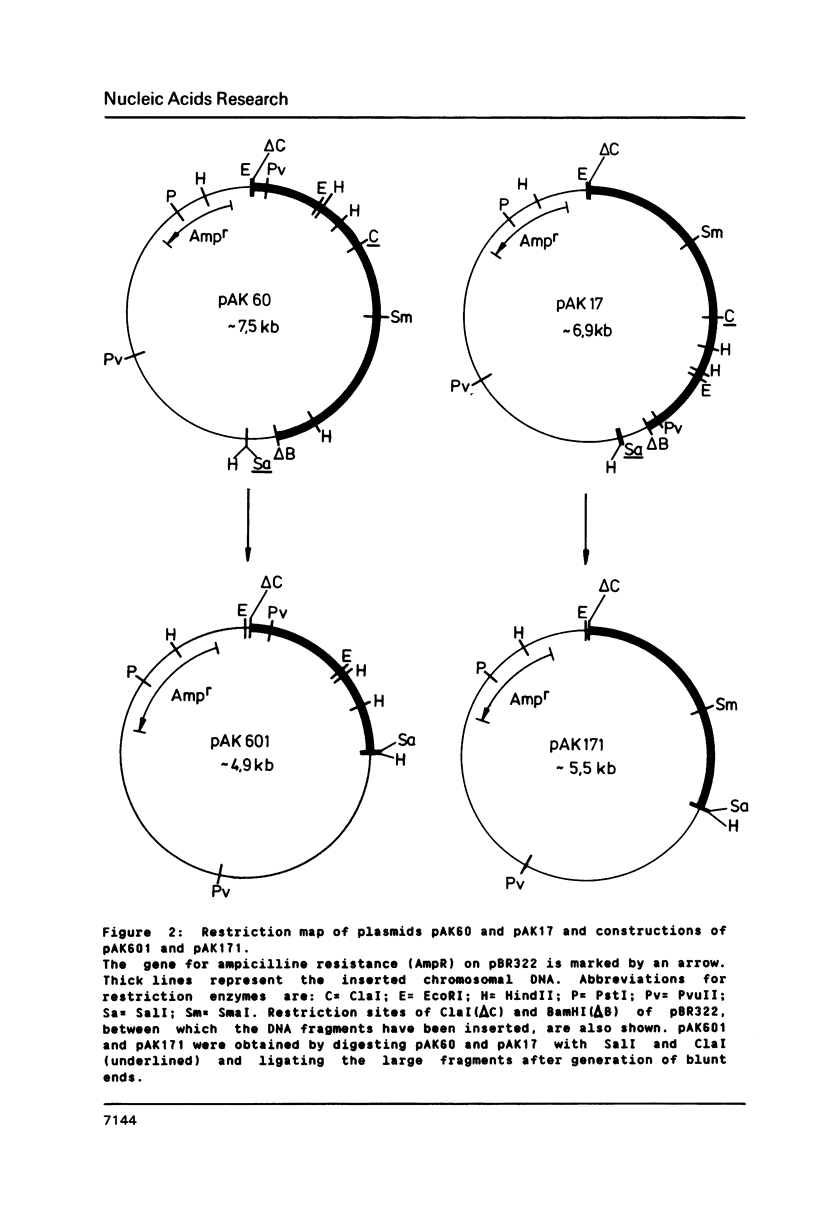
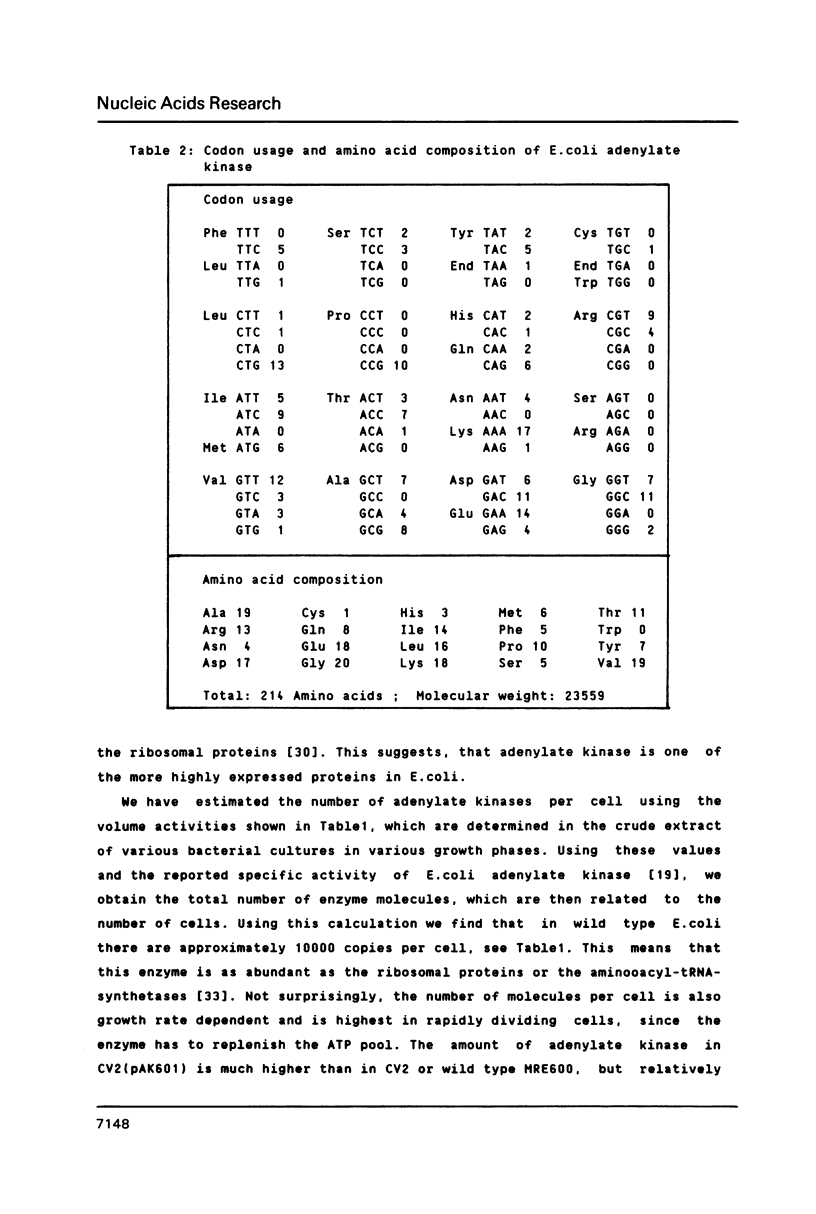
Adenylate kinase, the product of the adk locus in Escherichia coli K12, catalyzes the conversion of AMP and ATP to two molecules of ADP. The gene has been cloned by complementation of an adk temperature sensitive mutation. The DNA sequence of the complete coding region and of 5'- and 3'-untranslated regions were determined. The resulting protein sequence was found to contain several regions of high homology with cytosolic adenylate kinase of pig muscle (AK1), whose three-dimensional structure has been determined. The most significant of the amino acid exchanges is the replacement of histidine 36 with glutamine. This residue is believed to play a role in catalysis through metal ion binding. The codon usage pattern and the determination of adenylate kinase molecules per cell shows that the enzyme is one of the more abundant soluble proteins of the bacterial cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B. E., Kensil C. R., Cheng C. H., Glaser M. Genetic analysis of Escherichia coli mutants defective in adenylate kinase and sn-glycerol 3-phosphate acyltransferase. J Bacteriol. 1980 Jan;141(1):405–408. doi: 10.1128/jb.141.1.405-408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R., Trosin M., Tomasselli A. G., Schulz G. E., Schirmer R. H. Mitochondrial adenylate kinase (AK2) from bovine heart. Homology with the cytosolic isoenzyme in the catalytic region. Eur J Biochem. 1984 Jun 15;141(3):629–636. doi: 10.1111/j.1432-1033.1984.tb08238.x. [DOI] [PubMed] [Google Scholar]
- Gouy M., Grantham R. Polypeptide elongation and tRNA cycling in Escherichia coli: a dynamic approach. FEBS Lett. 1980 Jun 30;115(2):151–155. doi: 10.1016/0014-5793(80)81155-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heil A., Müller G., Noda L., Pinder T., Schirmer H., Schirmer I., von Zabern I. The amino-acid sequence of sarcine adenylate kinase from skeletal muscle. Eur J Biochem. 