Background: p40phox acquires PI(3)P-binding capabilities through arachidonic acid-induced and H2O2-induced conformational changes in phagocytes.
Results: In addition to conformational changes induced by H2O2 in the cytoplasm, p40phox can acquire PI(3)P binding following membrane targeting.
Conclusion: p40phox has novel mechanisms inducing its conformation changes, apart from p47phox.
Significance: This study demonstrates both p40phox and p47phox synchronously function as “carriers” and “adaptors” of Nox2-based NADPH oxidase assembly through their conformation changes.
Keywords: Intracellular Trafficking, Membrane Trafficking, Oxidase, Phagocytosis, Phosphatidylinositol, Reactive Oxygen Species (ROS), Signal Transduction, Phosphatidylinositol 3-Phosphate, NADPH Oxidase (Nox), p40phox, p47phox, PI(3)P, Phagocytosis
Abstract
During activation of the phagocyte (Nox2-based) NADPH oxidase, the cytoplasmic Phox complex (p47phox-p67phox-p40phox) translocates and associates with the membrane-spanning flavocytochrome b558. It is unclear where (in cytoplasm or on membranes), when (before or after assembly), and how p40phox acquires its PI(3)P-binding capabilities. We demonstrated that in addition to conformational changes induced by H2O2 in the cytoplasm, p40phox acquires PI(3)P-binding through direct or indirect membrane targeting. We also found that p40phox is essential when p47phox is partially phosphorylated during FcγR-mediated oxidase activation; however, p40phox is less critical when p47phox is adequately phosphorylated, using phosphorylation-mimicking mutants in HEK293Nox2/FcγRIIa and RAW264.7p40/p47KD cells. Moreover, PI binding to p47phox is less important when the autoinhibitory PX-PB1 domain interaction in p40phox is disrupted or when p40phox is targeted to membranes. Furthermore, we suggest that high affinity PI(3)P binding of the p40phox PX domain is critical during its accumulation on phagosomes, even when masked by the PB1 domain in the resting state. Thus, in addition to mechanisms for directly acquiring PI(3)P binding in the cytoplasm by H2O2, p40phox can acquire PI(3)P binding on targeted membranes in a p47phox-dependent manner and functions both as a “carrier” of the cytoplasmic Phox complex to phagosomes and an “adaptor” of oxidase assembly on phagosomes in cooperation with p47phox, using positive feedback mechanisms.
Introduction
In phagocytic cells, reactive oxygen species (ROS)3 are produced by the phagocyte NADPH oxidase. The enzyme is a multiprotein complex assembled from the membrane-spanning flavocytochrome b558 (composed of Nox2 (gp91phox) and p22phox) and four cytoplasmic components (p47phox, p67phox, p40phox, and Rac) (1–3). In unstimulated phagocytes, the oxidase is dissociated and inactive; the flavocytochrome b558 is stored on the membranes of intracellular granules (4), and the other Phox proteins associate in a separate cytoplasmic ternary complex (p47phox-p67phox-p40phox) (5) in a dephosphorylated state (6–8). During phagocyte activation, intracellular granules containing flavocytochrome b558 fuse to newly forming phagosomes, and the cytoplasmic ternary complex binds to these membranes; p47phox is phosphorylated (9, 10), thereby inducing conformational changes that promote interaction of the ternary complex with p22phox (11), and p40phox also undergoes conformational changes by disruption of the intramolecular PX-PB1 domain interaction to enable the ternary complex to bind through the p40phox PX domain to PI(3)P (12, 13), which is enriched in phagosomes (14–16).
Chronic granulomatous disease (CGD), characterized by defective microbial killing by phagocytic cells, is caused by defects or deficiencies in any one of five oxidase components: Nox2, p22phox, p47phox, p67phox, or p40phox. An essential role for Rac in NADPH oxidase activation was also demonstrated in an oxidase-deficient patient who expressed mutated Rac2 (1) and in mice rendered genetically deficient in Rac2 or in Rac1 plus Rac2 (17). p47phox is called a “carrier,” “adaptor,” or “organizer” component because it binds to membrane lipids (PI(3,4)P2, phosphatidic acid, and phosphatidylserine) through its PX domain (18), is tethered to the flavocytochrome b558 through direct interactions between p22phox and its tandem SH3 domains, and is linked to other cytoplasmic Phox proteins in this complex (19, 20). CGD patients who lack p47phox show impaired translocation of p67phox to the particulate fraction or phagosomes in response to PMA (21, 22), fMLP (22), or opsonized zymosan (23), whereas CGD patients who lack p67phox show normal translocation of p47phox to the particulate fraction (21, 22). p40phox was shown to act as an essential positive regulator of Nox2 in studies in p40phox-deficient mice (24), in p40phoxR58A/− knock-in mice (25), or in FcγIIa receptor-reconstituted cells (26). In recent work, we described a model in which p47phox functions as an early stage carrier and adaptor protein of the cytoplasmic ternary complex, whereas p40phox functions as a late stage carrier or adaptor protein that links the cytoplasmic ternary complex to closed phagosomes and prolongs retention of the complex on phagosomes using PI(3)P binding during FcγR-mediated oxidative burst (12, 27). Although mounting evidence suggested that p40phox functions as an essential positive regulator of the Nox2-based NADPH oxidase, only recently was p40phox deficiency described in a CGD patient, who has compound heterozygosity for a missense mutation predicting a R105Q substitution in the PX domain and a frameshift mutation at codon 52 (K52R) with a premature stop at codon 79 and exhibited a severe defect in FcγR-mediated oxidative burst but not in PMA- or fMLP-stimulated extracellular ROS release (28). Contrary to views on the role of p40phox serving as a carrier of the cytoplasmic Phox complex (12, 27, 29–31), a recent report suggested that p40phox primarily functions in sustaining Nox2 activity on phagosomes rather than in translocation of the cytoplasmic Phox complex to phagosomes (32). Another report suggested that although p40phox acts as a carrier of the Phox complex, this function is PX domain-dependent but PI(3)P-independent in PMA-stimulated permeabilized PLB-985 neutrophil cores (31). Thus, where (in the cytoplasm or on membranes), when (before or after assembly), and how p40phox acquires its PI(3)P-binding capabilities is unsolved, and how p40phox cooperates with p47phox during oxidase assembly or activation is also unclear. To address these questions, we used membrane-targeted mutants of p40phox and p47phox to delineate contributions of various intra- and intermolecular domain interactions affecting their targeting to phagosomes and oxidase activation. Here we show that in addition to acquiring PI(3)P-binding capabilities following exposure to H2O2 in the cytoplasm, p40phox can acquire PI(3)P binding following membrane targeting, either directly by itself or indirectly in a p47phox-dependent manner through interactions in the p47phox-p67phox-p40phox complex. We found that the dependence on p40phox PI(3)P binding for Nox2 activity is determined by the phosphorylation status of p47phox. p40phox is essential during FcγR-mediated oxidase activation; however, p40phox is less critical under conditions when p47phox is adequately phosphorylated, using phosphorylation/activation-mimicking p47phox mutants. Moreover, PI binding of p47phox is less important when the autoinhibitory PX-PB1 domain interaction in p40phox is disrupted or when p40phox is targeted to membranes. Taken together, these results indicate that p40phox and p47phox cooperate in executing the carrier function directing the cytoplasmic ternary Phox complex to phagosomes and the adaptor function for assembly of the Nox2 complex during the FcγR-mediated oxidative burst.
EXPERIMENTAL PROCEDURES
Materials
Goat polyclonal antibody (pAb) against p47phox or p67phox and rabbit pAb against p40phox were described previously (33, 34). Rabbit pAb against mouse p40phox and mouse monoclonal Ab (mAb) against p67phox were from Millipore and BD Biosciences, respectively. Mouse mAb against the C terminus of p47phox (196–390 aa) and rabbit mAb against the C-terminal end of p40phox were from Santa Cruz Biosciences and Abcam, respectively. Mouse mAb against gp91phox or p22phox was a kind gift from Drs. Roos and Verhoeven (35). Goat pAb against FcγRIIa and mouse mAb against early endosome antigen-1 (EEA1) were from R&D Systems and BD Biosciences, respectively. H2O2 was from Wako Pure Chemical Industries.
Cell Culture
HEK293 cells (ATCC) were maintained in Eagle's minimal essential medium (Wako) containing 10% heat-inactivated FBS (Invitrogen), 100 μm nonessential amino acids (Invitrogen), and antibiotics at 37 °C in 5% CO2. RAW264.7 cells were described previously (36). For establishing clonally derived HEK293 lines with stable expression of human Nox2 and human FcγRIIa (HEK293Nox2/FcγRIIa), Nox2 in pcDNA3.1(Neo) and FcγRIIa in pcDNA3.1(Neo) were transfected into HEK293 cells using FuGENE 6 (Roche Applied Science) and followed by selection in the presence of 1 mg/ml G418 (Calbiochem). Establishment of the cloned lines was confirmed by immunoblotting and immunostaining using α-Nox2 Ab (or α-p22phox Ab) and α-FcγRIIa Ab (supplemental Fig. 1, A and B), and by a ROS assay using IgG-opsonized glass beads (BIgG) after transfection of p47phox + p67phox with/without p40phox (supplemental Fig. 1C). A clonally derived HEK 293 line with stable expression of human p67phox (HEK293p67phox) was established in the same way as described above (supplemental Fig. 5A).
