Abstract
OBJECTIVE
We carried out a secondary analysis in high-risk patients with a previous myocardial infarction (MI) and diabetes in the Alpha Omega Trial. We tested the hypothesis that in these patients an increased intake of the n-3 fatty acids eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and α-linolenic acid (ALA) will reduce the incidence of ventricular arrhythmias and fatal MI.
RESEARCH DESIGN AND METHODS
A subgroup of 1,014 post-MI patients with diabetes aged 60–80 years was randomly allocated to receive one of four trial margarines, three with an additional amount of n-3 fatty acids and one placebo for 40 months. The end points were ventricular arrhythmia–related events and fatal MI. The data were analyzed according to the intention-to-treat principle, using multivariable Cox proportional hazards models.
RESULTS
The patients consumed on average 18.6 g of margarine per day, which resulted in an additional intake of 223 mg EPA plus 149 mg DHA and/or 1.9 g ALA in the active treatment groups. During follow-up, 29 patients developed a ventricular arrhythmia–related events and 27 had a fatal MI. Compared with placebo patients, the EPA-DHA plus ALA group experienced less ventricular arrhythmia–related events (hazard ratio 0.16; 95% CI 0.04–0.69). These n-3 fatty acids also reduced the combined end-point ventricular arrhythmia–related events and fatal MI (0.28; 0.11–0.71).
CONCLUSIONS
Our results suggest that low-dose supplementation of n-3 fatty acids exerts a protective effect against ventricular arrhythmia–related events in post-MI patients with diabetes.
There is strong evidence from prospective cohort studies and randomized trials that >250 mg/day of the fish fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) will reduce fatal coronary heart disease (CHD) by as much as 36% (1). There is also evidence, although less conclusive, that EPA-DHA reduces sudden death (2,3). Prospective cohort studies have provided evidence that the plant food–derived n-3 fatty acid α-linolenic acid (ALA) may reduce fatal CHD (4). Animal experiments showed that n-3 fatty acids reduce the vulnerability to cardiac arrhythmias (5). The Alpha Omega Trial tested the hypothesis that an additional intake of 0.4 g/day of EPA-DHA or 1.9 g/day of ALA will reduce fatal CHD and ventricular arrhythmia–related events in stable postmyocardial infarction (post-MI) patients (6). However, this hypothesis was not confirmed in the main analysis of the trial (7).
A post hoc analysis of the Alpha Omega Trial showed that in post-MI patients with diabetes, EPA-DHA reduced both fatal CHD and ventricular arrhythmia–related events by 49% (7). The reduction in these end points was in accord with that obtained in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)-Prevenzione trial for fatal coronary and sudden death (8). In the Alpha Omega Trial, an even stronger reduction of 61% of ventricular arrhythmia–related events was observed for ALA. This evokes the question of whether post-MI patients with diabetes are particularly susceptible to protective effects of n-3 fatty acids on fatal CHD and ventricular arrhythmia–related events.
In a cohort study of diabetic women, a dose-response relation was observed between fish consumption and CHD mortality (9). Women who consumed fish five or more times per week had a 64% lower risk of CHD mortality compared with those who consumed fish less than once per month. In a trial of heart failure patients with diabetes, a supplement of 0.9 g EPA-DHA per day reduced the composite end point of all-cause mortality or admission to the hospital for cardiovascular reasons significantly with 11% (10). Although evidence of the effect of fish consumption and EPA-DHA supplementation on fatal CHD in patients with diabetes is small, the available data are compatible with the hypothesis that EPA-DHA may protect against fatal CHD.
The life expectancy of a 50-year-old patient with diabetes is 6 years shorter than that of a person without diabetes (11). This difference is largely due to an increased risk of macrovascular diseases among diabetic patients. In addition, they have an increased risk of fatal CHD (12) and an increased risk of sudden death (13,14). A previous MI in combination with diabetes especially makes patients prone to fatal CHD and ventricular arrhythmia–related sudden death (12,15). Therefore, post-MI patients with diabetes are a suitable group to test the hypothesis that n-3 fatty acids protect against fatal CHD and ventricular arrhythmia–related events.
