Abstract
Limited research suggests a relationship between Restless Legs Syndrome and hypertension. We, therefore, assessed the relationship between restless legs syndrome and hypertension among middle-aged women.
This is a cross-sectional study including 65,544 women (aged 41-58 years) participating in Nurses Health Study II. The participants with diabetes and arthritis were excluded as these conditions can mimic restless legs syndrome. Restless legs syndrome was assessed by a self-administered questionnaire based on the International Restless Legs Study Group criteria. Information on diagnosis of hypertension and blood pressure values were collected via questionnaires. Multivariable logistic regression models were used to analyze the relation between restless legs syndrome and hypertension, with adjustment for age, race, body mass index, physical activity, menopausal status, smoking, use of analgesics, and intake of alcohol, caffeine, folate, and iron.
Compared to women with no restless legs symptoms, the multiple adjusted odds of having hypertension were 1.20 (95% CI: 1.10-1.30; P<0.0001) times higher among women with restless legs symptoms. The adjusted odds ratios for women who reported restless legs symptoms 5-14 times/month and ≥15 times/month were 1.06 (95% CI 0.94-1.18) and 1.41 (95% CI 1.24-1.61) respectively, compared to those without the symptoms (P trend <0.0001). Greater frequency of restless legs symptoms was associated with higher concurrent systolic and diastolic blood pressures (P trend<0.0001 for both).
Women with restless legs syndrome have a higher prevalence of hypertension, and this prevalence increases with more frequent restless legs symptoms.
Keywords: Restless legs syndrome, Hypertension, Sleep, Cardiovascular disease, Women
Introduction
Restless legs syndrome (RLS) is a common, yet under recognized sensory motor disorder characterized by intense, unpleasant leg sensations, and an irresistible urge to move the legs1. The symptoms of RLS can impair sleep onset, sleep maintenance, and overall quality of life2, 3. Most studies have reported a prevalence of approximately 5-15% of the adult population4. RLS affects both men and women, with a female preponderance of almost 2:15,6. Epidemiologic studies have suggested a possible association between RLS and cardiovascular diseases7,8, however the association between RLS and hypertension remains controversial. Previous studies have suggested that individuals with RLS are at increased risk of developing hypertension because of the presence of periodic limb movements of sleep (PLMS), seen in 80% of patients with RLS. The population based studies have also suggested that hypertension may act as an intermediary risk factor leading to cardiovascular diseases 9,10 in people with RLS.
Despite improvements in awareness and treatment, the prevalence of hypertension has increased over the last decade. According to the National Center for Health Statistics, one out of three US adults suffer from hypertension and it has been listed as a primary or contributing cause of death for 326,000 Americans in 200611,12. Because RLS may be associated with hypertension, further work into the possible clinical impact of RLS is required.
We, therefore, sought to test the hypothesis that RLS would be associated with prevalence of hypertension in a large ongoing cohort of US middle-age women.
Methods
Study population
The Nurses’ Health Study (NHS) II is a large prospective cohort of 116,430 female registered nurses who were 25-42 years old at the start of the study in 1989. Follow-up questionnaires are mailed to the participants every 2 years. The institutional review board at Brigham and Women’s Hospital approved this study and completion of the questionnaires was considered participant’s consent.
Assessment of RLS
In 2005, we asked NHS II participants (n=97,642, mean age 50.4 years) about RLS symptoms using questions based on the International Restless Legs Study Group Criteria13. The following question was asked:
“Do you have unpleasant leg sensations like crawling, paresthesia, or pain combined with motor restlessness and an urge to move”?
The possible responses were as follows: no, less than once/month, 2-4 times/month, 5-14 times/month, and ≥15 times/month. The participants who answered yes were asked the following two questions:
Do these symptoms occur only at rest and does moving improve them?
Are these symptoms worse in the evening/night compared with the morning?
79,992 (82%) women completed the questions regarding RLS. A probable diagnosis of RLS was thought to be present if participants answered “yes” to all of the above questions and if symptom frequency was ≥5 times/month14 . To reduce the misclassification of RLS, participants who had ever reported diabetes, had current arthritis, or who were currently pregnant were excluded, leaving 65,544 women in the primary analysis.