For establishing clonally derived RAW264.7 lines with stable knockdown of p40phox alone (RAW264.7p40KD) or both p40phox and p47phox (RAW264.7p40/p47KD), empty pSUPER(puro) vector (OrigoEngine) or pSUPER(puro) vector(s) containing a target sequence (three sequences each) was transfected into RAW264.7 cells using FuGENE HD (Promega) and followed by selection in the presence of 1.5 mg/ml puromycin (Wako). Establishment of the cloned lines was confirmed by immunoblotting and ROS assays using BIgG (supplemental Fig. 2). The most efficient target sequence for p40phox knockdown among three tested was GCAAATTGGAGCTAAGTTTCA (nucleotide 554–574 from ATG), and that for p47phox among three tested was GCGAAGAAGCCTGAGACATAC (nucleotide 397–417 from ATG), respectively.
Construction of Plasmids
Human FcγRIIa, a low affinity to IgG and monomeric type of Fcγ receptor, was amplified by PCR using the first-strand cDNA from leukocyte (BD Biosciences) and cloned into XbaI/EcoRI sites of pcDNA3.1 vector (Invitrogen). Human Nox2, p47phox, p67phox, and p40phox in pcDNA3.1 were described previously (12, 27, 37). p67phox(K355A), which does not bind p40phox (29) in pcDNA3.1, was made by site-directed mutagenesis using a QuikChange II XL site-directed mutagenesis kit (Stratagene). GFP-p47phox, GFP-p67phox, GFP-p40phox, and GFP-p40phox(PX) were also described previously (12, 27, 38). For p47phox(ΔAIR: Δ298–340 aa) construction, two fragments (p47phox(1–297 aa) with BamHI/ApaI sites and p47phox(298–390 aa) with ApaI/EcoRI sites) were amplified by PCR and cloned into BamHI/EcoRI sites of pcDNA3.1 or BglII/EcoRI sites of pEGFP(C1) (BD Biosciences). The plasma membrane (PM)-targeted mutant of p67phox (p67phoxpp) in pcDNA3.1, which is adapted with the C-terminal, polybasic motif (KKRKRK; 183–188 aa) and isoprenylation motif (CLLL; 189–192 aa) of Rac1, was described previously (37), and GFP-p67phoxpp was made by transfer of p67phoxpp into BglII/SalI sites of pEGFP(C3). p47phox(R90K), p47phox(S303D), p47phox(S304D), p47phox(S328D), p47phox(S303D/S304D), p47phox(S303D/S328D), p47phox(S304D/S328D), p47phox(S303D/S304D/S328D) in pcDNA3.1 were made using QuikChange. p40phox(R105K), in pcDNA3.1, which does not bind PI(3)P (39), and p40phox(F320A), in pcDNA3.1 or in pEGFP(C1), which disrupts the intermolecular PX-PB1 interaction within p40phox (12, 13, 27), were also made using QuikChange. For Noxo1-p47phox and Noxo1(R40Q)-p47phox construction, two fragments (Noxo1(PX: 1–122 aa) or Noxo1(PX,R40Q) with BamHI/AflII and p47phox(123–390 aa) with AflII/EcoRI) were amplified by PCR (40) and cloned into BamHI/EcoRI sites of pcDNA3.1 or into BglII/EcoRI sites of pEGFP(C1). An endomembrane-targeted mutant of p40phox (p40phoxp in pcDNA3.1 or pEGFP(C1)), which is adapted with the isoprenylation motif (CLLL) alone, and the PM-targeted mutant of p40phox (p40phoxpp in pcDNA3.1 or pEGFP(C1)), which is adapted with both the polybasic and isoprenylation motif (KKRKRKCLLL), were made using QuikChange. p40phoxpp deleted of its PX domain (1–136 aa) (p40phox(ΔPX)pp) with BamHI/EcoRI sites was amplified by PCR and cloned into BglII/EcoRI sites of pEGFP(C1), and GFP-p40phox(R105K)p was made using QuikChange. N-terminally monomeric Kusabira-Orange (mKO; excitation, 548 nm; emission, 561 nm) (27)-tagged p40phox (mKO-p40phox) was made by replacement of EGFP with mKO in pEGFP(C1) vector, and mKO-p40phox(R105K) was made using QuikChange. For FYVE-p40phox construction, two fragments (mouse FYVE domain (147–297 aa) of hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) (41) with BamHI/NdeI and p40phox(137–341 aa) with NdeI/EcoRI) were amplified by PCR and cloned into BamHI/EcoRI sites of pcDNA3.1 or BglII/EcoRI sites of pEGFP(C1), and FYVE-p40phoxp was made using QuikChange. For PH(TAPP1)-p40phox construction, two fragments (human PH domain (180–291 aa) of tandem PH domain containing protein-1 (TAPP1) (41) with BamHI/NdeI and p40phox(137–341 aa) with NdeI/EcoRI) were amplified by PCR and cloned into BamHI/EcoRI sites of pcDNA3.1 or BglII/EcoRI sites of pEGFP(C1). All plasmids were sequenced to confirm their identities. Properties of p47phox, p67phox and p40phox mutants are summarized in Table 1.
TABLE 1.
Summary of properties of p47phox, p67phox, and p40phox mutants used in the present study
| Name of expressed mutant | Properties of expressed protein |
|---|---|
| p47phoxp | Endomembrane-targeted mutant which is adapted with CLLL motif of Rac1 at its C terminus |
| Noxo1-p47phox | PM-targeted mutant of p47phox in which its PX domain is replaced with the PX domain of Noxo1 |
| Noxo1(R40Q)-p47phox | Noxo1-p47phox with R40Q mutation |
| p40-p47phox | p47phox mutant in which its PX domain is replaced with the PX domain of p40phox |
| p40(R105K)-p47phox | p40-p47phox with R105K mutation |
| p47phox(R90K) | PI binding-deficient and membrane targeting-defective mutant |
| p47phox(R90K)p | p47phox(R90K) with CLLL motif at its C terminus |
| p47phox(ΔAIR) | AIR-deleted mutant that constitutively interacts with p22phox |
| p47phox(S303D), p47phox(S304D), p47phox(S328D) | One-phosphorylation site-mimicking mutants |
| p47phox(S303D/S304D), p47phox(S303D/S328D), p47phox(S304D/S328D) | Two-phosphorylation site-mimicking mutants |
| p47phox(S303D/S304D/S328D) | Three-phosphorylation site-mimicking mutant that can interact with p22phox |
| p67phox(K355A) | p40phox binding-deficient mutant |
| p67phoxpp | PM-targeted mutant that is adapted with a KKRKRK + CLLL motif of Rac1 at its C terminus |
| p40phox(R105K) | PI(3)P binding-deficient mutant |
| p40phox(F320A) | Intramolecular PX-PB1 domain interaction-defective mutant |
| p40phox(R105K/F320A) | p40phox(F320A) with R105K mutation |
| p40phoxpp | PM-targeted mutant that is adapted with a KKRKRK + CLLL motif at its C terminus |
| p40phox(ΔPX)pp | p40phoxpp with deletion of its PX domain |
| p40phoxp | Endomembrane-targeted mutant that is adapted with a CLLL motif at its C terminus |
| p40phox(R105K)p | p40phoxp with R105K mutation |
| FYVE-p40phox | p40phox mutant in which its PX domain is replaced with the FYVE domain of Hrs |
| FYVE-p40phoxp | FYVE-p40phox with a CLLL motif at its C terminus |
| PH(TAPP1)-p40phox | p40phox mutant in which its PX domain is replaced with the PH domain of TAPP1 |
In Vitro Binding (Pull-down) Assay
The purified His6-p40phox(PX: 1–167 aa) protein was described previously (12). To avoid dimerization by H2O2, p40phox(PB1: 237–339 aa) in pGEX-6P-1 (12) with a C242F mutation (GST-p40phox(PB1)) was made using the QuikChange. The purified GST-p40phox(PB1) and full-length His6-p40phox proteins were obtained as described previously (12).
The purified (His)6-p40phox(PX) (100 nm) was mixed with the purified GST-p40phox(PB1) (100 nm) in 400 μl of the binding buffer (12). After 10 min of rotation at 4 °C, 0.01 mm H2O2 was added in the solution and incubated for 10 min at 4 °C. Then anti-His tag magnetic beads (MBL International) were added to the solution and rotated for 30 min at 4 °C. The precipitates were washed three times using a magnetic rack with the buffer, the material absorbed to beads was eluted in Laemmli sample buffer, and the magnetic beads were removed using a magnetic rack. The aliquots of eluants were subjected to SDS-PAGE and followed by immunoblotting using anti-GST pAb (Santa Cruz Biotechnology, Inc.; 1:1000, room temperature for 2 h). Bound antibodies were detected with secondary antibody-HRP conjugates using the ECL detection system (GE Healthcare).
The purified full-length His6-p40phox protein (300 nm) was mixed with biotin-coupled PI(3)P-containing polymerized liposomes (100 μm) (PI(3)P PolyPIPsomesTM: Y-P003, Echelon) in 50 μl of the binding buffer (12). After 10 min of agitation at 4 °C, 0.01 mm H2O2 was added in the solution and incubated for 10 min at 4 °C. Then streptavidin-coupled magnetic beads (Dynabeads® M-280 Streptavidin, Invitrogen) were added to the solution and agitated for 30 min at 4 °C. The precipitates were washed three times using a magnetic rack with the buffer, the material absorbed to beads was eluted in Laemmli sample buffer, and the magnetic beads were removed using a magnetic rack. The aliquots of eluants were subjected to SDS-PAGE and followed by immunoblotting using mouse mAb against His6(9C11)-peroxidase-conjugated (Wako; 1:1000 at room temperature for 2 h).