1974 Mar 15;43(1):131–144. doi: 10.1111/j.1432-1033.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Singer M. F. Purification and characterization of adenylate kinase as an apparent adenosine triphosphate-dependent inhibitor of ribonuclease II in Escherichia coli. J Biol Chem. 1973 Mar 25;248(6):2014–2021. [PubMed] [Google Scholar]
- Huss R. J., Glaser M. Identification and purification of an adenylate kinase-associated protein that influences the thermolability of adenylate kinase from a temperature-sensitive adk mutant of Escherichia coli. J Biol Chem. 1983 Nov 10;258(21):13370–13376. [PubMed] [Google Scholar]
- Kalbitzer H. R., Marquetant R., Rösch P., Schirmer R. H. The structural isomerisation of human-muscle adenylate kinase as studied by 1H-nuclear magnetic resonance. Eur J Biochem. 1982 Sep 1;126(3):531–536. doi: 10.1111/j.1432-1033.1982.tb06813.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipmann F. The ATP-phosphate cycle. Curr Top Cell Regul. 1981;18:301–311. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonald G. G., Cohn M. Proton magnetic resonance spectra or porcine muscle adenylate kinase and substrate complexes. J Biol Chem. 1975 Sep 10;250(17):6947–6954. [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Sachsenheimer W., Schulz G. E. Two conformations of crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):23–36. doi: 10.1016/0022-2836(77)90280-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Mildvan A. S. Nuclear magnetic resonance studies of the nucleotide binding sites of porcine adenylate kinase. Biochemistry. 1982 Nov 23;21(24):6119–6123. doi: 10.1021/bi00267a014. [DOI] [PubMed] [Google Scholar]
- Wieland B., Tomasselli A. G., Noda L. H., Frank R., Schulz G. E. The amino acid sequence of GTP:AMP phosphotransferase from beef-heart mitochondria. Extensive homology with cytosolic adenylate kinase. Eur J Biochem. 1984 Sep 3;143(2):331–339. doi: 10.1111/j.1432-1033.1984.tb08376.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



