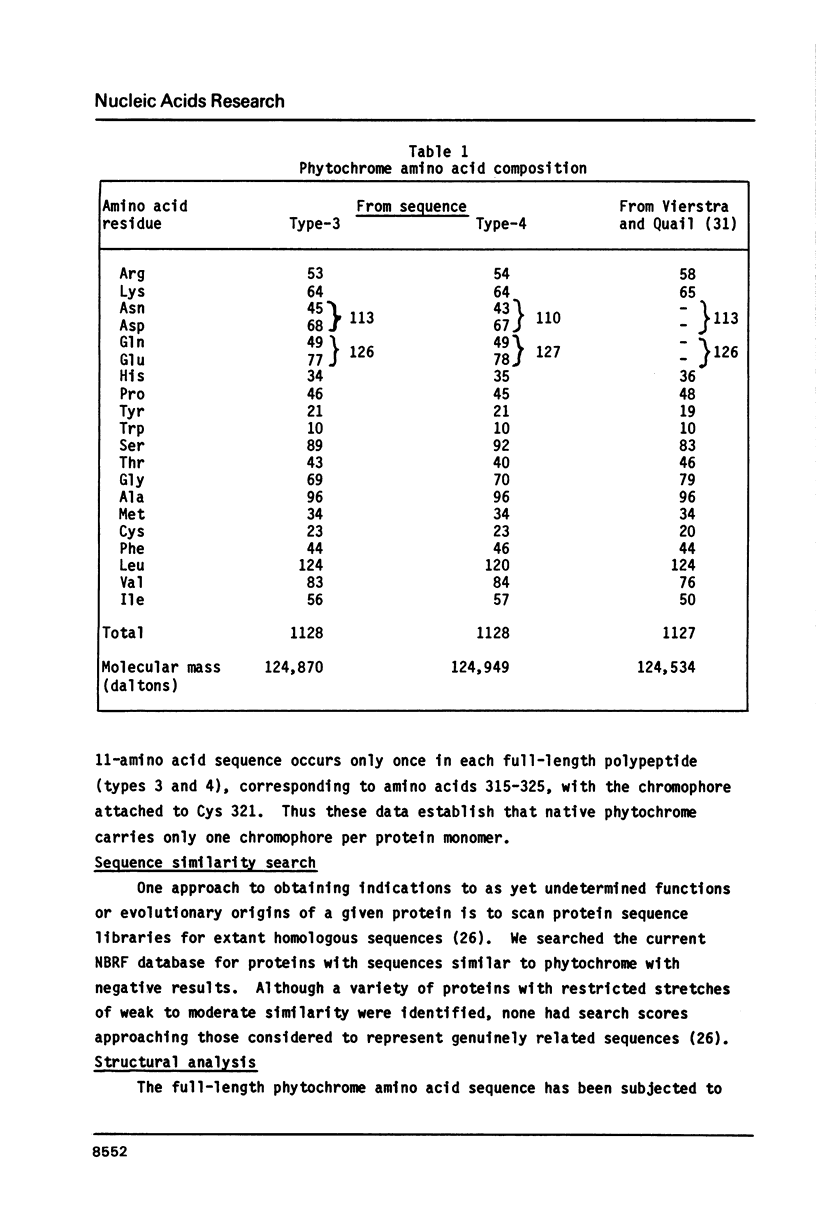
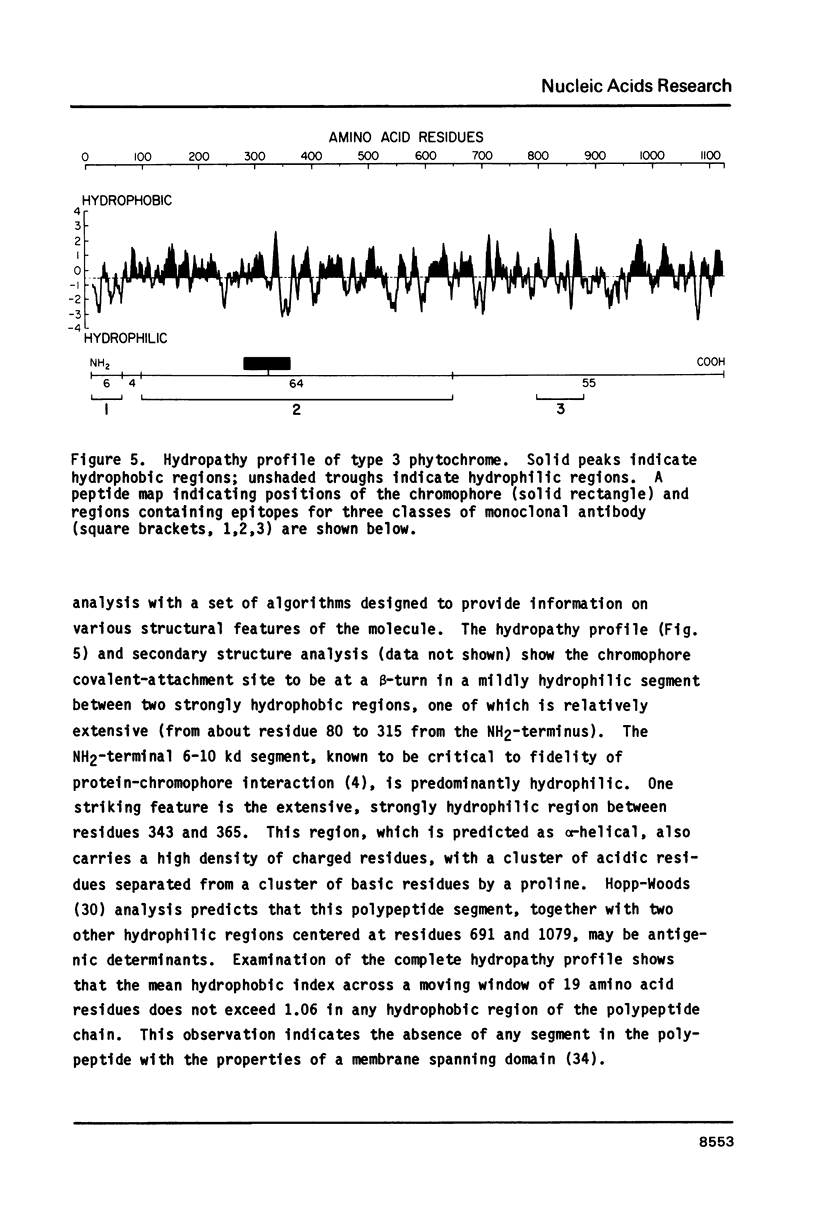
Abstract
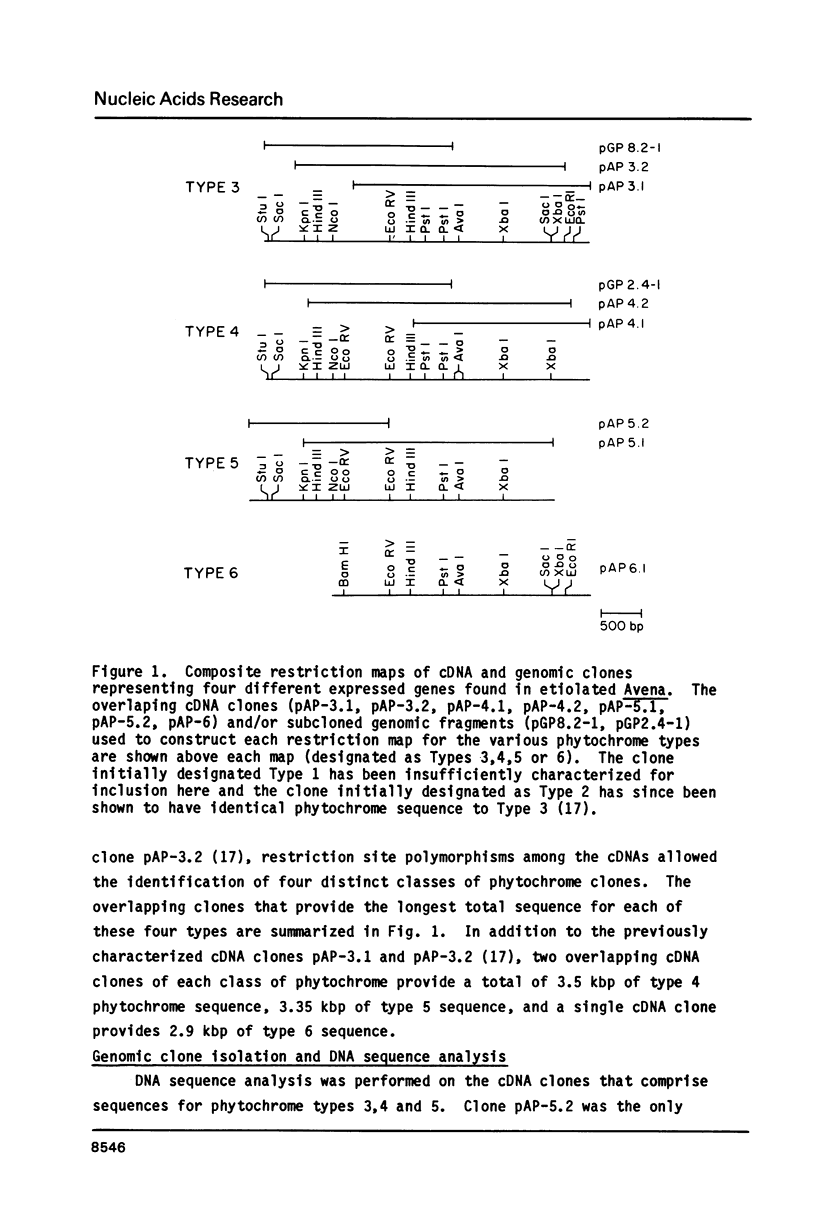
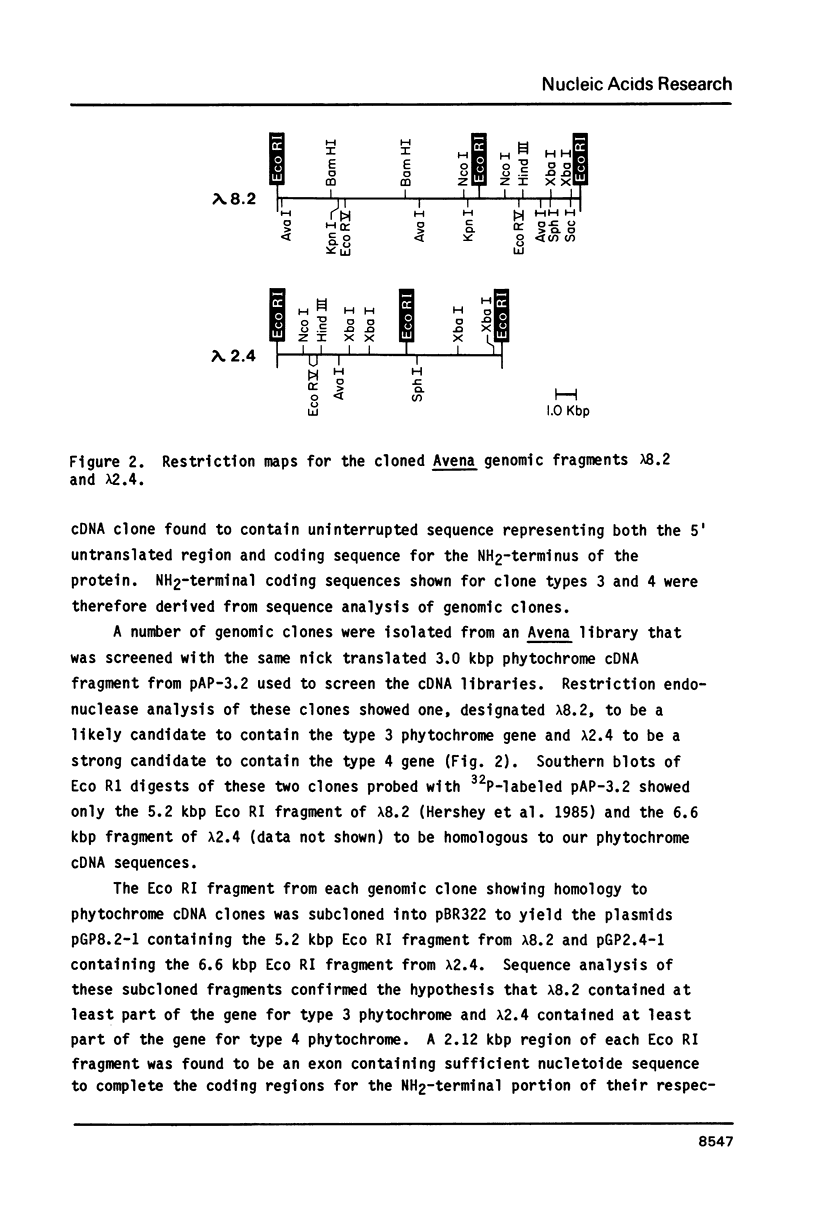
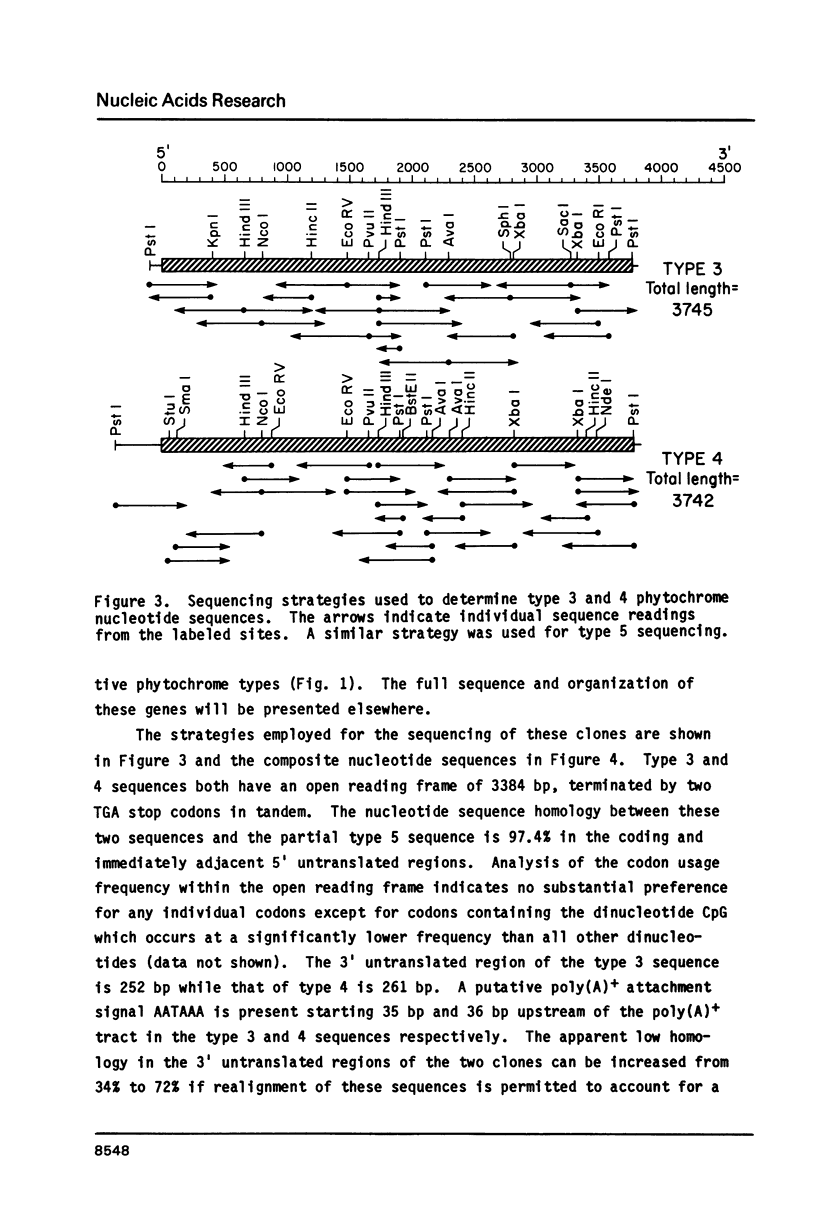
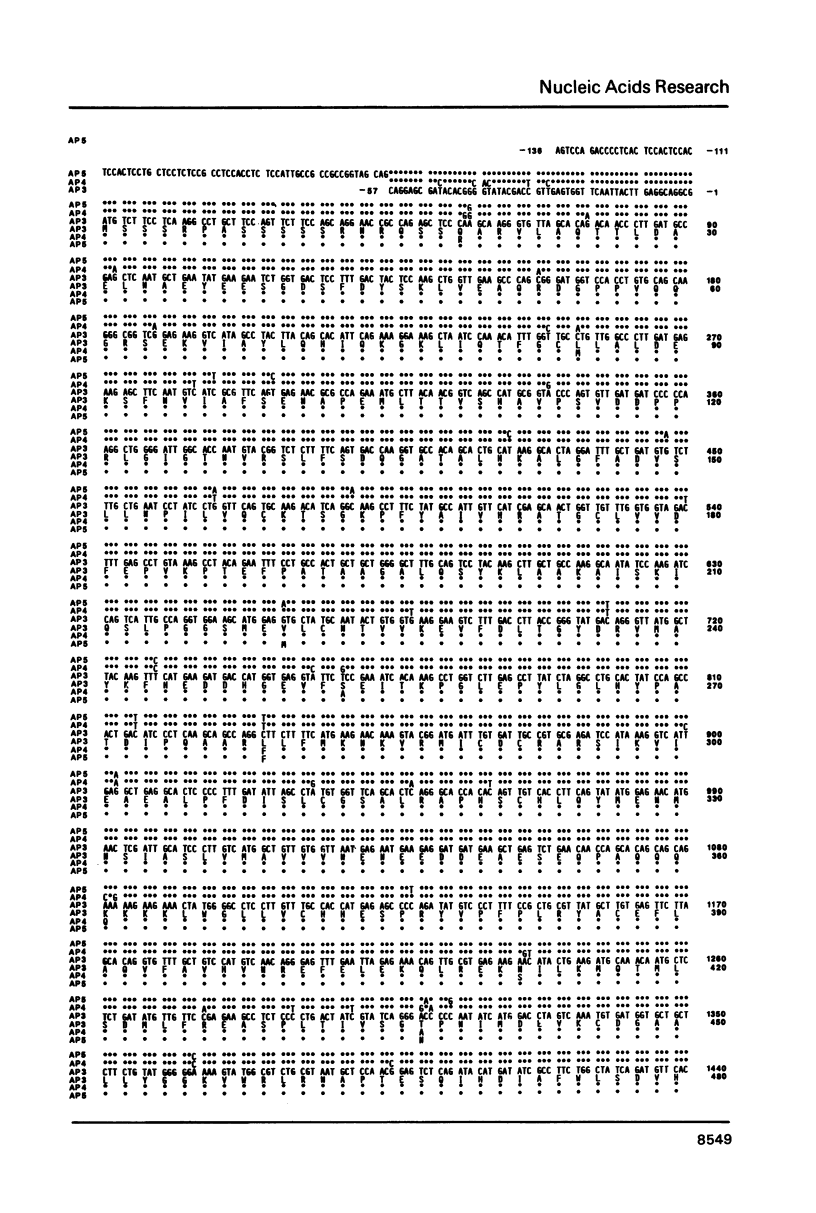
Cloned cDNA and genomic sequences have been analyzed to deduce the amino acid sequence of phytochrome from etiolated Avena. Restriction endonuclease site polymorphism between clones indicates that at least four phytochrome genes are expressed in this tissue. Sequence analysis of two complete and one partial coding region shows approximately 98% homology at both the nucleotide and amino acid levels, with the majority of amino acid changes being conservative. High sequence