Confocal Fluorescence Imaging Studies Using Fixed Cells or Live Cells
A total of 2.5 × 105 cells (HEK293, HEK293Nox2/FcγRIIa, or HEK293p67phox) were seeded on 35-mm glass bottom dishes (MatTek chambers) 48 h prior to transfection and transfected using FuGENE 6. 25–30 h after the transfection, cells were fixed using 4% paraformaldehyde in HEPES buffer solution, permeabilized as described previously (37), and stained using primary Abs at room temperature for 2 h. Primary Abs were visualized by a confocal laser-scanning fluorescence microscope (LSM510 or LSM700, Zeiss) using Alexa-conjugated anti-IgG (Invitrogen; 1:2000, 0.5 h at room temperature). BIgG was prepared using 5-μm glass beads (Duke Scientific Corp.) at 10 mg/500 μl of HBSS++ (Invitrogen), as described previously (42). 25–30 h after the transfection, the culture medium was replaced with HBSS++. After HBSS++ containing BIgG (five targets per cell) or H2O2 was added to each plate (12), images were collected at 5-s intervals for 15 min using a confocal laser-scanning fluorescence microscope with a heated stage and objective (38). The point of stimulant addition or the starting point of ingestion of added BIgG was chosen as time 0. All imaging experiments were performed in triplicate and were repeated in at least three independent transfection experiments (n ≥ 9).
ROS Production Assay
HEK293Nox2/FcγRIIa and RAW246.7 cells were seeded on 6-well dishes at 2.5 × 105 cells/well and 1.5 × 105 cells/well, respectively, 48 h prior to transfection. HEK293 and RAW246.7 cells were transfected using FuGENE 6 and FuGENE HD in complexes with various combinations of plasmids, respectively. The transfection to RAW246.7 cells was most efficient by FuGENE HD among several reagents tested, and the efficacy was about 70–80% based on the imaging experiments using various GFP-based plasmids, such as GFP-p40phox. The cells were fed 5 h post-transfection with complete medium and were used for assay 25–30 h after transfection. ROS release with or without stimulation (3 μl of BIgG or 200 ng/ml PMA; Sigma-Aldrich) from 2.0 × 105 trypsinized cells was measured by luminol-enhanced chemiluminescence methods in the presence of exogenous 10 units/ml HRP (Sigma-Aldrich) and 200 μm luminol (Sigma-Aldrich) for 20 min using a luminometer (Mithras LB940, Berthold). The ROS detected in the present study was the sum of extracellular ROS and intracellular ROS (probably including intraphagosomal ROS detected by luminol + exogenous HRP) but predominantly extracellular ROS (supplemental Fig. 1C). The assay (luminol-HRP without SOD + catalase; also luminol-HRP with SOD + catalase, luminol without HRP, and isoluminol-HRP) clearly shows p40phox dependence in response to BIgG (supplemental Fig. 1C). NADPH oxidase activity was inhibited by 10 min of prior incubation with 10 μm diphenylene iodonium (Sigma-Aldrich). Comparable expression of Phox proteins was adjusted and confirmed by immunoblotting using the total lysates from the same number of cells.
Statistical Analysis
Mean oxidase activities (ROS production) were calculated from at least three independent transfection experiments and were presented as percentages (mean ± S.E.). Significant differences were calculated by Student's t test, and results with p < 0.05 were considered significant.
RESULTS
H2O2 Induces Conformational Changes and Targeting of p40phox to Early Endosome (EE)
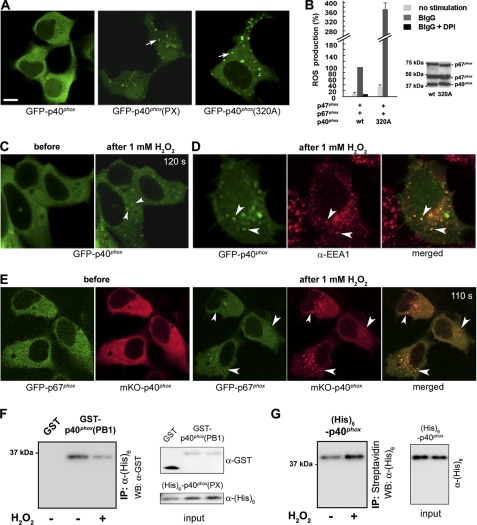
GFP-p40phox showed a diffuse cytoplasmic localization pattern (Fig. 1A, left), whereas in sharp contrast, the PX domain of p40phox was localized to dotlike, vesicular structures in resting wild type (WT) HEK293 cells (Fig. 1A, center), as reported previously in RAW 264.7 macrophages (12) and PBL-985 granulocytes (28). We (12, 27) and others (13, 31) reported that p40phox mutants, such as p40phox(11A:318–328), p40phox(4A:318–321), p40phox(F320A), p40phox(E259A), and p40phox(D269A), that disrupt the intermolecular PX-PB1 interaction in p40phox cause a redistribution of p40phox to EEs even in cells in a resting state. Consistent with those reports, p40phox(F320A) showed a dotlike localization pattern in resting WT HEK293 cells (Fig. 1A, right) and moderate constitutive (32.0 ± 6.2%) or high BIgG-stimulated (371.5 ± 25.4%) ROS production when compared with control HEK293Nox2/FcγRIIa cells (stably expressing Nox2 and FcγRIIa; supplemental Fig. 1) transiently expressing p47phox + p67phox + WT p40phox (Fig. 1B). The constitutive activity by p40phox(F320A) suggests that the EE is one of the sites where ROS production occurs in HEK293Nox2/FcγRIIa cells, in agreement with a recent report (43). In our recent studies using RAW macrophages, we demonstrated that p40phox itself or p40phox-containing ternary Phox complexes translocate to EEs in response to arachidonic acid, which induces conformational changes in p40phox that disrupt the intramolecular PX-PB1 domain interaction (12). In the present study, we examined the effects of H2O2, which is derived from the Nox2-based NADPH oxidase and can diffuse to the cytoplasm. GFP-p40phox translocated to dotlike structures in WT HEK293 cells (Fig. 1C) that co-stained with EEA1 Ab, an EE marker (Fig. 1D), in response to 1 mm H2O2, whereas neither GFP-p47phox nor GFP-p67phox showed translocation to membrane structures (data not shown). Importantly, GFP-p67phox also translocated to dotlike structures when co-expressed with mKO-p40phox, which has a red fluorescent protein tag (mKO) at the N terminus, after stimulation with H2O2 both in WT HEK293 cells (Fig. 1E) and in RAW macrophages (supplemental Fig. 3). Furthermore, inhibition of PI(3)P production on EEs by a PI 3-kinase inhibitor, wortmannin (100 nm for 15 min), showed no dotlike localization of GFP-p40phox(PX) or translocation of GFP-p40phox to dotlike structures in response to H2O2 in RAW macrophages (supplemental Fig. 4).
FIGURE 1.
H2O2 induces EE targeting of p40phox. A, transfected GFP-p40phox is localized in the cytoplasm (left); in contrast, GFP-p40phox(PX) (center; arrow) and p40phox(F320A) (right; arrow) are localized at dotlike structures, in resting WT HEK293 cells. Bar, 10 μm. B, p40phox(F320A) co-expressed with p47phox + p67phox supports moderate constitutive (32.0 ± 6.2%) and high BIgG-stimulated ROS production in HEK293Nox2/FcγRIIa cells when compared with cells expressing WT p40phox + p47phox + p67phox. Western blotting detects comparable levels of cytoplasmic Phox proteins in both transfection experiments. C, in response to exogenous 1 mm H2O2 (120 s after stimulation), GFP-p40phox expressed in WT HEK293 cells translocates to dotlike structures (arrowheads). D, the dotlike structures are co-stained by an EE marker, EEA1 (arrowheads). E, cytoplasmic GFP-p67phox co-expressed with mKO-p40phox in WT HEK293 cells translocates to dotlike structures (arrowheads) after stimulation (110 s) with 1 mm H2O2. Similar effects of H2O2 are observed in RAW 264.7 cells (supplemental Fig. 3). F, effect of H2O2 on the PX-PB1 domain interaction in vitro. The interaction between purified His6-p40phox(PX) and GST-p40phox(PB1) proteins detected by pull-down assays is weakened by the addition of 0.01 mm H2O2. Similar results are obtained in three independent experiments. G, effect of H2O2 on binding of p40phox to PI(3)P in vitro. The interaction between purified full-length His6-p40phox protein and PI(3)P-containing liposomes detected by pull-down assays is strengthened by the addition of 0.01 mm H2O2. Similar results were obtained in three independent experiments. Error bars, S.E.
These results suggest that H2O2 induces conformational changes within p40phox in the cytoplasm, enabling it to function as a carrier protein that directs the cytoplasmic Phox complex to PI(3)P-enriched membranes. To support this speculation, we performed a binding (pull-down) experiment using purified His6-p40phox(PX) and GST-p40phox(PB1) proteins. H2O2 (0.01 mm) weakened the interaction between His6-p40phox(PX) and GST-p40phox(PB1), further suggesting that H2O2 induces some conformational changes in the PX and/or the PB1 domain of p40phox (Fig. 1F). Furthermore, in an in vitro binding assay using purified full-length His6-p40phox protein and PI(3)P-containing liposomes, H2O2 (0.01 mm) strengthened the interaction between His6-p40phox and PI(3)P, suggesting that H2O2 induces disruption of the PX-PB1 domain interaction within p40phox (Fig. 1G).