Overlap in the definitions of ventricular arrhythmia–related events and in fatal CHD was present in the main publication of the results of the Alpha Omega Trial (7). Both end points included fatal cardiac arrest and sudden death. In the present analysis, mutually exclusive definitions will be used; therefore, fatal CHD is limited to fatal MI. In the main publication, the two groups that received EPA-DHA were compared with the two groups that did not receive EPA-DHA. The same strategy was used for ALA. This was done because in the analysis of the primary end-point major cardiovascular events, the cumulative incidence of the four treatment groups did not differ. Here we present the results of an in-depth analysis of the effects of different n-3 fatty acids compared with placebo on ventricular arrhythmia–related events and fatal MI in stable post-MI patients with diabetes.
RESEARCH DESIGN AND METHODS
Design and patients
The Alpha Omega Trial is a multicenter, double-blind, placebo-controlled trial with a 2 × 2 factorial design, which has been described in detail elsewhere (6,7). In collaboration with cardiologists from 32 hospitals in the Netherlands, we recruited 4,837 patients aged 60–80 years with a clinically diagnosed MI up to 10 years before randomization. For the current study, the results of 1,014 patients with diabetes were analyzed (Supplementary Fig. 1). These patients were randomly assigned to daily consumption of four different trial margarines for a period of 40 months.
The patients were randomly allocated to four groups and received either no additional n-3 fatty acids or ∼400 mg/day EPA+DHA, 2 g/day ALA, or the combination. The doses corresponded to recommended dietary allowances of those fatty acids. In trial margarines for active treatment groups, oleic acid was exchanged for the different n-3 fatty acids. The ratio of EPA to DHA in the spreads was 3:2. The margarines were developed by Unilever Research and Development and could not be distinguished from each other in taste, odor, texture, and color. The trial was conducted in accordance with the Helsinki Declaration and approved by one central medical ethics committee and by the committees of all participating hospitals. Written informed consent was obtained from all patients.
Procedures
For logistical reasons, all patients used placebo margarine during the first 4–6 weeks after randomization, after which actual treatment started. After randomization, the patients received eight blinded margarine tubs of 250 g every 12 weeks. Unused margarine tubs were returned. Based on this information, the daily intakes of margarine and n-3 fatty acids were calculated. An objective measure of compliance was obtained by determining fatty acids in plasma cholesteryl esters in random subgroups of patients at baseline and after 20 and 40 months of intervention.
Our definition of diabetes was based on a physician’s diagnosis, use of antidiabetic drugs, and/or elevated blood glucose, defined as a casual plasma glucose level of ≥7.0 mmol/L (126 mg/dL) for patients who had fasted >4 h or a level ≥11.1 mmol/L (200.0 mg/dL) in patients who did not fast. Demographic factors, lifestyle, drug use, and medical history were assessed by questionnaire (6). Medication was coded according to the Anatomical Therapeutic Chemical (ATC) Classification System (World Health Organization, 2009), including ATC code A10 for antidiabetic medication. Physical activity was assessed by the validated physical activity scale for the elderly (PASE) questionnaire. A patient was called physically active when he or she had at least 5 days per week of >30 min/day of physical activities of at least three metabolic equivalents of task. Anthropometric measures, blood pressure, and heart rate were taken, and nonfasting blood was collected for determining serum lipids, plasma cholesteryl esters, and glucose (6). Examinations were repeated in a random sample of 159 (15.7%) patients after 20 months and after 40 months in 476 patients (46.9%) who completed the trial before 1 January 2009. Due to budget constraints, the remainder of the cohort filled out mailed questionnaires only.
Outcome
All patients were followed for clinical events, including those who discontinued margarine use during the trial. We monitored vital status of all patients using a computerized link with municipal registers with 100% coverage. For fatal cases, death certificates were obtained from Statistics Netherlands and general practitioners filled out a standard form on primary and contributing causes of death. Additional information on fatal events was obtained from hospitals, nursing homes, and family members. Three members of the Endpoint Adjudication Committee who were blinded for patient identity, treating physician, and allocated treatment coded the fatal events independently according to the 10th revision of the International Classification of Diseases. In the cases of disagreement, information about the underlying causes of death was discussed until consensus was reached.