Assessment of Hypertension
Hypertension was self-reported by participants based on all biennial questionnaires; each questionnaire asked participants to report if a clinician has made a new diagnosis of hypertension. Responses from the 2005 questionnaire, the same year as the questions regarding RLS, were used in this cross-sectional study to determine hypertension status. The 2005 questionnaire also inquired about the participant’s blood pressure during the preceding 2 years. Blood pressure assessment was done using predefined categories for systolic and diastolic blood pressure. The categories of systolic blood pressure responses (mm Hg) were <105, 105-114, 115-124, 125-134, 135-144, 145-154, 155-164, 165 -174, 175+, and unknown or not checked within the past 2 years. The categories of diastolic pressure response were <65, 65-74, 75-84, 85-94, 95- 104, 105+, and unknown, or not checked within the past 2 years. For the purpose of present analyses, individual levels of blood pressure were assigned the midpoints of those ranges. Individuals who reported antihypertensive medication use were assigned to the highest category of blood pressure (SBP 175 and DBP 105).
The validity of self reported hypertension has been examined previously. Forman et al. compared relevant medical records from a subset of randomly selected nurses who reported a new diagnosis of hypertension on the 2005 biennial questionnaire with randomly selected participants who denied this diagnosis in 2005 and in every previous year. The sensitivity of self reported hypertension was 94% whereas the specificity was 85% 15.
Ascertainment of Covariates
Information on age, body mass index (BMI) (weight in kilograms divided by height in meters squared), smoking status, physical activity, menopausal status, use of aspirin and other non-steroidal anti-inflammatory drug (NSAID), and presence of stroke or myocardial infarction were obtained from the 2005 questionnaire. Dietary intake was assessed based on a validated food frequency questionnaire16, 17
The reliability and validity of self reported BMI and level of physical activity has been previously investigated18,19. Self reported weight and physical activity have suggested a correlation of 0.97 and 0.79 respectively20. Alcohol consumption measured by questionnaires, administered 1 year apart also provided highly reproducible results with a correlation of 0.9021. Similarly, the validity of the food frequency questionnaire for measurement of folate intake has also been demonstrated in previous studies20.
Based upon the diet prescribed in the Dietary Approaches to Stop Hypertension (DASH) trial22, we constructed a DASH score focusing on 8 components: high intake of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains and low intake of sodium, sweetened beverages, and red and processed meats23
Statistical Analyses
Statistical analyses were completed using SAS version 9.1 (SAS Institute, Inc, Cary, NC). We categorized participants into 3 groups: no RLS, RLS symptoms 5-14 times/month, and RLS symptoms ≥15 times/month. Logistic regression models were used to calculate the prevalence odds ratios (ORs) and 95% confidence intervals (95% CIs) of having hypertension among participants with RLS compared to those without RLS. Means (e.g., blood pressure) were compared using the General Linear Model procedure in SAS. The analyses were adjusted for age (in years), BMI (kg/m2), BMI squared, race (Caucasian, African American, or Asian and others), smoking (never smoked, former smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), physical activity (quintiles), alcohol intake (g/d: 0,0.1-4.9, 5.0-9.9, 10.0-14.9, and ≥15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), analgesic use, oral contraceptive use, DASH score (quintile) and total folate intake (food and supplements, quintiles). In the sensitivity analysis, we further adjusted caffeine and iron intake, the Crown-Crisp phobic anxiety index, antidepressant use, sleep duration and history of myocardial infarction or stroke.
We examined potential interactions of presence of RLS (no, 1-14, ≥15 times/mo) with age (<50 or ≥ 50 years, median value), obesity status (BMI <25, 25-29, and ≥ 30 kg/m2), and menopause status (yes/ no), by including multiplicative terms in the logistic regression models, with adjustment for other potential confounders.
Results
The characteristics of women participating in NHS II are shown in Table 1. Average age was 50.5 years. Greater intake of caffeine, analgesics, and tobacco was noted among women with RLS symptoms ≥15 times per month, relative to those without RLS. Similarly, women with less physical activity and post-menopausal status reported more frequent RLS symptoms.
Table1.