Direct or Indirect Membrane Targeting of p40phox Induces Recruitment of p40phox to EE
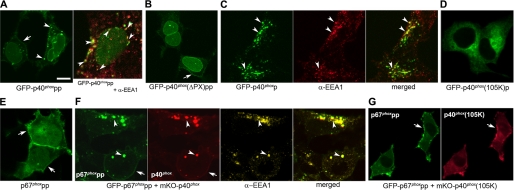
To examine the possibility that p40phox also develops PI(3)P-binding capabilities after membrane targeting, we utilized a PM-targeting motif consisting of the polybasic and prenylated (pp) C-terminal sequence of Rac1 (183–192 aa; KKRKRKCLLL) or the intracellular endomembrane-targeting prenylated (p) motif (189–192 aa; CLLL) of Rac1 fused onto GFP-tagged p40phox, according to previous reports (36, 37, 44–46). GFP-p40phoxpp was localized at EEs in addition to the PM (Fig. 2A), and this EE localization pattern was abolished with GFP-p40phox(ΔPX)pp, lacking the PX domain in WT HEK293 cells (Fig. 2B). GFP-p40phoxp was also targeted to EEA1-positive dotlike structures both in WT HEK293 (Fig. 2C) and HEK293Nox2/FcγRIIa cells (supplemental Video 1), and this EE localization was abolished in the case of the PX domain mutant (Fig. 2D), GFP-p40phox(R105K)p, which loses its capabilities to bind PI(3)P.
FIGURE 2.
Direct or indirect membrane targeting of p40phox induces EE-targeting of p40phox in WT HEK293 cells. A, transfected GFP-p40phoxpp is localized at dotlike structures (left; arrowheads) in addition to PM (left; arrow). The dotlike structures are co-stained with EEA1 (right; arrowheads). Bar, 10 μm. B, the dotlike localization of GFP-p40phoxpp disappears in the case of GFP-p40phox(ΔPX)pp. Arrow, PM. C, GFP-p40phoxp is co-stained with EEA1 (arrowheads). A movie of accumulation on phagosome of p40phoxp during ingestion of BIgG in HEK293Nox2/FcγRIIa cells is available (supplemental Video 1). D, the dotlike localization of GFP-p40phoxp disappears in the case of GFP-p40phox(R105K)p. E, PM-targeting mutant of cytoplasmic GFP-p67phox, GFP-p67phoxpp, shows PM localization (arrows) in addition to nuclear localization. F, when cytoplasmic mKO-p40phox is co-expressed with GFP-p67phoxpp, both proteins are co-localized at EEs stained by EEA1 (using Cy5-conjugated secondary Ab) (arrowheads) in addition to the PM (arrow). G, the dotlike localization of both proteins disappears in the case of mKO-p40phox(R105K) + GFP-p67phoxpp. Arrow, PM.
To further investigate the possibility that indirect membrane targeting of p40phox also induces conformational changes in p40phox promoting its binding to PI(3)P, we used a PM-targeted mutant of p67phox, GFP-p67phoxpp. GFP-p67phox is a cytoplasmic protein in resting cells (12); however, GFP-p67phoxpp showed PM localization in WT HEK293 cells (Fig. 2E). When cytoplasmic mKO-p40phox was co-expressed with GFP-p67phoxpp, mKO-p40phox and GFP-p67phoxpp co-localized at EEs in addition to the PM in WT HEK 293 cells (Fig. 2F). This EE but not PM localization of mKO-p40phox was abolished in the case of mKO-p40phox(R105K), which does not bind PI(3)P (39) (Fig. 2G).
These results suggest that PM or endomembrane targeting of p40phox, whether through direct or indirect means, caused recruitment of p40phox to PI(3)P-enriched EEs through subcellular membrane cycling (PM to EEs in the endocytic pathway (47, 48) and endomembranes to EEs in the retrograde-transport and anterograde-transport pathways (49)); finally, p40phox bound to PI(3)P and accumulated on the membranes of EE. In agreement with our data, a recent study reported that the membrane-spanning Phox protein (heterodimer of Nox2 and p22phox) was localized on the recycling endosomes as well as EEs and PM in CHO model cells and macrophages (43). Thus, p40phox probably develops PI(3)P-binding capabilities also through direct or indirect membrane targeting and may even promote these membrane cycling and trafficking pathways through PI(3)P binding.
Indirect Membrane Targeting of p40phox through p47phox-p67phox-p40phox Interaction Induces Recruitment of p40phox to EE
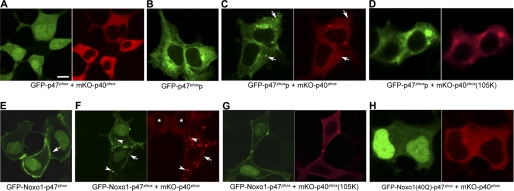
p47phox functions as a carrier and adaptor protein of the cytoplasmic ternary Phox complex (5, 21, 33, 50). We first examined the possibility that indirect membrane targeting of p40phox as a component of the ternary p47phox-p67phox-p40phox complex induces conformational changes within p40phox that enable it to bind to PI(3)P and localize at PI(3)P-enriched membranes. When GFP-p47phox and mKO-p40phox were co-expressed in HEK293p67phox cells (stably expressing p67phox; supplemental Fig. 5A), both were localized in the cytoplasm (Fig. 3A). Intriguingly, when cytoplasmic mKO-p40phox was co-expressed with an intracellular endomembrane-targeted mutant of GFP-p47phox, GFP-p47phoxp (Fig. 3B), dotlike structures containing GFP-p47phoxp, mKO-p40phox, and p67phox appeared (Fig. 3C and supplemental Fig. 5B) in HEK293p67phox cells, which were not observed in the case of mKO-p40phox(R105K) (Fig. 3D). To explore an alternative means for indirect p40phox membrane targeting through the p47phox-p67phox-p40phox interaction, a PM-targeted mutant of p47phox was used in which its PX domain was replaced with that of Noxo1, which constitutively localizes at the PM even in resting cells (40, 51). When mKO-p40phox was co-expressed with GFP-Noxo1-p47phox in HEK293p67phox cells (Fig. 3E), mKO-p40phox also showed a dotlike localization pattern, in addition to PM localization with GFP-Noxo1-p47phox (Fig. 3F). These dotlike structures were co-stained with EEA1 (data not shown). The dotlike localization, but not PM localization, of GFP-Noxo1-p47phox and mKO-p40phox disappeared in the case of mKO-p40phox(R105K) (Fig. 3G). GFP-Noxo1(R40Q)-p47phox, which loses its PM-targeting capabilities by mutation in the PX domain (40), showed no dotlike or PM localization with mKO-p40phox (Fig. 3H). Furthermore, neither the dotlike nor PM localizations of mKO-p40phox were observed in WT HEK293 cells when GFP-Noxo1-p47phox was co-expressed with p67phox(K355A), which disrupts interaction between p67phox and p40phox (supplemental Fig. 5C).
FIGURE 3.
Indirect membrane targeting of p40phoxby p47phoxp or Noxo1-p47phox through the p47phox-p67phox-p40phox interaction induces EE localization of p40phox in HEK293p67phox cells. A, both GFP-p47phox and mKO-p40phox are localized in the cytoplasm. Bar, 10 μm. B, GFP-p47phoxp has a reticular, nuclear membrane and Golgi complex localization. C, in the case of co-expression of GFP-p47phoxp and mKO-p40phox, dotlike structures with GFP-p47phoxp and mKO-p40phox appear (arrows). Co-staining of stably expressed p67phox at the dotlike structures is shown in supplemental Fig. 5B. D, the dotlike structures disappear in the case of co-expression of GFP-p47phoxp and mKO-p40phox(R105K). E, GFP-Noxo1-p47phox shows PM localization (arrow) in addition to nuclear localization. F, with co-expression of GFP-Noxo1-p47phox and mKO-p40phox, mKO-p40phox is localized at dotlike structures (arrowheads) in addition to PM (arrow) with GFP-Noxo1-p47phox. In contrast, without co-expression of GFP-Noxo1-p47phox, mKO-p40phox is localized in the cytoplasm (asterisks). G, the dotlike localization, but not PM localization, of both proteins disappears in the case of co-expression of GFP-Noxo1-p47phox and mKO-p40phox(R105K). H, both the dotlike and PM localizations of both proteins disappear with co-expression of GFP-Noxo1(R40Q)-p47phox and mKO-p40phox.
These data show that indirect targeting of p40phox to membranes through other Phox protein interactions enables p40phox to bind to PI(3)P, thereby redirecting the cytoplasmic ternary Phox complex to PI(3)P-enriched membranes.