In the original trial protocol, the primary end point was fatal CHD, and one of the secondary end points was sudden death, a proxy for fatal arrhythmias (6). Due to the low mortality rate, the Steering Committee approved the switch from primary to secondary end point for fatal CHD, and the extension with placement of cardioverter defibrillators for the definition of sudden death, as described in the Statistical Analysis Plan (7). Fatal CHD was defined as mortality from MI (I20–I25), cardiac arrest (I46), and sudden death undefined (R96) and the composite end-point ventricular arrhythmia–related events as nonfatal or fatal cardiac arrest (I46), sudden death (R96), and implantable cardioverter defibrillator placement (6,7). Sudden death was extended with placement of cardioverter defibrillators because ventricular arrhythmias are the main cause of sudden death and cardioverter defibrillators are implanted in patients who are at high risk for developing ventricular arrhythmias (16,17). Self-reported data on implantable cardioverter defibrillator placement were verified against hospital records by trained research nurses or the research physician. All indications for implantable cardioverter defibrillator placement could be retrieved and verified from hospital discharge letters or by telephone with the departments of cardiology where the placements had taken place. The definitions of fatal CHD and of the composite end-point ventricular arrhythmia–related events include both fatal cardiac arrest (I46) and sudden death undefined (R96) (7). To prevent overlap in these definitions, we limited in the present analysis fatal CHD to death from MI (I20–I25).
Statistical analysis
Baseline characteristics were compared among the four treatment groups of patients using ANOVA or χ2 tests, when appropriate. Analyses were carried out according to the intention-to-treat principle. Time-to-event data were analyzed with the Kaplan-Meier method and the log-rank test. The three treatment groups (i.e., EPA-DHA, ALA, and EPA-DHA plus ALA groups) were compared with the placebo group for each outcome. Hazard ratios (HRs) and 95% CIs were computed for ventricular arrhythmia–related events, fatal MI, and the combination of these two end points, using Cox proportional hazard models. Multivariable Cox models included age, sex, and smoking. No adjustments were made for multiple comparisons because only a priori–formulated hypotheses were tested based on animal experiments of the effects of n-3 fatty acids on atherosclerosis and cardiac arrhythmias (5). Two-sided P values <0.05 were considered statistically significant. Data were analyzed with SPSS 17.0 statistical software (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics
The definition of diabetes was made in 72.4% on the combination of elevated blood glucose levels, physician-diagnosed self-report, and drug treatment, in 14.4% on elevated glucose levels only, in 9.9% on self-report only, and in 3.4% on drug treatment only. Insulin was used by 273 (26.9%) patients and 577 (56.9%) used other antidiabetic medication, of whom 110 used both insulin and other antidiabetic medication. Baseline characteristics of post-MI patients with diabetes are presented in Table 1. Randomization was successful, though small group differences were found for sex and smoking. The proportion of women was lowest in the placebo group and highest in the ALA group, and 5 (1.9%) of the 267 women used hormone replacement therapy. The percentage of smokers was highest in the placebo group and lowest in the EPA-DHA plus ALA group.
Table 1.
Baseline characteristics of the 1,014 patients with an MI and diabetes,* according to n-3 fatty acid supplementation group
| Variables | Placebo (N = 249) | ALA (N = 258) | EPA-DHA (N = 262) | EPA-DHA plus ALA (N = 245) | P value |
