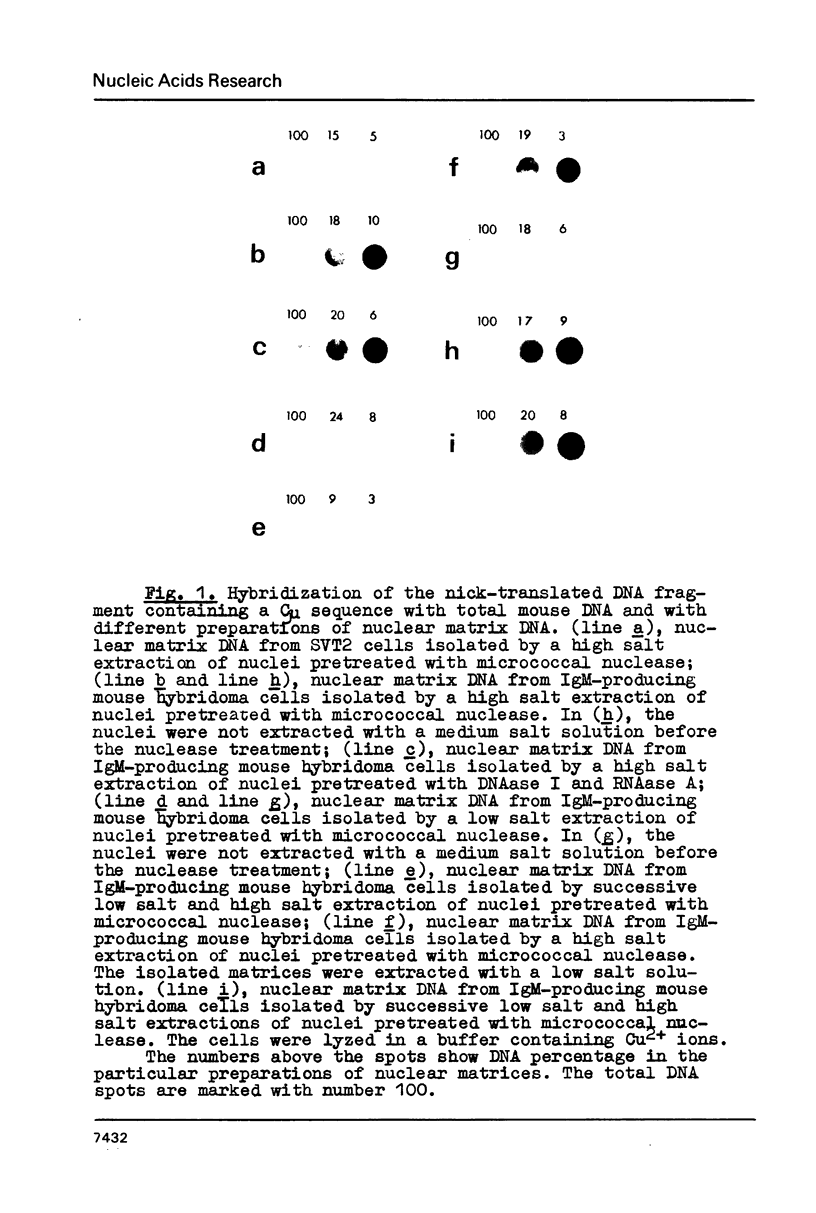
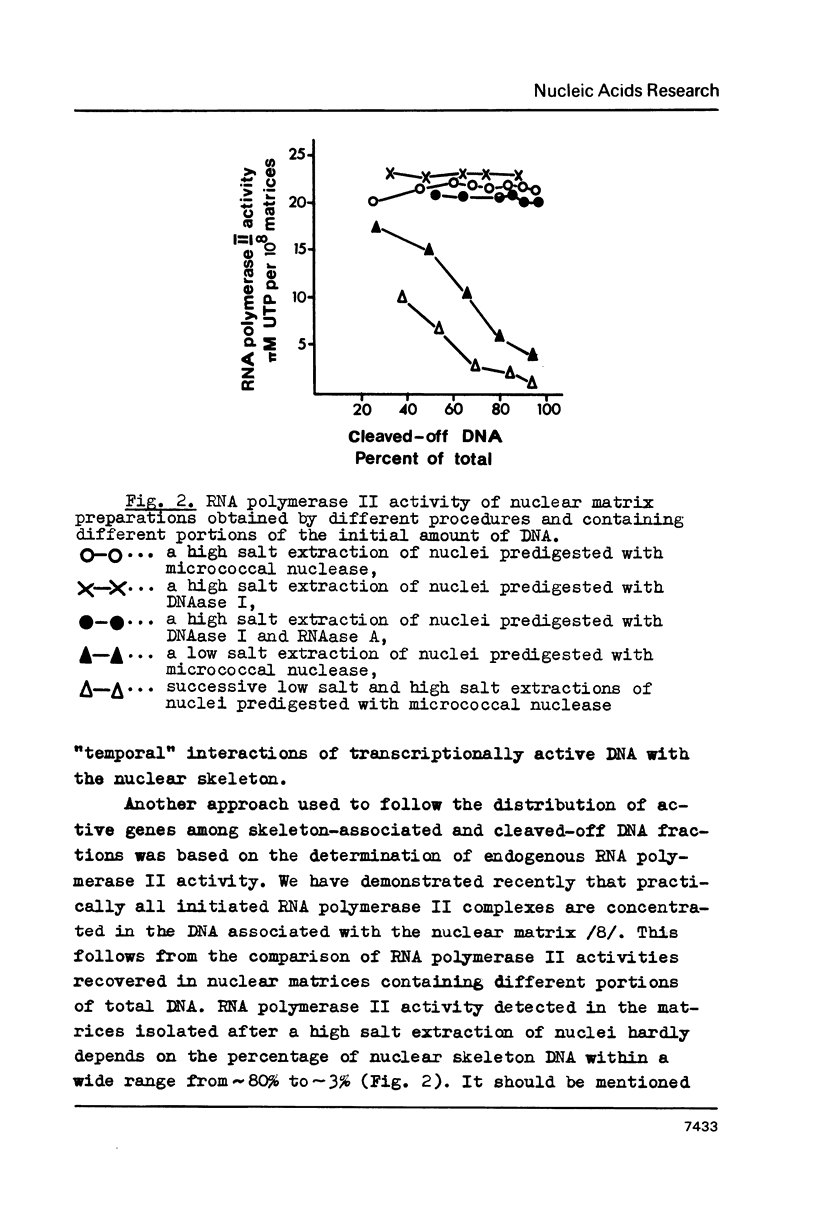
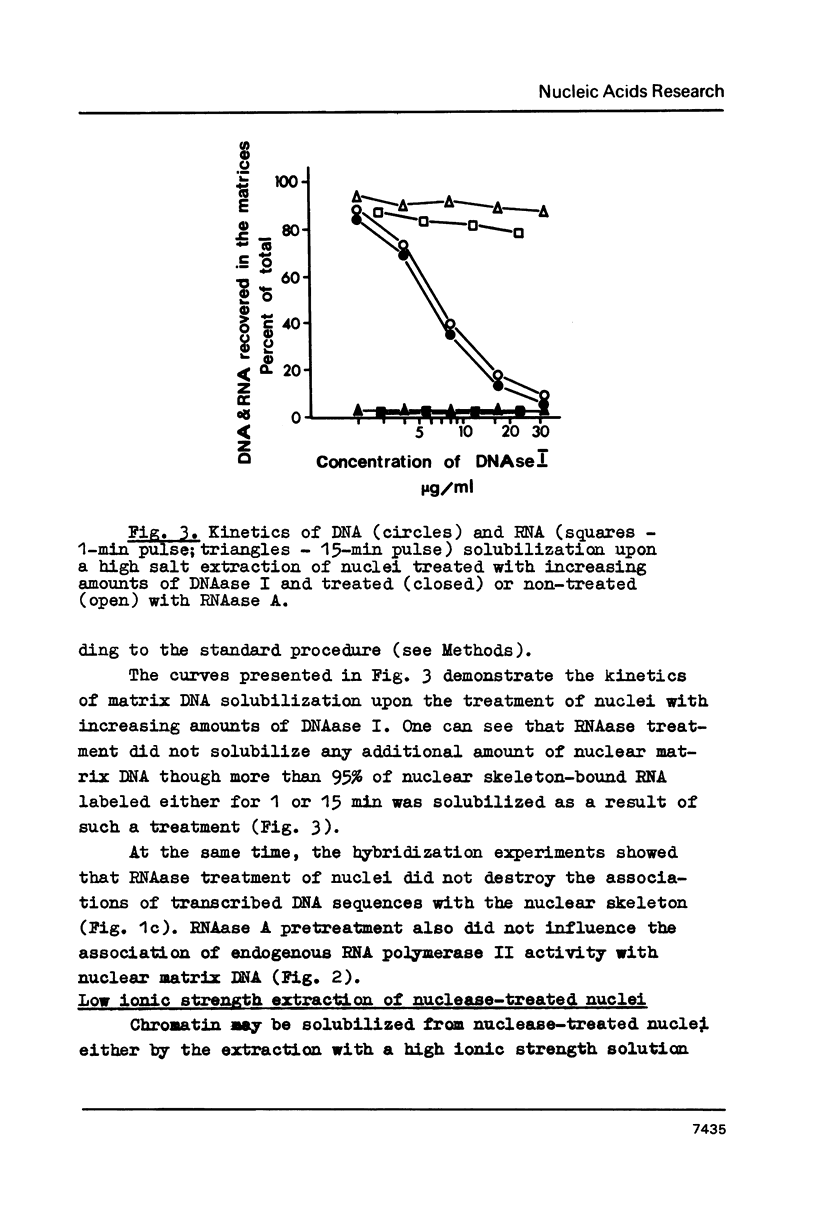
Abstract
We have studied how the conditions in which the nuclear matrix is isolated influence the association of transcribing DNA with the nuclear matrix. Extraction of nuclease-treated nuclei with a low ionic strength solution before a high salt nuclei with a low ionic strength solution before a high salt extraction completely abolishes this association. However, RNA removal by RNAase treatment does not affect the binding of transcribing DNA to the nuclear matrix. The nature of the association of active genes with the nuclear matrix is discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler J., Hastie N. D., Pietras D., Matsui S. I., Sandberg A. A., Berezney R. Hybridization of nuclear matrix attached deoxyribonucleic acid fragments. Biochemistry. 1981 Nov 24;20(24):6921–6929. doi: 10.1021/bi00527a027. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Mapping sequences in loops of nuclear DNA by their progressive detachment from the nuclear cage. Nucleic Acids Res. 1980 Jul 11;8(13):2895–2906. doi: 10.1093/nar/8.13.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlaczky G., Sumner A. T., Ross A. Protein-depleted chromosomes. II. Experiments concerning the reality of chromosome scaffolds. Chromosoma. 1981;81(4):557–567. doi: 10.1007/BF00285849. [DOI] [PubMed] [Google Scholar]