homology is also found in the 5'-untranslated region but significant divergence occurs in the 3'-untranslated region. The phytochrome polypeptides are 1128 amino acid residues long corresponding to a molecular mass of 125 kdaltons. The known protein sequence at the chromophore attachment site occurs only once in the polypeptide, establishing that phytochrome has a single chromophore per monomer covalently linked to Cys-321. Computer analyses of the amino acid sequences have provided predictions regarding a number of structural features of the phytochrome molecule.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batschauer A., Apel K. An inverse control by phytochrome of the expression of two nuclear genes in barley (Hordeum vulgare L.). Eur J Biochem. 1984 Sep 17;143(3):593–597. doi: 10.1111/j.1432-1033.1984.tb08411.x. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S. M., Quail P. H. Monoclonal antibodies to three separate domains on 124 kilodalton phytochrome from Avena. Plant Physiol. 1984 Nov;76(3):622–626. doi: 10.1104/pp.76.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry K. T., Mumford F. E. Isolation and partial characterization of a chromophore-peptide fragment from pepsin digests of phytochrome. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1466–1473. doi: 10.1016/0006-291x(71)90185-9. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hahn T. R., Song P. S., Quail P. H., Vierstra R. D. Tetranitromethane oxidation of phytochrome chromophore as a function of spectral form and molecular weight. Plant Physiol. 