|---|---|---|---|---|---|
| Age (years) | 69.1 ± 5.8 | 69.4 ± 5.7 | 69.3 ± 5.7 | 70.0 ± 5.5 | 0.31 |
| Male sex (%) | 197 (79.1) | 176 (68.2) | 197 (75.2) | 177 (72.2) | 0.04 |
| Diabetes* | |||||
| Diagnosis from a physician (%) | 202 (81.5) | 215 (84.0) | 212 (80.9) | 198 (81.8) | 0.81 |
| Use of antidiabetic drugs (%) | 184 (73.9) | 192 (74.4) | 184 (70.2) | 180 (73.5) | 0.70 |
| Elevated plasma glucose level (%) | 115 (46.2) | 100 (38.8) | 118 (45.0) | 108 (44.1) | 0.34 |
| Time since MI (years) | 4.7 ± 3.2 | 4.5 ± 3.3 | 4.4 ± 3.0 | 4.5 ± 3.1 | 0.77 |
| Self-reported history of stroke (%) | 13 (5.2) | 23 (8.9) | 18 (6.9) | 24 (9.8) | 0.22 |
| Use of cardiovascular medication (%) | |||||
| Antithrombotic drugs | 241 (96.8) | 250 (96.9) | 256 (97.7) | 234 (95.5) | 0.58 |
| Blood pressure–lowering drugs | 232 (93.2) | 238 (92.2) | 247 (94.3) | 227 (92.7) | 0.82 |
| Lipid-lowering drugs | 213 (85.5) | 217 (84.1) | 223 (85.1) | 205 (83.7) | 0.93 |
| Antiarrhythmic drugs | 11 (4.4) | 11 (4.3) | 9 (3.4) | 9 (3.7) | 0.93 |
| Systolic blood pressure (mmHg) | 142.1 ± 20.3 | 143.4 ± 22.5 | 143.5 ± 21.1 | 142.6 ± 23.2 | 0.88 |
| Serum lipids (mmol/L)† | |||||
| Total cholesterol | 4.65 ± 0.95 | 4.65 ± 0.97 | 4.63 ± 0.92 | 4.65 ± 1.01 | 1.00 |
| LDL cholesterol | 2.49 ± 0.82 | 2.40 ± 0.82 | 2.47 ± 0.78 | 2.41 ± 0.84 | 0.63 |
| HDL cholesterol | 1.18 ± 0.31 | 1.22 ± 0.33 | 1.22 ± 0.36 | 1.22 ± 0.33 | 0.51 |
| Triglycerides | 2.25 ± 1.35 | 2.34 ± 1.27 | 2.13 ± 1.12 | 2.21 ± 1.20 | 0.31 |
| BMI‡ | |||||
| Mean | 29.2 ± 4.6 | 29.3 ± 4.3 | 28.9 ± 4.3 | 29.4 ± 4.8 | 0.58 |
| ≥30 (%) | 93 (37.5) | 97 (37.7) | 93 (35.8) | 96 (39.2) | 0.89 |
| Current smoker (%) | 54 (21.7) | 44 (17.1) | 41 (15.6) | 30 (12.2) | 0.04 |
| Consumption of ≥1 glass of alcohol/week (%) | 165 (66.3) | 165 (64.2) | 181 (69.1) | 155 (63.3) | 0.52 |
| Physically active (%)** | 40 (16.2) | 36 (14.2) | 53 (20.4) | 47 (19.3) | 0.24 |
Data are means ± SD unless otherwise indicated. As data for a number of patients were missing from several variables (BMI, 4 patients; alcohol use, 1 patient; and physical activity, 9 patients), some percentages are based on a smaller number than the column total.
*Diabetes was considered to be present if a patient reported having received the diagnosis from a physician, was taking antidiabetic drugs, or had an elevated plasma glucose level (≥7.0 mmol/L [126 mg/dL] in the case of patients who had fasted more than 4 h or ≥11.1 mmol/L [200.0 mg/dL] in the case of nonfasting patients).
†To convert the values for cholesterol to milligrams per deciliter, divide by 0.02586. To convert the values for triglycerides to milligrams per deciliter, divide by 0.01129.
‡The BMI is the weight in kilograms divided by the square of the height in meters.
**Greater than or equal to three metabolic equivalent of task (indicating at least moderate intensity for at least 30 min/day) during 6 or 7 days/week.
n-3 Fatty acid supplementation and compliance
The mean intake (±SD) of the trial margarine was 18.6 (±5.2) g/day; 86.7% of the patients adhered fully to the protocol and consumed a mean of 20.7 (±4.2) g/day. Patients in the two EPA-DHA groups received, on average, an additional amount of 223 (±62) mg EPA and 149 (±42) mg of DHA per day and those in the two groups of ALA 1.9 (±0.5) g/day.
Plasma cholesteryl esters were determined as a measure of compliance. At the final examination, the effect of n-3 fatty acid supplementation on fatty acids in serum cholesteryl esters was compared with placebo (Supplementary Fig. 2). ALA supplementation increased ALA by 64.2% and EPA by 40.8%; EPA-DHA supplementation increased plasma EPA by 49.8% and DHA by 30.9%; and EPA-DHA plus ALA supplementation increased plasma ALA by 67.4%, EPA by 118.4%, and DHA by 44.0%.