The p40phox High Affinity PI(3)P-binding PX Domain Is Critical for BIgG-stimulated Nox2 Activation
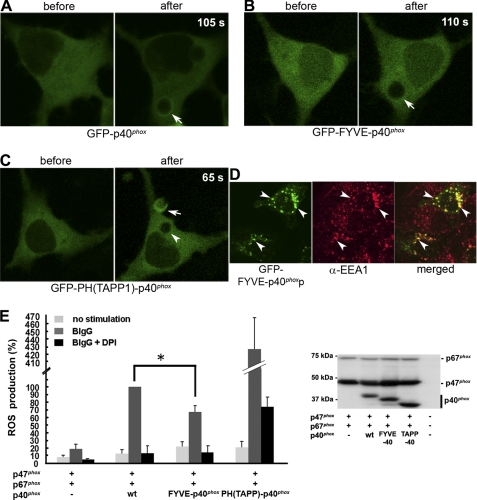
To examine the importance of PI(3)P binding to p40phox in Nox2 activation, we substituted its PX domain with other PI(3)P-specific binding domains, the FYVE domain from Hrs in addition to the PI(3,4)P2-specific PH domain from TAPP1 and the PI(3,4,5)P3-specific PH domain from GRP1 (general receptor for phosphoinositides-1), which were expressed in HEK293Nox2/FcγRIIa cells. GFP-FYVE showed no accumulation on phagosomes (data not shown), as described previously (52). In contrast, GFP-2xFYVE, which has about a 10-fold higher affinity for PI(3)P than GFP-FYVE (53), was localized at EE and accumulated on phagosomes by fusion with EE during ingestion of BIgG (data not shown), as observed with the p40phox PX domain (12), indicating that a threshold affinity for PI(3)P drives this translocation. GFP-PH(TAPP1) and GFP-PH(GRP1), which are primarily localized in the cytoplasm, also showed accumulation on phagosomes (data not shown). We then made chimeric mutants of p40phox replacing the PX domain with the FYVE, PH(TAPP1), or PH(GRP1) domains (FYVE-p40phox, PH(TAPP1)-p40phox, or PH(GRP1)-p40phox). It is unlikely that the FYVE or PH domains in these chimeric proteins are masked by the PB1 domain of p40phox, because the three-dimensional structures of these domains are quite different from that of the PX domain of p40phox (54, 55). Weak accumulation of GFP-p40phox on phagosomes was sometimes observed in HEK293Nox2/FcγRIIa cells without co-expression of p47phox or p67phox (Fig. 4A and supplemental Video 2), consistent with our previous report (12). The PX domain of p40phox has a high affinity for PI(3)P (Kd to 0.5% PI(3)P lysosome: 40 ± 7 nm (56)) but is masked by the PB1 domain. Accumulation of GFP-FYVE-p40phox, which has a low affinity domain (FYVE) for PI(3)P (Kd to 0.5% PI(3)P lysosome: 420 ± 8 nm (57); more than 10 times lower affinity than that of PX domain of p40phox (58)) but is not masked by the PB1 domain, was never observed (Fig. 4B and supplemental Video 3). In contrast, GFP-PH(TAPP1)-p40phox (Fig. 4C and supplemental Video 4) and GFP-PH(GRP1)-p40phox (data not shown), containing PH domains with a high affinity for PI(3,4)P2 (Kd of TAPP1 to 0.5% PI(3,4) P2 lysosome: 1.5 ± 0.5 nm (18); Kd of TAPP1 to PI(3,4)P2: 40.1 nm (41)) and for PI(3,4,5)P3 (Kd of GRP1 to PI(3,4,5)P3: 59.2 nm (41)), respectively, were readily detected on nascent phagosomes. A membrane-targeted mutant of FYVE-p40phox, FYVE-p40phoxp, showed EE localization (Fig. 4D) and accumulated on phagosomes by fusion of EE during ingestion of BIgG. (supplemental Video 5). From the standpoint of ROS production, PH(TAPP1)-p40phox showed about 4-fold higher ROS production compared with p40phox, whereas FYVE-p40phox showed statistically lower ROS production than p40phox (67.4 ± 9.6%) (Fig. 4E). Comparable expression of p47phox, p67phox, p40phox, and p40phox chimeric mutants was confirmed by immunoblotting (Fig. 4E).
FIGURE 4.
High affinity PI(3)P-binding PX domain is a critical determinant of p40phox phagosomal accumulation and oxidase function in HEK293Nox2/FcγRIIa cells. A, weak accumulation of GFP-p40phox on phagosome 105 s after the start of BIgG ingestion is shown (arrow). A movie is available (supplemental Video 2). B, no accumulation of GFP-FYVE-p40phox on phagosome is observed (arrow). A movie is available (supplemental Video 3). C, accumulation of GFP-PH(TAPP1)-p40phox on nascent phagosomal cup is observed 65 s after starting BIgG ingestion (arrow). No accumulation of GFP-PH(TAPP1)-p40phox on mature phagosome is observed (arrowhead). A movie is available (supplemental Video 4). D, FYVE-p40phoxp is localized at dotlike structures that co-stained with EEA1 (arrowheads). A movie of accumulation on phagosome of FYVE-p40phoxp during ingestion of BIgG is available (supplemental Video 5). E, BIgG-stimulated ROS production is enhanced 4-fold by p47phox + p67phox + PH(TAPP1)-p40phox and is statistically decreased by p47phox + p67phox + FYVE-p40phox when compared with p47phox + p67phox + p40phox. *, p < 0.05. Error bars, S.E.
These results indicate that the high affinity of the PX domain of p40phox for PI(3)P, even when masked by the PB1 domain in the resting state, initiates or dictates its accumulation on phagosomes. Despite the limitations of FYVE-p40phox detection by imaging these fluorescent-tagged proteins, even the basal level affinity for PI(3)P binding of the PX domain of p40phox or FYVE domain appears to influence ROS production.
The Phosphorylation Status of p47phox Influences the Dependence of Nox2-ROS Production on PI(3)P Binding by p40phox
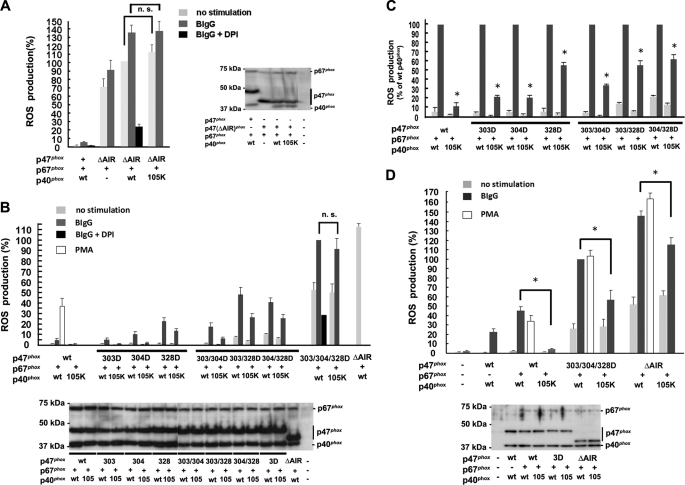
To further examine the consequences of indirect membrane targeting of p40phox through its interactions with p47phoxand p67phox, we used a mutant, p47phox(ΔAIR), in which the autoinhibitory region (AIR) is deleted. p47phox(ΔAIR) renders p47phox constitutively active and capable of membrane binding, supporting Nox2-based ROS production without stimulation because of its constitutive interaction with p22phox in resting cells (59). ROS production using p47phox(ΔAIR) HEK293Nox2/FcγRIIa cells was constitutive, was only slightly enhanced by BIgG stimulation, and was effectively inhibited by diphenylene iodonium (Fig. 5A). Enhanced ROS production by the addition of p40phox to p47phox(ΔAIR) + p67phox was probably due to enhanced stabilization of p67phox protein levels by p40phox (Fig. 5A). Most importantly, ROS production by p47phox(ΔAIR) + p67phox + p40phox was independent of the PI(3)P-binding capabilities of p40phox (Fig. 5A). Comparable expression levels of proteins were confirmed by immunoblotting (Fig. 5A). These results indicate that deletion of the AIR of p47phox leads to constitutive binding to p22phox, thereby inducing constitutive oxidase activity and only minor enhancing effects of BIgG, which are independent of the PI(3)P-binding capabilities of p40phox. To investigate the relationship between the phosphorylation status of p47phox and the dependence on p40phox-PI(3)P binding for ROS production by the Nox2-based oxidase, we used seven phosphorylation site-mimicking mutants of p47phox. ROS production using p47phox(S303D/S304D/S328D), which is a phosphorylation-mimicking mutant of p47phox sufficient for access to p22phox without cell stimulation (60), was not affected by the R105K mutation of p40phox in HEK293Nox2/FcγRIIa cells and was therefore independent of PI(3)P binding (Fig. 5B), as in the case of p47phox(ΔAIR). However, ROS production in response to BIgG using one or two phosphorylation site-mimicking mutants of p47phox (S303D, S304D, S328D, S303D/S304D, S303D/S328D, or S304D/S328D) was dependent on p40phox PI(3)P binding (Fig. 5B), and all of the phosphorylation-mimicking mutants showed higher ROS production (Fig. 5B) and less PI(3)P dependence than in the case of WT p47phox (Fig. 5C). Interestingly, the mimicking mutants having S328D (S328D, S303D/S328D, and S304D/S328D) showed constitutive activity without BIgG stimulation, higher ROS production, and less PI(3)P dependence than in the case of S303D, S304D, or S303D/S304D (Fig. 5C). PI(3)P binding independence in p47phox(S303D/S304D/S328D) and p47phox(ΔAIR), but not in two-site phosphorylation-mimicking mutants of p47phox (S303D/S304D, S303D/S328D, and S304D/S328D), was also detected by a ROS production assay using luminol without exogenous HRP (supplemental Fig. 6). Phosphorylation of WT p47phox in response to BIgG in HEK293Nox2/FcγRIIa cells was confirmed using 32P label (supplemental Fig. 7). Comparable expression of p47phox, p47phox mutants, p67phox, p40phox, and p40phox(R105K) was confirmed by immunoblotting (Fig. 5B). Taken together, it appears that the dependence on p40phox PI(3)P binding for ROS production by Nox2 is determined by the phosphorylation status of p47phox, which governs the interaction between p47phox and p22phox; complete constitutive (high affinity) interaction with p22phox results in maximum ROS production with PI(3)P independence, whereas incomplete (low affinity) interaction with p22phox favors PI(3)P dependence involving p40phox.