Characteristics according to restless legs syndrome status in 2005 in the Nurses’ Health Study II*
| Restless legs syndrome status in 2005 |
|||
|---|---|---|---|
| Baseline Characteristics | No RLS | RLS 5-14 times/mo |
RLS 15+ times/mo |
| n | 61321 | 2475 | 1748 |
| Age, y | 50.0 | 50.6 | 50.9 |
| Current smokers, % | 7.2 | 8.8 | 9.1 |
| Past smokers, % | 26.3 | 28.5 | 29.0 |
| African Americans, % | 1.2 | 0.4 | 0.7 |
| Asian & other ethnicity, % | 4.3 | 2.7 | 2.4 |
| BMI, kg/m2 | 26.0 | 27.2 | 28.0 |
| Physical activity, Mets/wk | 24.3 | 21.2 | 20.0 |
| Alcohol intake, g/d | 5.9 | 5.5 | 5.2 |
| Caffeine intake, mg/d | 164 | 164 | 171 |
| Iron intake, mg/d | 19.8 | 19.6 | 19.2 |
| Total folate intake, mcg/d | 701 | 688 | 702 |
| DASH Score | 23.76 | 23.28 | 23.16 |
| Pre-menopause, % | 47.5 | 43.1 | 41.3 |
| Current analgesic use, % | 60.7 | 71.4 | 74.1 |
| Current antidepressant use, % | 17.0 | 28.0 | 34.9 |
| Current oral contraceptive use, % | 5.0 | 4.0 | 4.4 |
| Current anti-hypertensive use % | 20.2 | 25.6 | 31.2 |
| History of stroke in or prior to 2005, % |
0.8 | 1.1 | 1.3 |
| History of myocardial infarction in or prior to 2005, % |
0.7 | 0.8 | 1.9 |
Values were standardized to the age distribution of the overall cohort.
The prevalence of hypertension was 33.0% (576/1748) among the group with more frequent RLS symptoms (>15 times/month), 26.0% (643/2475) within the group with RLS symptoms 5-14 times /month, and 21.4% (13104/61321) within the group with no RLS symptoms (Table 2). The age-adjusted prevalence odds of hypertension were 1.43 (95%CI: 1.33, 1.53; P < 0.0001) times higher among women with RLS symptoms. Significant associations between RLS and hypertension did not materially change (OR=1.20; 95% CI 1.10-1.30), after further adjustment for other potential covariates, such as BMI, BMI squared, menopausal status, DASH score, smoking, race, use of analgesics, oral contraceptives, alcohol, folate, and iron intake (a surrogate of iron deficiency). There was a significant relationship between hypertension and RLS severity as measured by symptom frequency: women suffering from RLS symptoms ≥15 times per month had higher odds of having hypertension compared with the group with less frequent symptoms (Table 2). The multivariate adjusted ORs for hypertension were 1.06 and 1.41 (95% CI: 0.94- 1.18 and 1.24- 1.61, P for trend < 0.0001) for women with RLS symptoms of 5-14 times/mo, and ≥15 times/mo, respectively, relative to those without RLS (Table 2). Subgroup analysis after stratifying age, BMI, and menopausal status suggested higher odds of having hypertension amongst women with RLS symptoms in all subgroups (Table 2). Women within the higher BMI category (≥30 kg/m2) and with RLS symptoms ≥15 times/mo had higher odds of having hypertension (OR 1.57, 95% CI: 1.28-1.93) compared to women with less frequent RLS symptoms (OR 1.15, 95% CI: 0.96-1.37). Similarly, women who reported sleeping less than 8 hours/day along with more frequent RLS symptoms had higher odds of having hypertension (Table 2). Women with RLS symptoms ≥15 times/mo and who were sleeping less than 6 hours/day had OR of 1.49 (95% CI: 1.19-1.87) for having hypertension compared to women without RLS symptoms and who reported similar sleep duration. In contrast, women with RLS symptoms ≥15 times/mo who reported sleeping 8 hours/day or more had OR of 1.24 (95% CI: 0.96-1.60) compared to women without RLS symptoms and with similar sleep duration. However, we did not find a significant interaction between the presence of RLS and age, obesity, menopause status, and sleep duration (P for interaction > 0.35 for all), in relation to likelihood of having hypertension (Table 2).
Table 2.
