Abstract
Glucose readily supplies the brain with the majority of carbon needed to sustain neurotransmitter production and utilization., The rate of brain glucose metabolism can be computed using 13C nuclear magnetic resonance (NMR) spectroscopy by detecting changes in 13C contents of products generated by cerebral metabolism. As previously observed, scalar coupling between adjacent 13C carbons (multiplets) can provide additional information to 13C contents for the computation of metabolic rates. Most NMR studies have been conducted in large animals (often under anesthesia) because the mass of the target organ is a limiting factor for NMR. Yet, despite the challengingly small size of the mouse brain, NMR studies are highly desirable because the mouse constitutes a common animal model for human neurological disorders. We have developed a method for the ex vivo resolution of NMR multiplets arising from the brain of an awake mouse after the infusion of [1,6-13C2]glucose. NMR spectra obtained by this method display favorable signal-to-noise ratios. With this protocol, the 13C multiplets of glutamate, glutamine, GABA and aspartate achieved steady state after 150 min. The method enables the accurate resolution of multiplets over time in the awake mouse brain. We anticipate that this method can be broadly applicable to compute brain fluxes in normal and transgenic mouse models of neurological disorders.
INTRODUCTION
The majority of biological processes of man and animals are intimately coupled to the supply of carbon and energy derived from glycolysis and the tricarboxylic acid (TCA) cycle. In the brain, glucose metabolism provides both carbon and energy to fuel neural signaling operating via neurotransmitter fluxes and consequent changes in cell membrane potential (Schurr, 2002). Differences in brain functional states are thus dependent on changes in metabolic flux, whereas brain disorders inexorably alter brain function. Neurological disorders can thus be characterized by understanding the relation between fuel supply, neurotransmitter synthesis, and resting metabolic rates. However, achieving this goal in vivo is difficult.
The measurement of relative or absolute metabolic fluxes in any tissue requires information about the fate of a carbon tracer and suitable mathematical models for analysis of the data. Because of the complexity of carbon metabolism in the brain, various tracer methods have been introduced. Positron emission tomography, 14C tracer methods, and 13C studies with detection by mass spectrometry generally provide limited information about the fate of the tracer, and some are impractical in the mouse. Compared to these alternative methods, 13C NMR spectroscopy, in principle, offers far greater information about the fate of a carbon tracer because of information encoded in the chemical shift and in 13C-13C spin coupled multiplets. The ability to measure brain TCA cycle and neurotransmitter flux rates in the mouse, a common animal model for human neurological disorders would be an important advance. However, detection of 13C multiplets has proven elusive because of the small mass of the brain, the generally low levels of 13C enrichment achievable in vivo, the low sensitivity of 13C (compared to 1H) for NMR detection, and uncertainty about conditions required to achieve isotopic and metabolic steady state after infusion of the 13C-labeled substrate. In the anesthetized mouse brain (175 µl at most), 13C NMR spectroscopy is practicable but suboptimal: glutamate and glutamine are detectable in vivo with a temporal resolution of 8.6 min, but accurate multiplet detection is not yet feasible (Nabuurs et al., 2008).
The purpose of this report is to describe a technique to obtain high-quality 13C NMR spectra from extracts of the brain from conscious mice at different points during infusion of 13C - enriched glucose. This method enables quantitation of 13C-13C spin coupled multiplets over time in multiple neurotransmitters and other key intermediates including glutamate, glutamine, γ-aminobutiric acid (GABA) and aspartate. The 13C multiplets of each cerebral isotopomer achieved steady state after 150 min infusion. The evolution of the fractional amount of multiplets of interrelated molecules (i.e., glutamate and glutamine) was similar to each other. In combination with modeling, this method may allow the computation of brain metabolic fluxes in the normal and the diseased mouse brain.