Effects of n-3 fatty acids on study end points
The median follow-up period was 40.7 months (IQR 36.6–41.6 months), during which 3,195 person-years of follow-up were accumulated. During follow-up, 29 patients developed a ventricular arrhythmia–related event, 2 died suddenly, 1 had a nonfatal cardiac arrest, 11 had a fatal cardiac arrest, and 15 had a cardioverter defibrillator implanted. Indications for these implantable cardioverter defibrillator placements were a left ventricular ejection fraction ≤35% (n = 13; 87%), resuscitated ventricular fibrillation (n = 1), and ventricular tachycardia with a left ventricular ejection fraction of 45% (n = 1). In addition, 27 patients died of MI, 23 from other vascular diseases, 34 from cancer, and 26 from other causes.
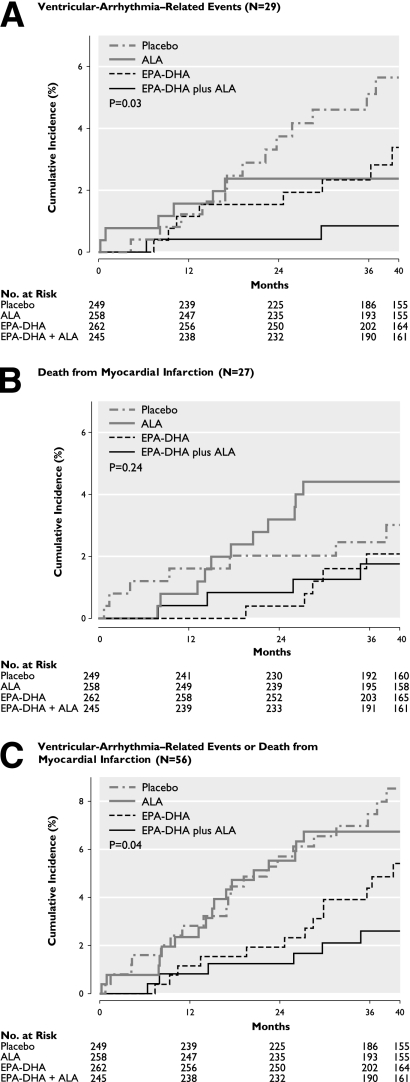
The Kaplan-Meier curves showed that both EPA-DHA and ALA reduced ventricular arrhythmia–related events compared with placebo (Fig. 1A). The lowest incidence was observed in patients who were randomized to the combined supplementation of EPA-DHA plus ALA. After adjustment for imbalances in age, sex, and current smoking, the combined supplementation of n-3 fatty acids reduced the ventricular arrhythmia–related events by 84% compared with placebo (cumulative incidence at median follow-up 0.9 vs. 5.6%, Fig. 1A; HR 0.16; 95% CI 0.04–0.69; Table 2). Similar effects were observed for the combined end-point cardiac arrest and sudden death (0.13; CI 0.02–1.09) and for placement of cardioverter defibrillators (0.19: 0.02–1.55).
Figure 1.
Kaplan-Meier curves of ventricular arrhythmia–related events (A), death from MI (B), or both end points combined (C). The cumulative incidence of end points is shown in 1,014 patients with an MI and diabetes. Patients were randomly assigned to receive a margarine containing supplemental EPA combined with DHA, a margarine containing supplemental ALA, a margarine containing both EPA-DHA and ALA, or a placebo margarine. P value by log-rank (Mantel-Cox) test.
Table 2.