Association between restless legs syndrome and hypertension
| Variables | No RLS |
RLS 5-14 times/month |
RLS 15+ times/month |
P for trend |
P for interaction |
|---|---|---|---|---|---|
| Hypertension cases # | 13104 | 643 | 576 | ||
| Hypertension prevalence (%) | 21.4 | 26.0 | 33.0 | ||
| Age adjusted OR (95% CI) | 1 | 1.24 (1.13- 1.36) |
1.73 (1.56-1.92) | <0.0001 | |
| Multivariate adjusted OR* | 1 | 1.06 (0.94- 1.18) |
1.41(1.24-1.61) | <0.0001 | |
| Age* | |||||
| <50 years | 1.0 | 1.06 (0.87- 1.30) |
1.52 (1.21-1.91) | 0.0007 | |
| ≥50 years | 1.0 | 1.05 (0.91- 1.21) |
1.37 (1.17-1.60) | 0.0003 | |
| 0.67 | |||||
| Body mass index * | |||||
| <25 kg/m2 | 1.0 | 0.93 (0.75- 1.17) |
1.31 (1.02-1.68) | 0.14 | |
| 25-29 kg/m2 | 1.0 | 1.06 (0.87, 1.29) |
1.33 (1.06, 1.67) | 0.02 | |
| ≥30 kg/m2 | 1.0 | 1.15 (0.96- 1.37) |
1.57 (1.28-1.93) | 0.02 | |
| 0.52 | |||||
| Menopause status† | |||||
| Pre | 1.0 | 0.96 (0.79, 1.18) |
1.33 (1.06-1.67) | 0.06 | |
| Post | 1.0 | 1.13 (0.98- 1.30) |
1.42 (1.21-1.67) | <0.0001 | |
| 0.35 | |||||
| Sleep duration (hours/day) | |||||
| ≤ 6 | 1.0 | 1.003 (0.81- 1.24) |
1.49 (1.19-1.87) | 0.003 | |
| 7 | 1.0 | 1.17 (0.98- 1.39) |
1.47 (1.18-1.81) | 0.0001 | |
| ≥8 | 1.0 | 1.05 (0.83- 1.33) |
1.24 (0.96-1.60) | 0.11 | |
| 0.57 |
Adjustment for age (years), Body mass index (BMI, kg/m2), BMI squared, race (Caucasian, African American, or Asian and other), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1–14 or ≥15), physical activity (quintiles), alcohol intake (g/d: 0,0.1-4.9, 5.0-9.9, 10.0-14.9, and >15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), analgesic use (yes/no), oral contraceptive use (yes/no), total folate intake(quintiles)and DASH score (quintile).
Adjusted above variables except menopause status.
We conducted several sensitivity analyses and obtained similar significant results. Multiple-adjusted ORs comparing women with RLS symptoms ≥15 times/month with those without RLS were 1.41 (95% CI: 1.24, 1.61) after excluding participants with stroke or MI and 1.35 (95% CI: 1.15, 1.60) after excluding antidepressant users. Further adjustment for intake of caffeine and iron, the Crown-Crisp phobic anxiety index, antidepressant use, sleep duration and history of MI or stroke did not materially change the association between RLS and HTN: adjusted OR comparing the women with RLS symptoms ≥15 times/month with those without RLS was 1.31 (95% CI: 1.15, 1.50). We did another sensitivity analysis to exclude the current antihypertensive users. The multiple-adjusted OR comparing women with RLS symptoms ≥15 times/month with those without the symptoms were 1.84 (95%CI: 1.48, 2.27) after excluding the antihypertensive users.
We also observed a significant relationship between RLS severity, as assessed by frequency of the symptoms, and blood pressure. The multivariate adjusted mean systolic blood pressure was 130 mm Hg amongst the group with no RLS, 131 amongst the group with RLS symptoms 5-14 times per month, and 133 mm Hg amongst the group with symptoms ≥15 times per month (p trend <0.0001) (Fig. 1A). The adjusted mean diastolic blood pressure was 80, 81, and 82 mm Hg, respectively, across three RLS categories (P trend <0.0001) (Fig. 1B).
Figure 1.