FIGURE 5.
Dependence of PI(3)P-binding of p40phox in ROS production by Nox2 is determined by the phosphorylation status of p47phox in both HEK293Nox2/FcγRIIa (A–C) and RAW264.7p40/p47KD cells (D). A, ROS production using p47phox(ΔAIR) is constitutive, shows small BIgG dependence, and is effectively inhibited by 10 μm diphenylene iodonium. The addition of p40phox enhances ROS production by p47phox(ΔAIR) and p67phox. ROS production by p47phox(ΔAIR) + p67phox + p40phox is independent of the PI(3)P-binding capabilities of p40phox (p40phox(R105K)). n.s., not statistically significant. B, ROS production using one- or two-site phosphorylation-mimicking mutants of p47phox as well as WT p47phox shows PI(3)P-binding of p40phox dependence; however, p47phox(S303D/S304D/S328D) is independent of PI(3)P binding to p40phox. All mimicking mutants showed higher ROS production than WT p47phox. 3D, S303D/S304D/S328D. C, PI(3)P dependence in ROS production using WT or one- or two-site phosphorylation-mimicking mutants of p47phox. Data shown in B are normalized to activities supported by WT p40phox. All mimicking mutants show less PI(3)P dependence than WT p47phox. The mimicking mutants having Asp-328 (S328D, S303D/S328D, and S304D/S328D) show constitutive activity without BIgG and less PI(3)P dependence than S303D, S304D, or S303D/S304D. *, p < 0.01. D, in RAW264.7p40/p47KD cells, ROS production using WT p47phox shows almost complete dependence on PI(3)P binding to p40phox. In contrast, reconstituted ROS production by p47phox(S303D/S304D/S328D) or p47phox(ΔAIR) shows only moderate PI(3)P dependence. *, p < 0.01. 3D, S303D/S304D/S328D. Error bars, S.E.
These results were confirmed in similar experiments using RAW264.7p40/p47KD cells (stable knockdown of both p40phox and p47phox; supplemental Fig. 2). Although PI(3)P dependence was still seen in reconstituted ROS production with p47phox(S303D/S304D/S328D) or p47phox(ΔAIR) (Fig. 5D), the order of PI(3)P dependence was WT p47phox ≫ p47phox(S303D/S304D/S328D) > p47phox(ΔAIR) in three different assays (Fig. 5D and supplemental Fig. 8, A and B). Comparable expression of p47phox, p47phox mutants, p67phox, p40phox, and p40phox(R105K) was confirmed by immunoblotting (Fig. 5D).
Membrane-associating Mutants of p40phox Can Rescue the PI-binding Function of the PX Domain of p47phox
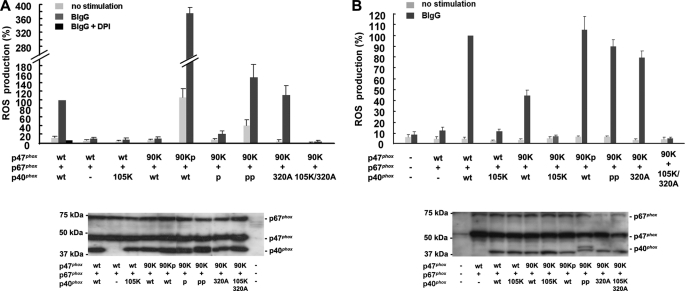
Finally, to examine the contribution of the adaptor functions of p40phox in the Nox2-based oxidase, we used membrane-targeted mutants of p40phox and p47phox(R90K), which is a PI binding-deficient (50, 61) and membrane targeting-defective mutant protein (12, 18, 56). ROS production reconstituted by p47phox(R90K) + p67phox + p40phoxv was significantly decreased in HEK293Nox2/FcγRIIa cells, consistent with previous studies (50) (Fig. 6A); however, ROS production was dramatically rescued by the membrane-targeted version of p47phox(R90K), p47phox(R90K)p (Fig. 6A). Although p40phoxp rescued some ROS production by p47phox(R90K) + p67phox + p40phox, considerably higher ROS production was rescued using p40phoxpp (Fig. 6A). Intriguingly, p40phox(F320A), which is a mutant freely accessible to PI(3)P with a disrupted intermolecular PX-PB1 interaction, fully rescued ROS production by p47phox(R90K) + p67phox + p40phox, whereas p40phox(R105K/F320A) supported no activity (Fig. 6A). Comparable expression of p47phox, p47phox mutants, p67phox, p40phox, and p40phox mutants was confirmed by immunoblotting (Fig. 6A). These findings complement observations on ROS production using p47phox(ΔAIR) or p47phox(S303D/S304D/S328D), which were not affected by the R105K mutation in p40phox (Fig. 5, A and B), and suggest that strong membrane associations in p40phox, seen in p40phoxpp or p40phox(F320A), could restore ROS production in the absence of PI binding to p47phox in HEK293Nox2/FcγRIIa cells.
FIGURE 6.
Membrane-associating mutants of p40phox can rescue the function of the PX domain of p47phox. A, in HEK293Nox2/FcγRIIa cells, ROS production by p47phox(R90K) + p67phox + p40phox is almost absent, whereas p47phox(R90K)p dramatically rescues reconstituted ROS production. p40phoxpp, but not p40phoxp, is able to completely rescue ROS production by p47phox(R90K) + p67phox + p40phox. p40phox(F320A), which is targeted to EE by disrupted PX-PB1 intermolecular interaction (Fig. 1A), effectively rescues ROS production by p47phox(R90K) + p67phox + p40phox, whereas p40phox(R105K/F320A) does not. B, in RAW264.7p40/p47KD cells, WT p40phox, but not p40phox(R105K), moderately rescues ROS production with p47phox(R90K). Membrane-associating mutants, p47phox(R90K)p, p40phoxpp, and p40phox(F320A) almost completely rescue reconstituted ROS production with p47phox(R90K). Error bars, S.E.
These results were confirmed in similar experiments using RAW264.7p40/p47KD cells in which WT p40phox, but not p40phox(R105K), showed moderate restoring capabilities in reconstituted ROS production by p47phox(R90K) (Fig. 6B). Comparable expression of p47phox, p47phox mutants, p67phox, p40phox, and p40phox mutants was confirmed by immunoblotting (Fig. 6B).
DISCUSSION
It has been well recognized that p47phox serves as a carrier of the cytoplasmic ternary Phox complex to membranes, using its PX domain, and also serves as an adaptor between the cytoplasmic Phox complex and the membrane-spanning flavocytochrome b558; thus, p47phox is called as an “organizer” of the Nox2 complex (2, 3). Although we and others reported that p40phox also acts as a carrier of the ternary Phox complex during FcγR-mediated (12, 27), PMA-stimulated (29–31), and fMLP-stimulated oxidative bursts (30), another report suggests that p40phox primarily functions in Nox2 activation on phagosomes (32). We showed here that the dependence on p40phox PI(3)P binding for Nox2 activity is determined, at least partly, by the phosphorylation status of p47phox. p40phox is essential during FcγR-mediated oxidase activation; however, p40phox is less critical under conditions when p47phox is adequately phosphorylated and binds to p22phox efficiently, as revealed when examining phosphorylation/activation-mimicking p47phox mutants (i.e. p47phox(ΔAIR) and p47phox(S303D/S304D/S328D)) (Fig. 5 and supplemental Figs. 6 and 8). In the present study, we showed the dependence on p40phox PI(3)P binding for Nox2 activity (both for extracellular and intracellular ROS) during FcγR-mediated oxidative burst, based on the following methods and observations: 1) in assays using luminol with HRP (measuring total ROS but predominantly extracellular ROS), in assays using luminol with HRP + (SOD + catalase) (measuring intracellular ROS), in assays using luminol without HRP (measuring intracellular ROS), or in assays using isoluminol with HRP (measuring extracellular ROS) in HEK293Nox2/FcγRIIa cells (Fig. 5, A and B, and supplemental Figs. 1 and 6); 2) in assays using RAW264.7p40/p47KD cells (Fig. 5D and supplemental Fig. 8); and 3) based on a report demonstrating p40phox and PI(3)P binding dependence during FcγR-mediated oxidative burst (measured using luminol with HRP) in COS7 cells stably expressing Phox proteins and FcγRIIa (32). A series of stepwise phosphorylation events at eight distinct phosphorylated sites within p47phox were reported (8, 9), of which only four are prominently phosphorylated in membrane-bound p47phox fractions in early phases of stimulation in normal neutrophils but not in flavocytochrome b558-deficient CGD neutrophils (8). We demonstrated that PI binding (i.e. membrane targeting) (12, 18, 56), by p47phox is less critical (Fig. 6) when the autoinhibitory (PX-PB1) interaction within p40phox is released and allows binding to PI(3)P or when p40phox is targeted to membranes by other means, as seen with p40phox(F320A) and p40phoxpp. These observations strongly support the proposed carrier function of p40phox in delivering the ternary Phox complex to phagosomes in cooperation with p47phox under conditions when p47phox is only partially phosphorylated (e.g. in the initial stages of translocation of the Phox complex to phagosomes) or when PI levels are insufficient to bind p47phox (e.g. in late stages of phagocytosis) (12). Furthermore, although the function of p40phox as a carrier and adaptor seems to be less prominent than p47phox (Fig. 6A), we propose that both p40phox and p47phox are required for orchestrating optimal phagosome-targeting of the cytoplasmic Phox complex and also for stable assembly and retention of the Nox2 complex on phagosomes during the FcγR-mediated oxidative burst. Several reports support this concept of a functional partnership of both proteins: p47phox is required for phagosome-targeting of p67phox and ROS production in p47phox-deficient neutrophils (23); p40phox-deficient neutrophils exhibit a severe defect in FcγR-mediated oxidative burst but not in PMA- or fMLP-stimulated ROS production (28); PI(3)P-binding capabilities of p40phox are required for prolonged retention of p40phox (28) and the p40phox-p67phox-p47phox complex (27) on phagosomes; autosomal recessive CGD patients, including a p40phox-deficient patient, suffer less severe clinical phenotypes than X-linked CGD patients (62); phosphorylation of p40phox on Thr-154 is required for phagosome targeting of p47phox and ROS production in reconstituting p40phox-deficient (or knockdown) neutrophils (63); and both p40phox and p47phox are required for ROS production in microvascular endothelial cells (64).
