Abstract
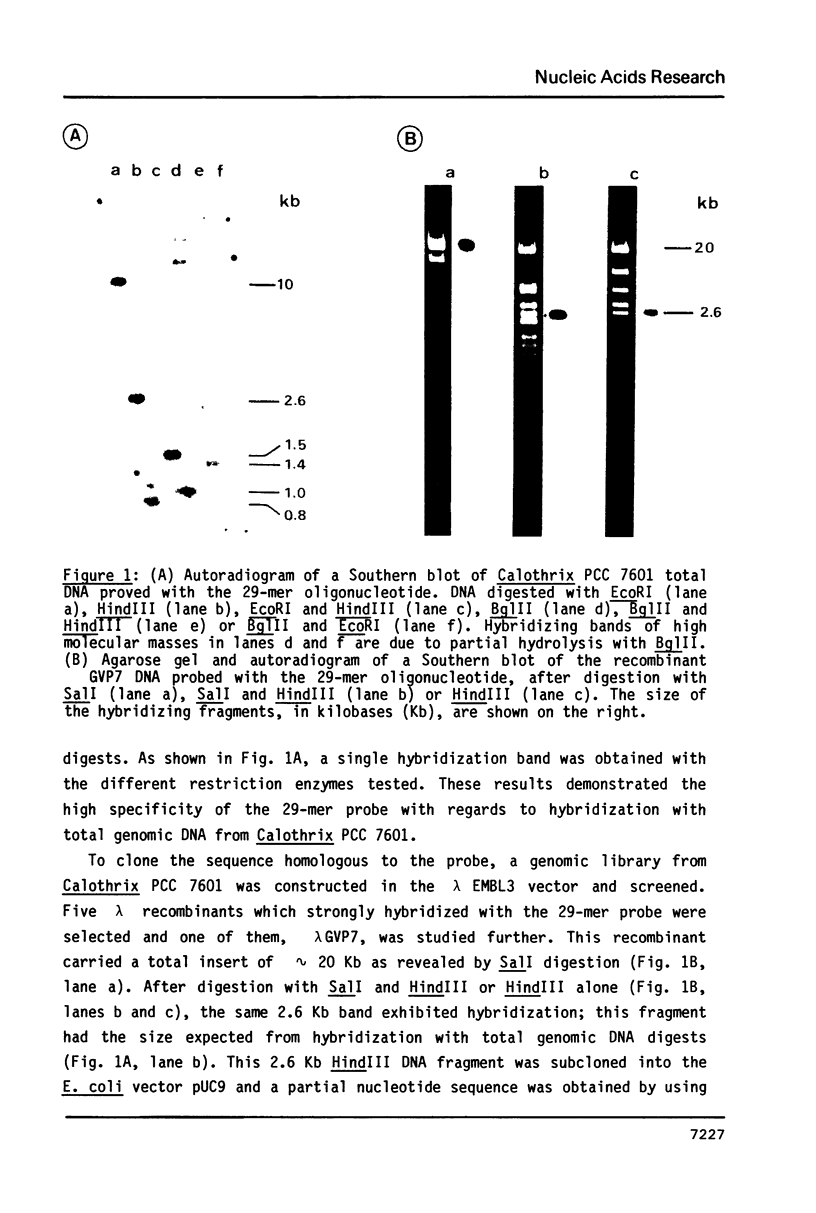
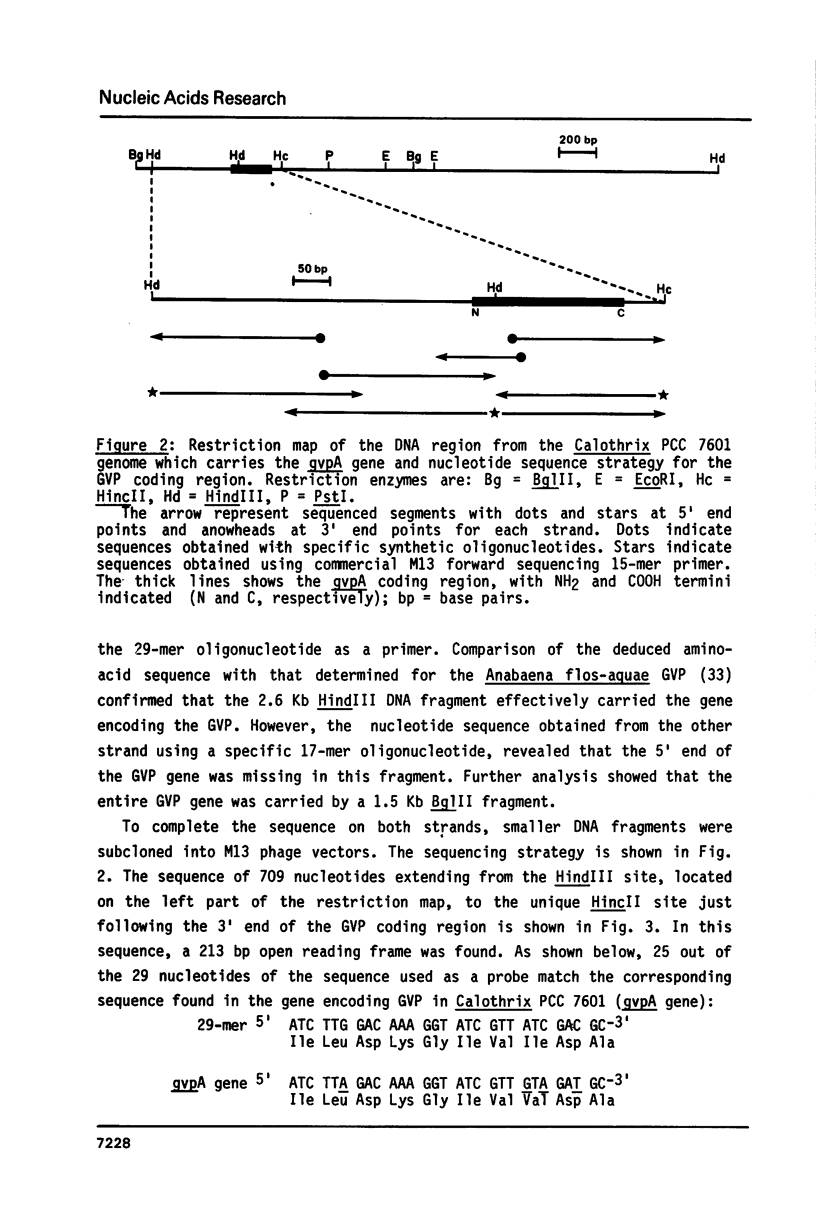
Since the gas vesicle protein (GVP) is highly conserved among the different gas-vacuolate prokaryotes, a 29-mer oligonucleotide corresponding to a portion of the Anabaena flos-aquae GVP gene was synthesized and used to isolate the GVP structural gene from Calothrix PCC 7601 (= Fremyella diplosiphon). Gas vacuole production in this filamentous cyanobacterium is restricted to hormogonia which occur at a specific stage during the developmental cell cycle. The GVP gene (gvpA) was localized on a 709 bp HindIII-HincII fragment. Nucleotide sequence analysis revealed a 213 bp open reading frame whose deduced amino-acid sequence shows a very high homology with that of the Anabaena flos-aquae GVP. Assuming that the first methionine residue is proteolytically processed, the molecular mass of the Calothrix GVP is 7375 daltons. Sequences resembling the Escherichia coli consensus promoter were found upstream from the gvpA gene. The initiator codon of the gvpA gene is preceded by a polypurine sequence assumed to be the ribosome binding site. Southern hybridizations with a probe specific for the gvpA gene indicated that this gene is not plasmid-borne, and that another homologous gene is present in the Calothrix genome.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E., Walsby A. E. Crystalline structure of the gas vesicle wall from Anabaena flos-aquae. J Mol Biol. 1976 Aug 5;105(2):183–199. doi: 10.1016/0022-2836(76)90106-6. [DOI] [PubMed] [Google Scholar]