- Hancock R., Boulikas T. Functional organization in the nucleus. Int Rev Cytol. 1982;79:165–214. doi: 10.1016/s0074-7696(08)61674-5. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R., Patel S. B. Attachment of repeated sequences to the nuclear cage. Nucleic Acids Res. 1984 Sep 11;12(17):6709–6726. doi: 10.1093/nar/12.17.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Kirov N., Djondjurov L., Tsanev R. Nuclear matrix and transcriptional activity of the mouse alpha-globin gene. J Mol Biol. 1984 Dec 15;180(3):601–614. doi: 10.1016/0022-2836(84)90029-9. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Maxwell E. S., Puvion E., Scherrer K. The nuclear matrix of duck erythroblasts is associated with globin mRNA coding sequences but not with the major proteins of 40S nuclear RNP. Exp Cell Res. 1981 Dec;136(2):435–445. doi: 10.1016/0014-4827(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Ueda K. Association of actin with the nuclear matrix from bovine lymphocytes. Exp Cell Res. 1983 Jan;143(1):55–62. doi: 10.1016/0014-4827(83)90108-8. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Rzeszowska-Wolny J., Moreau J., Scherrer K. Lokalizatsiia uchastkov prikrepleniia DNK k iadernomu skeletu v ramkakh domena alpha-globinovykh genov kur v funktsional'no aktivnykh i funktsional'no neaktivnykh iadrakh. Mol Biol (Mosk) 1985 Mar-Apr;19(2):456–466. [PubMed] [Google Scholar]
- Razin S. V., Yarovaya O. V. Initiated complexes of RNA polymerase II are concentrated in the nuclear skeleton associated DNA. Exp Cell Res. 1985 May;158(1):273–275. doi: 10.1016/0014-4827(85)90451-3. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Small D., Idzerda R., McKnight G. S., Vogelstein B. The association of transcriptionally active genes with the nuclear matrix of the chicken oviduct. Nucleic Acids Res. 1983 Aug 11;11(15):5113–5130. doi: 10.1093/nar/11.15.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. M., Garrard W. T. Differentiation-dependent chromatin alterations precede and accompany transcription of immunoglobulin light chain genes. J Biol Chem. 1984 Jul 10;259(13):8534–8544. [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolunay H. E., Yang L., Anderson W. F., Safer B. Isolation of an active transcription initiation complex from HeLa cell-free extract. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5916–5920. doi: 10.1073/pnas.81.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]