Unadjusted and adjusted HRs of n-3 fatty acid supplementation on end points in 1,014 patients with diabetes, according to n-3 fatty acid supplementation group
| Variables | Placebo (N = 249) | ALA (N = 258) | EPA-DHA (N = 262) | EPA-DHA plus ALA (N = 245) |
|---|---|---|---|---|
| Ventricular arrhythmia–related events (N = 29) | ||||
| No./total no. (%) | 13/249 (5.2%) | 6/258 (2.3%) | 8/262 (3.1%) | 2/245 (0.8%) |
| Crude* | 1.0 (ref.) | 0.45 (0.17–1.18); 0.10 | 0.57 (0.24–1.38); 0.21 | 0.15 (0.04–0.68); 0.01 |
| Adjusted*† | 1.0 (ref.) | 0.47 (0.18–1.24); 0.13 | 0.58 (0.24–1.39); 0.22 | 0.16 (0.04–0.69); 0.01 |
| Death from MI (N = 27) | ||||
| No./total no. (%) | 7/249 (2.8%) | 11/258 (4.3%) | 5/262 (1.9%) | 4/245 (1.6%) |
| Crude* | 1.0 (ref.) | 1.52 (0.59–3.93); 0.39 | 0.67 (0.21–2.10); 0.49 | 0.57 (0.17–1.96); 0.38 |
| Adjusted*† | 1.0 (ref.) | 1.45 (0.56–3.75); 0.45 | 0.66 (0.21–2.07); 0.47 | 0.53 (0.15–1.81); 0.31 |
| Ventricular arrhythmia–related events or death from MI (N = 56) | ||||
| No./total no. (%) | 20/249 (8.0%) | 17/258 (6.6%) | 13/262 (5.0%) | 6/245 (2.4%) |
| Crude* | 1.0 (ref.) | 0.82 (0.43–1.57); 0.56 | 0.60 (0.30–1.21); 0.15 | 0.30 (0.12–0.74); 0.009 |
| Adjusted*† | 1.0 (ref.) | 0.81 (0.43–1.56); 0.54 | 0.60 (0.30–1.20); 0.15 | 0.28 (0.11–0.71); 0.007 |
| All-cause mortality (N = 110) | ||||
| No./total no. (%) | 31/249 (12.4%) | 28/258 (10.9%) | 26/262 (9.9%) | 25/245 (10.2%) |
| Crude* | 1.0 (ref.) | 0.88 (0.53–1.47); 0.62 | 0.78 (0.47–1.32); 0.36 | 0.81 (0.48–1.37); 0.43 |
| Adjusted*† | 1.0 (ref.) | 0.87 (0.52–1.46); 0.60 | 0.80 (0.47–1.34); 0.39 | 0.78 (0.46–1.33); 0.37 |
*Data are HRs with 95% CIs and P values, with the use of Cox proportional hazards models.
†Adjusted for age, sex, and smoking status.
For fatal MI, the Kaplan-Meier curves did not differ significantly among the four groups (Fig. 1B). After adjustment for confounders, the strongest, although not significant, effect was observed for EPA-DHA plus ALA (HR 0.53; 95% CI 0.15–1.81; Table 2). The Kaplan-Meier curves for the composite end-point ventricular arrhythmia–related events and fatal MI showed the strongest effect for EPA-DHA plus ALA (Fig. 1C). After adjustment, these three n-3 fatty acids together reduced this combined end point by 72% (cumulative incidence at median follow-up 2.6 vs. 8.5%, Fig. 1C; 0.28; 0.11–0.71). n-3 Fatty acids did not reduce all-cause mortality (Table 2).
CONCLUSIONS
Low-dose supplementation of n-3 fatty acids EPA-DHA plus ALA significantly reduced ventricular arrhythmia–related events in post-MI patients with diabetes. The combined supplementation of these three fatty acids also reduced the composite end-point ventricular arrhythmia–related events plus fatal MI but not fatal MI alone.
A limitation of the current study is the small number of patients who developed ventricular arrhythmias (n = 29) or died of MI (n = 27). The study had enough power to detect significant effects of combined supplementation of EPA-DHA and ALA on the end-point ventricular arrhythmia–related events whether or not in combination with fatal MI. Significant effects on these end points were not observed for either EPA-DHA or ALA supplementation. The combined effects of EPA-DHA and ALA on the two composite end points are compatible with additive effects of either EPA-DHA or ALA. Assuming an additive model, an HR of 0.47 × 0.58 = 0.27 is expected for ventricular arrhythmia–related events and of 0.81 × 0.60 = 0.49 for the combination of ventricular arrhythmias and fatal MI. These results fit well with the 95% CI of these end points and are compatible with the hypothesis that all three n-3 fatty acids reduce the risk of ventricular arrhythmias. This is also in accord with the results of animal experiments suggesting that ALA, EPA, and DHA have similar antiarrhythmic effects by influencing the Na+ and L-type Ca2+ channels of cardiomyocytes (5).