- Borbely G., Simoncsits A. 3'-Terminal conserved loops of 16S rRNAs from the cyanobacterium Synechococcus AN PCC 6301 and maize chloroplast differ only in two bases. Biochem Biophys Res Commun. 1981 Aug 14;101(3):846–852. doi: 10.1016/0006-291x(81)91827-1. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Lambert D. H., Dubbs J. M., Stirewalt V. L., Stevens S. E., Jr, Porter R. D., Tam J., Jay E. Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1985 May;82(10):3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg P., Buckland B., Walsby A. E. Chemical composition of gas vesicles isolated from Anabaena flos-aquae. Arch Mikrobiol. 1972;85(4):304–309. doi: 10.1007/BF00549268. [DOI] [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jones D. D., Jost M. Isolation and chemical characterization of gas-vacuole membranes from Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1970;70(1):43–64. doi: 10.1007/BF00691059. [DOI] [PubMed] [Google Scholar]
- Kallas T., Rebière M. C., Rippka R., Tandeau de Marsac N. The structural nif genes of the cyanobacteria Gloeothece sp. and Calothrix sp. share homology with those of Anabaena sp., but the Gloeothece genes have a different arrangement. J Bacteriol. 1983 Jul;155(1):427–431. doi: 10.1128/jb.155.1.427-431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A. E., Lara J. C., Staley J. T. Isolation and characterization of gas vesicles from Microcyclus aquaticus. Arch Microbiol. 1977 Mar 1;112(2):133–140. doi: 10.1007/BF00429325. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Staley J. T., Lara J. C. Gas vesicle assembly in Microcyclus aquaticus. J Bacteriol. 1975 Jun;122(3):1301–1309. doi: 10.1128/jb.122.3.1301-1309.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt B. Y., Delaney S. F. The nucleotide sequence for the large subunit of ribulose 1,5-bisphosphate carboxylase from a unicellular cyanobacterium, Synechococcus PCC6301. DNA. 1983;2(2):121–129. doi: 10.1089/dna.1983.2.121. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rippka R., Herdman M. Division patterns and cellular differentiation in cyanobacteria. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):33–39. doi: 10.1016/s0769-2609(85)80018-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Porter R. D. Enhanced recombination between lambda plac5 and mini-F-lac: the tra regulon is required for recombination enhancement. Mol Gen Genet. 1984;193(2):269–274. doi: 10.1007/BF00330679. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yamada C., Takahata N., Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. D. Halobacterium strain 5 contains a plasmid which is correlated with the presence of gas vacuoles. Nature. 1978 May 25;273(5660):314–317. doi: 10.1038/273314a0. [DOI] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Walsby A. E. Molecular weight of gas-vesicle protein from the planktonic cyanobacterium Anabaena flos-aquae and implications for structure of the vesicle. Biochem J. 1983 Mar 1;209(3):809–815. doi: 10.1042/bj2090809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. The isolation of gas vesicles from blue-green algae. Methods Enzymol. 1974;31:678–686. doi: 10.1016/0076-6879(74)31073-7. [DOI] [PubMed] [Google Scholar]
- Weathers P. J., Jost M., Lamport D. T. The gas vacuole membrane of Microcystis aeruginosa. A partial amino acid sequence. Arch Biochem Biophys. 1977 Jan 15;178(1):226–244. doi: 10.1016/0003-9861(77)90188-6. [DOI] [PubMed] [Google Scholar]
- Weidinger G., Klotz G., Goebel W. A large plasmid from Halobacterium halobium carrying genetic information for gas vacuole formation. Plasmid. 1979 Jul;2(3):377–386. doi: 10.1016/0147-619x(79)90021-0. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]