MATERIALS AND METHODS
13C infusions
This study was performed under Institutional Animal Care and Use Committee of UT Southwestern Medical Center at Dallas guidelines. Female C57BL/6J mice were used for the study (n=7, weight: 23.7 ± 2.6 g, age: 8.6 ± 1.3 m; all ranges and errors hereafter are expressed as standard deviation unless otherwise indicated). The right jugular vein was aseptically cannulated under intraperitoneal anesthesia provided by ketamine (100mg/kg) and xylazine (10mg/kg). Outer catheter diameter was 0.025 mm (Braintree Scientific, Braintree, MA). The catheters were filled with glycerol and heparin (500 U/ml) to prevent clotting. After cannulation, the animals were individually housed under standard animal care conditions with ad libitum access to water. Seven days post-cannulation, the mice were habituated and confined to a cylindrical Lucite cage to prevent ambulation and the catheter purged with 2 µL saline solution. [1,6-13C2]glucose (1-13C, 99% enrichment; 6-13C, 97% enrichment, Cambridge Isotope Laboratories, Cambridge, MA) was administered as a bolus containing 0.4mg/g (in 0.2 ml of saline) infused over 30 sec, followed by continuous infusion of 0.012 mg/g/min (in 0.375 ml of saline) at 150 µl/hr during increasing periods of time (one per animal) at room temperature in an unperturbed environment. Infusion time periods were: 20, 30, 50, 75, 150 and 300 min. Animals were decapitated at the end of infusion and forebrains were rapidly removed (in less than 15 sec) after blood collection, weighed (forebrain weight: 314.03 ± 14 mg), frozen in liquid nitrogen, and stored at −85°C. Blood was simultaneously collected from the cervical stump, weighed (190 ± 10 µL), frozen in liquid nitrogen, and stored at −85°C.
Preparation of tissue extracts
A modified version of perchloric acid extraction protocol was applied (Walton et al., 2003). In brief, frozen brain or blood samples were finely grounded in a mortar under liquid nitrogen. Perchloric acid (4%; 1:4 w/v) was added to each sample, followed by centrifugation at 47800 g for 15 min. The supernatant was transferred to a new tube where chloroform / tri-n-octylamine (78% / 22%; v/v) was added in 1:2 volumetric ratio to a pH of 6. The samples were centrifuged at 3300 g for 15 min, the aqueous phase removed and transferred to a microfuge tube and then lyophilized. 200 µL of deuterium oxide (99.96%, Cambridge Isotope Laboratories) was added to each sample and the pH adjusted to 7.0 with 2–3 µL of 1 M sodium deuteroxide (99.5%, Cambridge Isotope Laboratories). The pH-neutral samples were then centrifuged at 18,400 g for 1 min and the supernatant removed and placed into a 3-mm NMR tube for subsequent NMR analysis. In addition NMR study of blood samples, total blood glucose was measured by enzyme , Sigma, St Louis, MO).
NMR Spectroscopy
Proton decoupled 13C spectra were acquired on a 600 MHz Oxford magnet and Varian VNMRS Direct Drive console using a 3 mm broadband probe (Varian Inc., Palo Alto, CA) at room temperature. Proton decoupling was performed at 2.3 kHz using a Waltz-16 sequence. 13C NMR spectroscopy parameters included a 45° flip angle per transient, a relaxation delay of 1.5 s, an acquisition time of 1.5 s, and a spectral width of 36.7 kHz. Samples were spun at 20 Hz and 25°C. A heteronuclear 2H lock was used to compensate for magnet drift during data acquisition. To achieve adequate signal-to-noise in brain spectra, the number of scans acquired for short infusions (20–75 min) were typically 10,000 to 12,000 and for long infusions (150–300 min) were between 4,000 and 6,000. Proton spectra were similarly acquired to characterize the fractional enrichment of the α-anomer of the C1 proton resonance of D-glucose in blood. 1H NMR parameters included a 45° flip angle per transient, a relaxation delay of 1 s, an acquisition time of 2 s, and a spectral width of 20 kHz. Samples were spun and the temperature maintained as above. A heteronuclear 2H lock was also used. For proton NMR in blood samples, the number of scans acquired to achive favorable signal-to-noise was 120.