In the GISSI-Prevenzione trial, patients with a recent MI (<3 months ago) were supplemented with 0.9 g of EPA-DHA per day, which reduced their risk of sudden death by 45% (8). In later trials, smaller reductions were obtained for sudden death. Recent meta-analyses quantified the effect of EPA-DHA supplementation on sudden death at a 19% (HR 0.81; 95% CI 0.52–1.25) (2) and 13% (0.87; 0.76–0.99) reduction (3). An explanation for the smaller effect of EPA-DHA in the more recent trials could be improved drug treatment of cardiovascular risk factors (18). This is supported by the recently published results of the OMEGA trial in which patients were randomized 3–14 days after acute MI. In these high-risk patients, a supplement of 0.8 g EPA-DHA per day did not reduce sudden cardiac death (19). In this trial and in the Alpha Omega Trial, almost all patients received antithrombotic drugs, antihypertensive drugs, and statins (7). The patients in the GISSI-Prevenzione trial were also well treated with antithrombotic and antihypertensive drugs but only 29% received statins (8). The Alpha Omega Trial started in 2002, the OMEGA trial in 2003, and the GISSI-Prevenzione in 1993. This illustrates the increase in statin treatment over 10 years.
One of the secondary end points in the Alpha Omega Trial was ventricular arrhythmia–related events. Ventricular arrhythmias are the major cause of sudden death and cardiac arrest and cardioverter defibrillators were generally implanted in post-MI patients with a low ejection fraction who are at high risk for these arrhythmias (16,17). The common denominator of this end point is the high risk of ventricular fibrillation. The protective effect of the combined supplementation of the three n-3 fatty acids on this end point (HR 0.16; 95% CI 0.04–0.69) contrasts with the absence of an effect of EPA-DHA in cardiac patients with implanted cardioverter defibrillators (who all had had a severe arrhythmic event before placement) (2). These discrepant results suggest that n-3 fatty acids may reduce primary arrhythmias in post-MI patients with diabetes with a low ejection fraction but not secondary arrhythmias in cardiac patients with cardioverter defibrillators.
EPA-DHA supplementation reduced fatal MI by 47% but this reduction was not significant, possibly due to the small number of events. A possible protective effect of EPA-DHA on fatal MI would be in accord with the results of a prospective study carried out in postmenopausal women (20). In that study, smaller increases in stenosis were observed in diabetic women who consumed greater than two servings of fish or greater than one serving of fatty fish compared with diabetic women who consumed less fish. An intervention study carried out in Japan showed that diabetic patients who were supplemented with 1.8 g EPA per day in contrast with the control group did not show an increase in intima-media thickness during 2.1 years of follow-up (21). These results suggest that in diabetic patients, fish and EPA supplementation may reduce progression of atherosclerosis and the eventual risk of fatal MI.
Diabetes alters smooth muscle function through atherosclerotic lesion formation, plaque instability, and cardiovascular events. Basic research shows that peroxisome proliferator–activated receptor γ (PPAR-γ) plays a critical role in the regulation of insulin sensitivity. To become functional, PPAR-γ should bind to an appropriate ligand. Cell culture studies provide evidence that EPA and DHA are potential ligands for PPAR-γ activation (22). Recently published results of an in vivo experiment suggested that fish oil increases the expression of glucose uptake genes, leading to reduced glucose levels (23). EPA and DHA also have anti-inflammatory properties. Experiments in obese mice showed that activation of the G protein–coupled receptor 120 (GPR120) by EPA and DHA inhibited multiple inflammation cascades and reversed insulin resistance caused by a high-fat diet (24). These mechanistic findings support an important role for n-3 fatty acids in the etiology of diabetes, a major risk factor of fatal MI.
In conclusion, low doses of n-3 fatty acids reduced the risk of ventricular arrhythmia–related events in post-MI patients who also have diabetes. Ongoing trials in diabetic patients, e.g., ORIGIN (Outcome Reduction with an Initial Glargine Intervention; clinical trial reg. no. NCT00069784) (25) and ASCEND (A Study of Cardiovascular Events in Diabetes; NCT00135226; http://www.ctsu.ox.ac.uk/ascend/), evaluate the effect of EPA-DHA on sudden death and fatal CHD. The results of more trials on the effect of different n-3 fatty acids are needed before definitive conclusions can be drawn on their role in the etiology of ventricular arrhythmias and fatal CHD.