1984 Apr;74(4):755–758. doi: 10.1104/pp.74.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V., Zwar J. A. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Colbert J. T., Lissemore J. L., Barker R. F., Quail P. H. Molecular cloning of cDNA for Avena phytochrome. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2332–2336. doi: 10.1073/pnas.81.8.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983 Apr;20(4):483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Partial characterization of undegraded oat phytochrome. Biochemistry. 1980 Jan 22;19(2):390–394. doi: 10.1021/bi00543a022. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lagarias J. C., Mercurio F. M. Structure function studies on phytochrome. Identification of light-induced conformational changes in 124-kDa Avena phytochrome in vitro. J Biol Chem. 1985 Feb 25;260(4):2415–2423. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Kennard W. C., Drong R. F., Slightom J. L. Use of a recombination-deficient phage lambda system to construct wheat genomic libraries. Gene. 1984 Oct;30(1-3):237–240. doi: 10.1016/0378-1119(84)90126-4. [DOI] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. S. Protozoan and related photoreceptors: molecular aspects. Annu Rev Biophys Bioeng. 1983;12:35–68. doi: 10.1146/annurev.bb.12.060183.000343. [DOI] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Silverthorne J., Tobin E. M. Phytochrome Control of the Expression of Two Nuclear Genes Encoding Chloroplast Proteins in Lemna gibba L. G-3. Plant Physiol. 1983 Jul;72(3):717–724. doi: 10.1104/pp.72.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien W., Schopfer P. Control by Phytochrome of Cytoplasmic Precursor rRNA Synthesis in the Cotyledons of Mustard Seedlings. Plant Physiol. 1982 May;69(5):1156–1160. doi: 10.1104/pp.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Native phytochrome: Inhibition of proteolysis yields a homogeneous monomer of 124 kilodaltons from Avena. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5272–5276. doi: 10.1073/pnas.79.17.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]