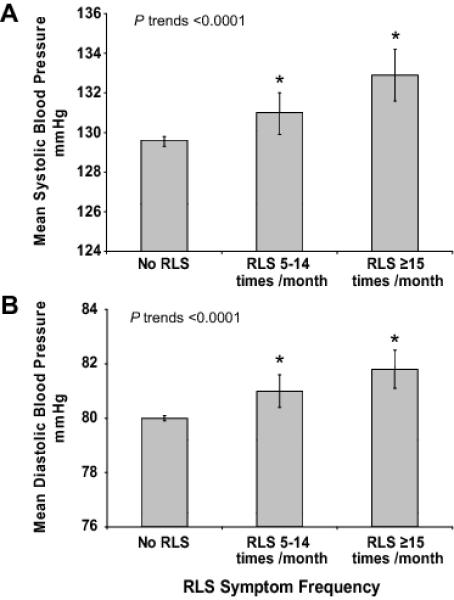
Mean systolic (Panel A) and diastolic (Panel B) blood Pressure (in mmHg), according to the frequency of restless legs symptoms (no RLS, 5-14 times per month, ≥15 times per month). Multivariable model was adjusted for age (years), Body mass index (BMI kg/m2), BMI squared, race (Caucasians, African American, or Asians and other), smoking status (never smoker, past smoker, or current smoker: cigarettes per day, 1-14 or >15), physical activity (quantiles,), alcohol drinking (g/d: 0, 0.1-4.9, 5.0-9.9, 10-14.9, >15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), analgesic use (yes/no), oral contraceptive use (yes/no), total folate intake(quintiles)and DASH score (quintile).
* P<0.01, Compared to No RLS
Discussion
Our results demonstrate increased prevalence of hypertension among women with RLS symptoms. The association was independent of age, BMI, smoking status, and presence of stroke or myocardial infarction. Similar associations were observed in the subgroup analyses. These findings are consistent with the previous literature suggesting a possible role of RLS in the pathogenesis of hypertension9,24. In a survey including 4000 men selected from the general population in central Sweden, participants with RLS symptoms were more likely to report hypertension (OR 1.5, 95% CI 0.9-2.4), after adjusting for age, smoking, and alcohol consumption 9. However, this study did not include women. Moreover, the study failed to exclude RLS mimics such as peripheral neuropathy, and anxiety. Since patients with RLS mimics meet all the essential diagnostic criteria, and do not actually have RLS25,26, it is extremely important to exclude these RLS mimics. Another cross sectional study of 18,980 participants, found a significant association between RLS and hypertension (OR 1.36, 95% CI 1.14-1.61)10, as did telephone survey which ascertained these two diseases and found direct relation(p<0.05) 24.
A number of possible biological mechanisms could account for the increased risk of hypertension in those with RLS. Roughly 80% of patients with RLS have periodic limb movements (PLMS) during sleep27. PLMS are rhythmic extensions of the big toe and dorsiflexion of the ankle, lasting 0.5-5 seconds, which occur up to 200-300 times per night. Such leg movements are associated with sympathetically mediated elevations in both heart rate and blood pressure, which may be responsible for the increased cardiovascular diseases seen in patients with RLS28-30. It is postulated that these repetitive blood pressure elevations at night lead to the development of daytime hypertension31. Electroencephalographic arousals resulting from PLMS may represent another risk factor for hypertension in persons affected by RLS. Interestingly, the arousals from sleep have also been shown to increase blood pressure through elevated peripheral sympathetic tone32 even in individuals without PLMS. Increased daytime pulse rate and blood pressure has also been shown to be associated with higher arousal rate in individuals without PLMS33. It is therefore possible that arousals associated with RLS with or without PLMS are responsible for hypertension. Moreover, the prevalence of PLMS in patients with grade III hypertension has been shown to be twice as frequent as in patients with grades I and II hypertension together (36.4% vs. 13.0%, respectively; p <0.02)34. PLMS are also concomitantly identified in patients with obstructive sleep apnea (OSA), an independent risk factor for hypertension35-38
In a large US sample, subjects with low sleep efficiency and average sleep duration of ≤ 5 hours per night were found to have increased risk of developing hypertension29. Additionally, insufficient sleep may be responsible for increased risk of cardiovascular disease as a result of increased blood pressure39. In the current study, when we adjusted for sleep duration, the association between RLS and hypertension was attenuated but remained significant, suggesting that RLS was not merely leading to hypertension through an effect on sleep duration.