It was reported that translocation of p67phox, involving the carrier function of p40phox, is dependent on the PX domain of p40phox but is PI(3)P-independent and that activation of Nox2 is PI(3)P-dependent in PMA-stimulated, permeabilized PLB-985 neutrophil cores (31). These authors speculated that moesin (65), a cytoskeletal protein, instead of PI(3)P may be a predominant target of the p40phox PX domain in the PMA-stimulated oxidative burst of permeabilized cores (31). In the present study using HEK293Nox2/FcγRIIa cells, we found that the PX domain of p40phox is much less critical in responses to PMA than the PX domain of p47phox (supplemental Fig. 9), indicating that PMA, an analog of diacylglycerol, triggers predominantly p47phox(PX)-dependent but p40phox-independent oxidase activation, consistent with studies in p40phox-deficient COSphoxFcγR cells and neutrophils (26, 28). Thus, the p40phox and PI(3)P dependence in Nox2 activation is determined by stimulus (e.g. BIgG versus PMA).
Cho and Stahelin (58) described a general mechanism of membrane-protein interactions in which membrane adsorption of PX domain-containing proteins such as p47phox and p40phox (18, 56) is initially driven by nonspecific electrostatic interactions (between anionic lipids in membranes and cationic surfaces of proteins) and by diffusion, which is then followed by specific interaction with PIs and interfacial penetration (66) of hydrophobic and aromatic residues located near its respective binding site of PIs. Crystallographic studies on p40phox, revealed that intramolecular PX-PB1 domain interactions are sterically inhibiting access of the PX domain with membrane-embedded PI(3)P, rather than completely masking the PI(3)P-binding site (13); in other words, the PX domain is able to access PI(3)P in certain conditions because three-dimensional positioning of membrane-embedded PI(3)P changes during phagosome formation. This speculation is supported by reports that full-length p40phox binds to soluble PI(3)P to the same extent as the PX domain (39) and that full-length p40phox binds to PI(3)P in surface plasmon resonance and lipid monolayer assays in which PI(3)P is flexible in the lipid monolayer (56). Considering our finding that p40phox possessing the high affinity binding PX domain for PI(3)P accumulated on phagosomes (Fig. 4A), even if sterically inhibited, whereas FYVE-p40phox possessing low affinity PI(3)P binding did not accumulate on phagosomes (Fig. 4B), the “semimasked” high affinity binding PX domain of p40phox probably fulfills important missions during its translocation, including initiation of translocation.
Enhanced protein tyrosine phosphorylation by H2O2-induced inhibition of phosphotyrosine phosphatases has been reported (67, 68). In addition, it was reported that H2O2 directly induces conformational changes in proteins (69). In the present study, p40phox tyrosine phosphorylation was not observed in response to H2O2 using anti-phosphotyrosine Ab (4G10) (data not shown), consistent with previous work (70), and conformational changes within p40phox induced by H2O2 were observed by in vitro binding assays (Fig. 1, F and G). The other adaptor protein, p47phox, showed no translocation to any membrane site in response to H2O2. Interestingly, PKCs, which are well known to phosphorylate p47phox (10, 38, 71) and accumulate on phagosomes (38), are reported to be activated by H2O2 both on membranes and in the cytoplasm (72, 73). Thus, H2O2 may induce several positive feedback effects on both p40phox and p47phox, both on membranes and in the cytoplasm during the FcγR-mediated oxidative burst. In addition to this positive feedback mechanism of p40phox directly acquiring PI(3)P binding capabilities by exposure to H2O2 (demonstrated in the present study) or by arachidonic acid (12), p40phox can also indirectly acquire PI(3)P binding (Figs. 2F and 3, C and F) on targeted membranes in a p47phox-dependent manner through its associations within the p47phox-p67phox-p40phox complex.
Supplementary Material
Acknowledgments
We are thankful to Mr. Takeshi Hamada for his technical assistance. We also thank Dr. Y Irino and Prof. T Takenawa (Kobe University Graduate School of Medicine) for providing the 2xFYVE domain of mouse Hrs and the PH domain of human TAPP1.
This work was supported in part by the National Institutes of Health, NIAID, Intramural Research Program. This work was also supported in part by Grants-in-aid for scientific research; on the Global Center of Excellence Program, on Priority Areas “Transportsome,” on Innovative Areas “Fluorescence Live Imaging” and (C), from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by the “Japan-Hungary Research Cooperative Program” grant from the Japan Society for the Promotion of Science and Hungarian Academy of Sciences, by a grant from the Naito Foundation, by a grant from the Suzuken Memorial Foundation, and by a grant from the Takeda Science Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–9 and Videos 1–5.
- ROS
- reactive oxygen species
- EE
- early endosome
- FcγR
- Fcγ receptor
- PI(3)P
- phosphatidylinositol 3-phosphate
- PI(3,4)P2
- phosphatidylinositol 3,4-bisphosphate
- PI
- phosphoinositide
- BIgG
- IgG-opsonized glass beads
- AIR
- autoinhibitory region
- PM
- plasma membrane
- p
- prenylation motif
- pp
- polybasic and prenylation motif
- CGD
- chronic granulomatous disease
- PMA
- phorbol 12-myristate 13-acetate
- fMLP
- formylmethionylleucylphenylalanine
- Ab
- antibody
- pAb
- polyclonal antibody
- aa
- amino acids
- EEA1
- early endosome antigen-1
- mKO
- monomeric Kusabira-Orange
- EGFP
- enhanced GFP
- PH
- pleckstrin homology.
REFERENCES
- 1. Quinn M. T., Gauss K. A. (2004) J. Leukoc. Biol. 76, 760–781 [DOI] [PubMed] [Google Scholar]
- 2. Sumimoto H. (2008) FEBS J. 275, 3249–3277 [DOI] [PubMed] [Google Scholar]
- 3. Leto T. L., Morand S., Hurt D., Ueyama T. (2009) Antioxid. Redox Signal. 11, 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borregaard N., Heiple J. M., Simons E. R., Clark R. A. (1983) J. Cell Biol. 97, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapouge K., Smith S. J., Groemping Y., Rittinger K. (2002) J. Biol. Chem. 277, 10121–10128 [DOI] [PubMed] [Google Scholar]
- 6. Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. (1985) Nature 316, 547–549 [DOI] [PubMed] [Google Scholar]
- 7. Bolscher B. G., van Zwieten R., Kramer I. M., Weening R. S., Verhoeven A. J., Roos D. (1989) J. Clin. Invest. 83, 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rotrosen D., Leto T. L. (1990) J. Biol. Chem. 265, 19910–19915 [PubMed] [Google Scholar]
- 9. El-Benna J., Dang P. M., Gougerot-Pocidalo M. A., Marie J. C., Braut-Boucher F. (2009) Exp. Mol. Med. 41, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontayne A., Dang P. M., Gougerot-Pocidalo M. A., El-Benna J. (2002) Biochemistry 41, 7743–7750 [DOI] [PubMed] [Google Scholar]
- 11. Boussetta T., Gougerot-Pocidalo M. A., Hayem G., Ciappelloni S., Raad H., Arabi Derkawi R., Bournier O., Kroviarski Y., Zhou X. Z., Malter J. S., Lu P. K., Bartegi A., Dang P. M., El-Benna J. (2010) Blood 116, 5795–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ueyama T., Tatsuno T., Kawasaki T., Tsujibe S., Shirai Y., Sumimoto H., Leto T. L., Saito N. (2007) Mol. Biol. Cell 18, 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honbou K., Minakami R., Yuzawa S., Takeya R., Suzuki N. N., Kamakura S., Sumimoto H., Inagaki F. (2007) EMBO J. 26, 1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellson C. D., Anderson K. E., Morgan G., Chilvers E. R., Lipp P., Stephens L. R., Hawkins P. T. (2001) Curr. Biol. 11, 1631–1635 [DOI] [PubMed] [Google Scholar]
- 15. Vieira O. V., Botelho R. J., Rameh L., Brachmann S. M., Matsuo T., Davidson H. W., Schreiber A., Backer J. M., Cantley L. C., Grinstein S. (2001) J. Cell Biol. 155, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillooly D. J., Simonsen A., Stenmark H. (2001) J. Cell Biol. 155, 15–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu Y., Filippi M. D., Cancelas J. A., Siefring J. E., Williams E. P., Jasti A. C., Harris C. E., Lee A. W., Prabhakar R., Atkinson S. J., Kwiatkowski D. J., Williams D. A. (2003) Science 302, 445–449 [DOI] [PubMed] [Google Scholar]