NMR spectrum analysis
NMR spectral analyses were performed with ACD/Spec Manager 11.0 software (Advanced Chemistry Development, Inc., Toronto ON, Canada). Time-series free induction decays were zero-filled and windowed with an exponential weighting function prior to Fourier transformation for analysis of spectral contents. Metabolite peaks were then identified based on chemical shift position referenced to the glutamate C4 singlet at 34.2 ppm. Each peak was then fitted with a Gauss-Lorentz function and the area measurements for each fitted resonance peak and their multiplets estimated. For each isotopomer, multiplet areas were defined as a fraction of the total atomic resonance area, here called multiplet fractional amount (Malloy, 1998). For example, the fractional amount of glutamate C4 singlet (C4S) represents the area of C4S relative to the total area of glutamate C4; the fractional amount of the doublet 34 (C4D34) refers to area of C4D34 due to J34 (coupling between 13C carbons in positions 3 and 4 of glutamate) relative to the total area of glutamate C4; the same contention applies to the doublet 45 (D45) and the quartet (Q) of glutamate C4. By convention, the sum of all multiplets arising from a given isotopomer equals unity; in this case glutamate C4S + C4D34 + C4D45 + C4Q = 1. The 13C enrichment of glucose in blood was computed by 1H-NMR as previously described (Dobbins and Malloy, 2003). The portion of blood glucose that included 13C at the carbon 1 position was defined as the area of the 13C doublets, predominantly derived from 13C coupling between carbon 1 and 6, relative to the total 1H resonance area (13C + 12C).
RESULTS
Animals tolerated and recovered from cannulation uneventfully and did not exhibit abnormal behavior during the infusion. All brain-derived NMR spectra were resolved with favorable signal-to-noise ratios. As shown by others for the microwave-fixed cerebral cortex (Walls et al., 2011), the brief time of dissection (<15 s) does not significantly impact metabolite abundance in the brain relative to other methods of brain fixation. The resonances ascribed to cerebral glutamate and glutamine in C1, C2, C3 and C4, together with aspartate C2 and C3, GABA C2, C3, C4, N-acetyl-aspartate C2, C3 and C6, lactate C2 and C3 and taurine C1 and C2 were readily identified. As expected from previous 13C-glucose studies in brain extracts (Alexander et al., 2005; Hassel et al., 1997), glutamate, glutamine and lactate constituted the largest resonances in the NMR spectrum consistent both with the preference of neurons and glia to produce glutamate and glutamine from glucose, respectively, and also with the high glycolytic capacity of brain cells, which are able to robustly metabolize glucose to produce lactate (Escartin et al., 2006; Hassel et al., 1995; Sonnewald et al., 1994; Tansey et al., 1991).
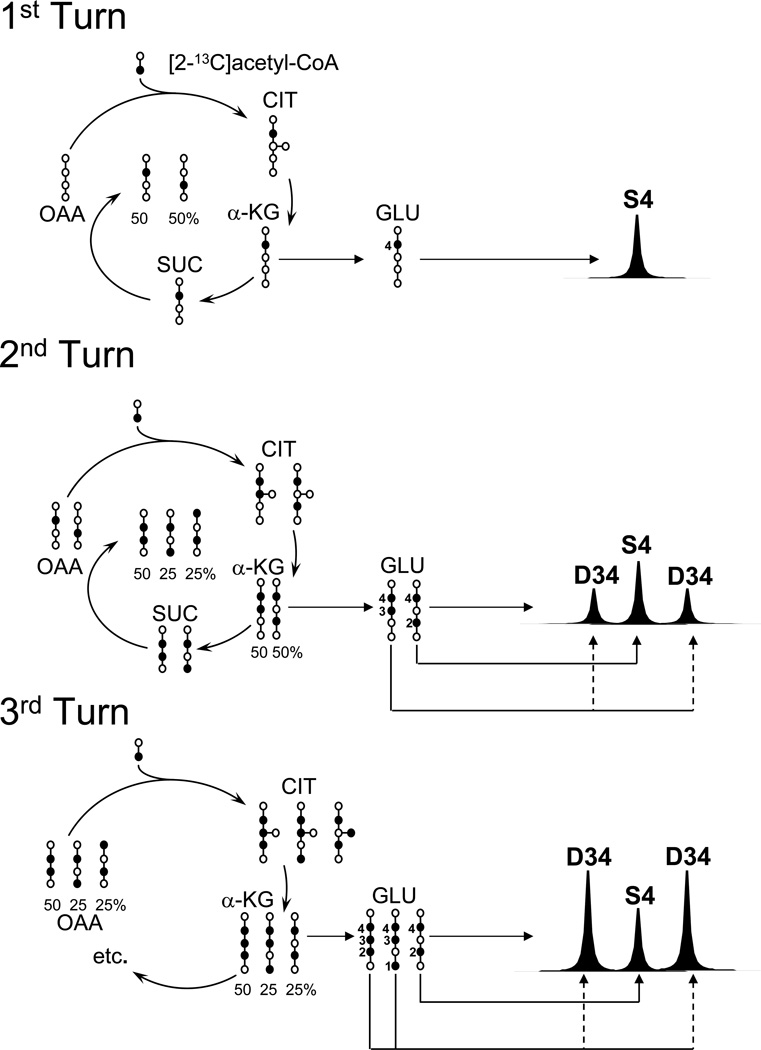