Our results represent an important addition to the literature because our sample size is considerably greater than previously published studies, 9,10, 24 and we were able to control rigorously for potential covariates and exclude patients in which misclassification is likely to occur. We acknowledge a number of limitations. First, the cross sectional nature of the study design prevent drawing conclusions about temporality or causality in the RLS-hypertension association. RLS can lead to hypertension because of its effect on sleep quality and duration or the coexisting PLMS. Alternatively hypertension can also cause RLS through vascular changes or secondary to effects of medications used to treat hypertension7. However, we obtained similar positive associations between RLS and hypertension when we excluded participants who reported use of anti-hypertensive medicines. Residual confounding is impossible to exclude because of observational nature of our study. Thus, conducting a prospective study to clarify whether individuals with RLS have an increased risk of developing hypertension is important in this context.
As discussed earlier, increased frequencies of patients with RLS have PLMS, which are also identified in patients with obstructive sleep apnea, an independent risk factor for hypertension. Although we controlled for snoring, a surrogate of OSA, residual confounding is certainly possible. Similarly, chronic renal failure, another risk factor for both RLS and hypertension 40 was not collected in our cohort. It remains possible that confounding by renal failure played a role in our results although the effects would be modest due to the low prevalence of chronic renal failure in the general populations. The status of iron deficiency; yet another risk factor for RLS, was not available for our cohort. However, further adjusting for the use of iron specific supplements, a surrogate for iron deficiency did not alter the observed association between RLS and hypertension.
The differences in actual blood pressure levels in this study were small which raises the concern about clinical relevance. However, Cook et al have suggested that a 2 mm Hg reduction in diastolic blood pressure could result in 17% decrease in the prevalence of hypertension as well as 6% and 15% reduction in the risk of coronary heart disease and stroke respectively41. Furthermore, random error in our questionnaire-based ascertainment of blood pressure could probably underestimate the association between RLS and blood pressure.
Because of the demographic of the Nurses II cohort, our findings lack generalizability (e.g. to men) and thus our conclusions are limited to the sample studied. Further, because the findings of our study depend mainly on self-reports, our results may have differed if all of our participants had undergone a rigorous neurological history and physical examination. However, such assessments are impractical in a sample size >97,000. Moreover, random misclassification of patients should bias towards the null hypothesis. Finally, RLS is by definition self-reported, yielding credibility to the diagnostic criteria used in the present study. Our study lacks the information on RLS treatment; therefore it is unclear if the association between RLS and hypertension would change after adequate treatment of RLS. However, there are limited studies on long-term efficacy of RLS treatment, and studies have reported under recognition and under treatment of RLS6, 42.
In conclusion, in this large-scale cross sectional study, we found that women with restless legs syndrome had a higher risk of having hypertension than those without RLS, independent of several known risk factors for hypertension. Future prospective studies are needed to confirm this observation.
Perspectives
The prevalence of hypertension continues to rise despite increased awareness and improved treatments. Therefore, there is an increasing need to better understand the potential risk factors leading to hypertension. The current study used a large cohort of women to examine the association between RLS and hypertension after adjusting for potential covariates. The results show that the prevalence of hypertension is higher among women with RLS symptoms. Moreover, there was a significant relationship between RLS severity, as measured by symptom frequency, and hypertension.
Because of the cross sectional nature of this study, it is difficult to determine causality. Therefore, future prospective studies are required to confirm these findings.
Acknowledgments
Nurses’ Health Study II participants and staff.
Alberto Ascherio, MD, DrPH
Gary Curhan, MD, ScD
Sources of Funding: The study was supported by NIH/NINDS grant 1R01NS062879-01A2 and NIH grant CA50385.
Footnotes
Conflict of Interest/Disclosure: A.M. received research grant from Philips, Sepracor, and Cephalon. A.M. has reported consultancy/advisory board relationship with SGS, SHC, Philips, Pfizer, Ethicon, Merck, Medtronic, and Apnex.
J.W received research grant from GlaxoSmithKline. J.W has also served as an expert for AVH-Insomnia medication. He holds consultancy/advisory board relationship with GlaxoSmithKline, Impax Laboratories, Luitpold pharmaceuticals, Pfizer, UCB, and Zeo Inc.