- 18. Karathanassis D., Stahelin R. V., Bravo J., Perisic O., Pacold C. M., Cho W., Williams R. L. (2002) EMBO J. 21, 5057–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5345–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leto T. L., Adams A. G., de Mendez I. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10650–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. (1991) J. Clin. Invest. 87, 352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dusi S., Donini M., Rossi F. (1996) Biochem. J. 314, 409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen L. A., DeLeo F. R., Gallois A., Toyoshima S., Suzuki K., Nauseef W. M. (1999) Blood 93, 3521–3530 [PubMed] [Google Scholar]
- 24. Ellson C. D., Davidson K., Ferguson G. J., O'Connor R., Stephens L. R., Hawkins P. T. (2006) J. Exp. Med. 203, 1927–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellson C., Davidson K., Anderson K., Stephens L. R., Hawkins P. T. (2006) EMBO J. 25, 4468–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suh C. I., Stull N. D., Li X. J., Tian W., Price M. O., Grinstein S., Yaffe M. B., Atkinson S., Dinauer M. C. (2006) J. Exp. Med. 203, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueyama T., Kusakabe T., Karasawa S., Kawasaki T., Shimizu A., Son J., Leto T. L., Miyawaki A., Saito N. (2008) J. Immunol. 181, 629–640 [DOI] [PubMed] [Google Scholar]
- 28. Matute J. D., Arias A. A., Wright N. A., Wrobel I., Waterhouse C. C., Li X. J., Marchal C. C., Stull N. D., Lewis D. B., Steele M., Kellner J. D., Yu W., Meroueh S. O., Nauseef W. M., Dinauer M. C. (2009) Blood 114, 3309–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuribayashi F., Nunoi H., Wakamatsu K., Tsunawaki S., Sato K., Ito T., Sumimoto H. (2002) EMBO J. 21, 6312–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J., He R., Minshall R. D., Dinauer M. C., Ye R. D. (2007) J. Biol. Chem. 282, 30273–30284 [DOI] [PubMed] [Google Scholar]
- 31. Bissonnette S. A., Glazier C. M., Stewart M. Q., Brown G. E., Ellson C. D., Yaffe M. B. (2008) J. Biol. Chem. 283, 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian W., Li X. J., Stull N. D., Ming W., Suh C. I., Bissonnette S. A., Yaffe M. B., Grinstein S., Atkinson S. J., Dinauer M. C. (2008) Blood 112, 3867–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Mendez I., Adams A. G., Sokolic R. A., Malech H. L., Leto T. L. (1996) EMBO J. 15, 1211–1220 [PMC free article] [PubMed] [Google Scholar]
- 34. Sathyamoorthy M., de Mendez I., Adams A. G., Leto T. L. (1997) J. Biol. Chem. 272, 9141–9146 [DOI] [PubMed] [Google Scholar]
- 35. Verhoeven A. J., Bolscher B. G., Meerhof L. J., van Zwieten R., Keijer J., Weening R. S., Roos D. (1989) Blood 73, 1686–1694 [PubMed] [Google Scholar]
- 36. Ueyama T., Eto M., Kami K., Tatsuno T., Kobayashi T., Shirai Y., Lennartz M. R., Takeya R., Sumimoto H., Saito N. (2005) J. Immunol. 175, 2381–2390 [DOI] [PubMed] [Google Scholar]
- 37. Ueyama T., Geiszt M., Leto T. L. (2006) Mol. Cell. Biol. 26, 2160–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ueyama T., Lennartz M. R., Noda Y., Kobayashi T., Shirai Y., Rikitake K., Yamasaki T., Hayashi S., Sakai N., Seguchi H., Sawada M., Sumimoto H., Saito N. (2004) J. Immunol. 173, 4582–4589 [DOI] [PubMed] [Google Scholar]
- 39. Bravo J., Karathanassis D., Pacold C. M., Pacold M. E., Ellson C. D., Anderson K. E., Butler P. J., Lavenir I., Perisic O., Hawkins P. T., Stephens L., Williams R. L. (2001) Mol. Cell 8, 829–839 [DOI] [PubMed] [Google Scholar]
- 40. Ueyama T., Lekstrom K., Tsujibe S., Saito N., Leto T. L. (2007) Free Radic. Biol. Med. 42, 180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furutani M., Tsujita K., Itoh T., Ijuin T., Takenawa T. (2006) Anal. Biochem. 355, 8–18 [DOI] [PubMed] [Google Scholar]
- 42. Larsen E. C., Ueyama T., Brannock P. M., Shirai Y., Saito N., Larsson C., Loegering D., Weber P. B., Lennartz M. R. (2002) J. Cell Biol. 159, 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Casbon A. J., Allen L. A., Dunn K. W., Dinauer M. C. (2009) J. Immunol. 182, 2325–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. (1999) Cell 98, 69–80 [DOI] [PubMed] [Google Scholar]
- 45. Miyano K., Ogasawara S., Han C. H., Fukuda H., Tamura M. (2001) Biochemistry 40, 14089–14097 [DOI] [PubMed] [Google Scholar]
- 46. Alloul N., Gorzalczany Y., Itan M., Sigal N., Pick E. (2001) Biochemistry 40, 14557–14566 [DOI] [PubMed] [Google Scholar]
- 47. Lindmo K., Stenmark H. (2006) J. Cell Sci. 119, 605–614 [DOI] [PubMed] [Google Scholar]
- 48. Seaman M. N. (2008) Cell. Mol. Life Sci. 65, 2842–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonifacino J. S., Rojas R. (2006) Nat. Rev. Mol. Cell Biol. 7, 568–579 [DOI] [PubMed] [Google Scholar]
- 50. Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4474–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng G., Lambeth J. D. (2004) J. Biol. Chem. 279, 4737–4742 [DOI] [PubMed] [Google Scholar]
- 52. Blatner N. R., Stahelin R. V., Diraviyam K., Hawkins P. T., Hong W., Murray D., Cho W. (2004) J. Biol. Chem. 279, 53818–53827 [DOI] [PubMed] [Google Scholar]
- 53. Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaullier J. M., Parton R. G., Stenmark H. (2000) EMBO J. 19, 4577–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mao Y., Nickitenko A., Duan X., Lloyd T. E., Wu M. N., Bellen H., Quiocho F. A. (2000) Cell 100, 447–456 [DOI] [PubMed] [Google Scholar]
- 55. Kutateladze T. G. (2007) Prog. Lipid Res. 46, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stahelin R. V., Burian A., Bruzik K. S., Murray D., Cho W. (2003) J. Biol. Chem. 278, 14469–14479 [DOI] [PubMed] [Google Scholar]
- 57. Stahelin R. V., Long F., Diraviyam K., Bruzik K. S., Murray D., Cho W. (2002) J. Biol. Chem. 277, 26379–26388 [DOI] [PubMed] [Google Scholar]
- 58. Cho W., Stahelin R. V. (2005) Annu. Rev. Biophys. Biomol. Struct. 34, 119–151 [DOI] [PubMed] [Google Scholar]
- 59. Cheng G., Ritsick D., Lambeth J. D. (2004) J. Biol. Chem. 279, 34250–34255 [DOI] [PubMed] [Google Scholar]
- 60. Ago T., Nunoi H., Ito T., Sumimoto H. (1999) J. Biol. Chem. 274, 33644–33653 [DOI] [PubMed] [Google Scholar]
- 61. Kanai F., Liu H., Field S. J., Akbary H., Matsuo T., Brown G. E., Cantley L. C., Yaffe M. B. (2001) Nat. Cell Biol. 3, 675–678 [DOI] [PubMed] [Google Scholar]
- 62. van den Berg J. M., van Koppen E., Ahlin A., Belohradsky B. H., Bernatowska E., Corbeel L., Español T., Fischer A., Kurenko-Deptuch M., Mouy R., Petropoulou T., Roesler J., Seger R., Stasia M. J., Valerius N. H., Weening R. S., Wolach B., Roos D., Kuijpers T. W. (2009) PLoS ONE 4, e5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chessa T. A., Anderson K. E., Hu Y., Xu Q., Rausch O., Stephens L. R., Hawkins P. T. (2010) Blood 116, 6027–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fan L. M., Teng L., Li J. M. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wientjes F. B., Reeves E. P., Soskic V., Furthmayr H., Segal A. W. (2001) Biochem. Biophys. Res. Commun. 289, 382–388 [DOI] [PubMed] [Google Scholar]
- 66. Málková S., Stahelin R. V., Pingali S. V., Cho W., Schlossman M. L. (2006) Biochemistry 45, 13566–13575 [DOI] [PubMed] [Google Scholar]
- 67. Rhee S. G. (2006) Science 312, 1882–1883 [DOI] [PubMed] [Google Scholar]
- 68. Tonks N. K. (2006) Nat. Rev. Mol. Cell Biol. 7, 833–846 [DOI] [PubMed] [Google Scholar]
- 69. Umada-Kajimoto S., Yamamoto T., Matsuzaki H., Kikkawa U. (2006) Biochem. Biophys. Res. Commun. 341, 101–107 [DOI] [PubMed] [Google Scholar]
- 70. Grandvaux N., Elsen S., Vignais P. V. (2001) Biochem. Biophys. Res. Commun. 287, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 71. Shiose A., Sumimoto H. (2000) J. Biol. Chem. 275, 13793–13801 [DOI] [PubMed] [Google Scholar]
- 72. Konishi H., Tanaka M., Takemura Y., Matsuzaki H., Ono Y., Kikkawa U., Nishizuka Y. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11233–11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ohmori S., Shirai Y., Sakai N., Fujii M., Konishi H., Kikkawa U., Saito N. (1998) Mol. Cell. Biol. 18, 5263–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.