Supplementary Material
Acknowledgments
Financial support was obtained from the Netherlands Heart Foundation, the National Institutes of Health (NIH), and Unilever Research and Development. The grant of the Netherlands Heart Foundation covered baseline examinations and mortality follow-up. The NIH grant covered mid-term and final examinations and the verification of nonfatal cardiovascular events. Unilever provided an unrestricted grant for distribution of trial margarines to the patients. The funding organizations had no role in the design of the study, data collection, data analysis, interpretation, writing of the report, or the decision to submit.
D.K. received grants from the Netherlands Heart Foundation, NIH, and Unilever Research and Development. J.M.G. obtained an unrestricted grant from the Alpro Foundation (Belgium) for epidemiologic analyses within a project on ALA, CHD, and stroke in the general population. P.L.Z. is an employee of Unilever. Unilever markets food products, some of which are enriched in omega-3 fatty acids. No other potential conflicts of interest relevant to this article were reported.
D.K. designed the study, performed statistical analysis, wrote the first draft of the manuscript, and was the guarantor of the manuscript. J.M.G. designed the study and collected data. J.d.G. and L.M.O.G. collected data. B.J.M.M. was the chair. M.-J.d.B. and P.L.Z. were observers of the Steering Committee. J.W.D. was a voting member. E.B. was the chair of the Data Safety and Monitoring Board of the Alpha Omega Trial. E.J.G. designed the study, performed statistical analysis, and collected data. All authors critically reviewed and edited the manuscript.
Footnotes
Clinical trial reg. no. NCT00127452, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0896/-/DC1.
References
- 1.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–1899 [DOI] [PubMed] [Google Scholar]
- 2.León H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ 2008;337:a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol 2009;32:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr 2004;134:919–922 [DOI] [PubMed] [Google Scholar]
- 5.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007;116:e320–e335 [DOI] [PubMed] [Google Scholar]
- 6.Geleijnse JM, Giltay EJ, Schouten EG, et al. ; Alpha Omega Trial Group Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J 2010;159:539–546, e2 [DOI] [PubMed] [Google Scholar]
- 7.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026 [DOI] [PubMed] [Google Scholar]
- 8.GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–455 [PubMed] [Google Scholar]
- 9.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003;107:1852–1857 [DOI] [PubMed] [Google Scholar]
- 10.Tavazzi L, Maggioni AP, Marchioli R, et al. ; GISSI-HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–1230 [DOI] [PubMed] [Google Scholar]
- 11.Seshasai SR, Kaptoge S, Thompson A, et al. ; Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 13.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation 1999;99:1978–1983 [DOI] [PubMed] [Google Scholar]
- 14.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord 2010;11:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila MJ, Barthel P, Myerburg RJ, et al. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm 2010;7:1396–1403 [DOI] [PubMed] [Google Scholar]
- 16.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001;345:1473–1482 [DOI] [PubMed] [Google Scholar]
- 17.Myerburg RJ. Implantable cardioverter-defibrillators after myocardial infarction. N Engl J Med 2008;359:2245–2253 [DOI] [PubMed] [Google Scholar]
- 18.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010;376:540–550 [DOI] [PubMed] [Google Scholar]
- 19.Rauch B, Schiele R, Schneider S, et al. ; OMEGA Study Group OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–2159 [DOI] [PubMed] [Google Scholar]
- 20.Erkkilä AT, Lichtenstein AH, Mozaffarian D, Herrington DM. Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr 2004;80:626–632 [DOI] [PubMed] [Google Scholar]
- 21.Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis 2007;191:162–167 [DOI] [PubMed] [Google Scholar]
- 22.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1–11 [DOI] [PubMed] [Google Scholar]
- 23.Yu YH, Wu SC, Cheng WT, Mersmann HJ, Shen TL, Ding ST. The function of porcine PPARγ and dietary fish oil effect on the expression of lipid and glucose metabolism related genes. J Nutr Biochem 2011;22:179–186 [DOI] [PubMed] [Google Scholar]
- 24.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J; Origin Trial Investigators Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008;155:26–32, e1–e6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.