X.G. has reported consultancy relationship with Teva.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith JE, Tolson JM. Recognition, diagnosis, and treatment of restless legs syndrome. Journal of the American Academy of Nurse Practitioners. 2008;20:396–401. doi: 10.1111/j.1745-7599.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom K, Ulfberg J. Restless legs syndrome. Journal of internal medicine. 2009;266:419–431. doi: 10.1111/j.1365-2796.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 3.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Archives of Internal Medicine. 2000;160:2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep medicine reviews. 2006;10:153–167. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005;65:239–246. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 6.Hogl B, Kiechl S, Willeit J, Saletu M, Frauscher B, Seppi K, Muller J, Rungger G, Gasperi A, Wenning G, Poewe W. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64:1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 7.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger I, Erikh I, Avizohar O, Sprecher E, Yarnitsky D. Cardiovascular risk factors in restless legs syndrome. Movement disorders: official journal of the Movement Disorder Society. 2009;24:1587–1592. doi: 10.1002/mds.22486. [DOI] [PubMed] [Google Scholar]
- 9.Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: An association with somatic disease and neuropsychiatric symptoms. Movement Disorders. 2001;16:1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. Journal of psychosomatic research. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics . Health, United States, 2008 [PDF 8.4M] National Center for Health Statistics; Hyattsville, MD: 2008. [Google Scholar]
- 12.Group M Writing, Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson B, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 13.Allen Rp KCAAMJ. The international restless legs syndrome study group validation of the international restless legs group rating scale. Sleep Med. 2003:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72:1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman JP, Curhan GC, Taylor EN. Plasma 25-Hydroxyvitamin D Levels and Risk of Incident Hypertension Among Young Women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens SH, Speizer FE. REPRODUCIBILITY AND VALIDITY OF A SEMIQUANTITATIVE FOOD FREQUENCY QUESTIONNAIRE. American Journal of Epidemiology. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-Based Validation of a Dietary Questionnaire: The Effects of Week-to-Week Variation in Food Consumption. International Journal of Epidemiology. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical Activity, Obesity, and Risk for Colon Cancer and Adenoma in Men. Annals of Internal Medicine. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire. International Journal of Epidemiology. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 20.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate Intake and the Risk of Incident Hypertension Among US Women. JAMA: The Journal of the American Medical Association. 2005;293:320–329. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 21.Glovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The Assessment of Alcohol Consumption by a Simple Self-administered Questionnaire. American Journal of Epidemiology. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. New England Journal of Medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 23.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 24.Phillips B, Hening W, Britz P, Mannino D. Prevalence and Correlates of Restless Legs Syndrome*. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 25.Trenkwalder C, Hogl B, Winkelmann J. Recent advances in the diagnosis, genetics and treatment of restless legs syndrome. Journal of neurology. 2009;256:539–553. doi: 10.1007/s00415-009-0134-9. [DOI] [PubMed] [Google Scholar]
- 26.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”) Sleep medicine. 2009;10:976–981. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: A study of 133 patients diagnosed with new standard criteria. Movement Disorders. 1997;12:61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 28.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–165. [PubMed] [Google Scholar]
- 29.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short Sleep Duration as a Risk Factor for Hypertension: Analyses of the First National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 30.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–580. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 31.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–1130. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 33.Ekstedt M, Akerstedt T, Soderstrom M. Microarousals During Sleep Are Associated With Increased Levels of Lipids, Cortisol, and Blood Pressure. Psychosom Med. 2004;66:925–931. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- 34.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry and clinical neurosciences. 1997;51:103–107. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 36.Chervin RD. Periodic Leg Movements and Sleepiness in Patients Evaluated for Sleep disordered Breathing. Am J Respir Crit Care Med. 2001;164:1454–1458. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 37.Scharf SM, Tubman A, Smale P. Prevalence of concomitant sleep disorders in patients with obstructive sleep apnea. Sleep and Breathing. 2005;9:50–56. doi: 10.1007/s11325-005-0014-1. [DOI] [PubMed] [Google Scholar]
- 38.Kapa S, Kuniyoshi FH Sert, Somers VK. Sleep Apnea and Hypertension: Interactions and Implications for Management. Hypertension. 2008;51:605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 39.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A Prospective Study of Sleep Duration and Coronary Heart Disease in Women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 40.Portaluppi F, Cortelli P, Buonaura GC, Smolensky MH, Fabbian F. Do restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) play a role in nocturnal hypertension and increased cardiovascular risk of renally impaired patients? Chronobiology international. 2009;26:1206–1221. doi: 10.3109/07420520903245276. [DOI] [PubMed] [Google Scholar]
- 41.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of Small Reductions in Diastolic Blood Pressure for Primary Prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 42.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Neurology. 2000;54:1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]