Figure 1 illustrates the distribution of 13C-label into intermediates of [1,6-13C2]glucose metabolism. [3-13C]pyruvate generated via glycolysis and converted into [2-13C]acetyl-CoA by pyruvate dehydrogenase. The condensation of [2-13C]acetyl-CoA with non-labeled oxaloacetate yields [4-13C]citrate, [4-13C]ketoglutarate and, consequently, [4-13C]glutamate in the first cycle (“turn”) of the TCA cycle, which appears in the 13C spectrum as a singlet glutamate C4 resonance (Figure 1). [2-13C]oxaloacetate produced in the first turn of the TCA cycle, can coalesce with another [2-13C]-acetyl-CoA molecule, yielding the first TCA cycle intermediate containing two adjacent 13C carbons, [2,3-13C2]citrate. This molecule is subsequently converted into [3,4-13C2]glutamate, which appears as a doublet (D34) surrounding the glutamate C4 singlet (Figure 1 and 2). A similar progression of enrichment can be expected for glutamate-derived molecules such as glutamine and GABA (Figure 2). As shown in Figure 3, the multiplets arising from all detected isotopomers gradually increased in signal area as the infusion progressed, reflecting the distribution of 13C amount the TCA cycle intermediates as the cycle turnover continues. Of note, the amplitude of the singlet glutamate C4 resonance decreased beyond 75 min of infusion (Figure 3). This phenomenon is probably the consequence of progressive enrichment of [2-13C]acetyl-CoA and [2-13C]oxaloacetate, with longer infusions resulting in reduced formation of [4-13C]citrate and [4-13C]glutamate. As previously noted (Malloy, 1998; Robitaille et al., 1993; Weiss et al., 1995), the intensity of multiplet resonances also depends on metabolite pool sizes, TCA cycle flux and on other metabolic exchanges. In glutamate C3 and glutamine C3, the area of the doublet (quantified as the sum of doublet 23 and 34, as they share the same J-J coupling constant 34 Hz) and the triplet evolve proportionally over time (Figure 4). The singlet C3 resonance appears to increase along the infusion time course probably due to the parallel raise of the triplet, which overlaps with the singlet resonance.
FIGURE 1. Evolution of glutamate labeling in the mouse brain TCA cycle.
Schematic of the key reactions of the TCA cycle and the consequent 13C-labeling and spectral pattern of brain glutamate (GLU) labeled in position 4 after the administration of [1,6-13C2]glucose. The intermediates citrate (CIT), α-ketoglutarate (α-KG), succinate (SUC) and oxaloacetate (OAA), are numbered from bottom (carbon 1) to top. This model assumes that the only source of 13C for the TCA cycle is acetyl-CoA and that 100% of the acetyl-CoA is [2-13C]acetyl-CoA. The resulting labeling pattern of glutamate via TCA cycle intermediate metabolism and the predicted 13C NMR spectrum of glutamate arising from each “turn” of the cycle (number to the left) are shown. Note that carbons 4 and 5 of glutamate are derived directly from acetyl-CoA and carbons 1, 2 and 3 originate from oxaloacetate.
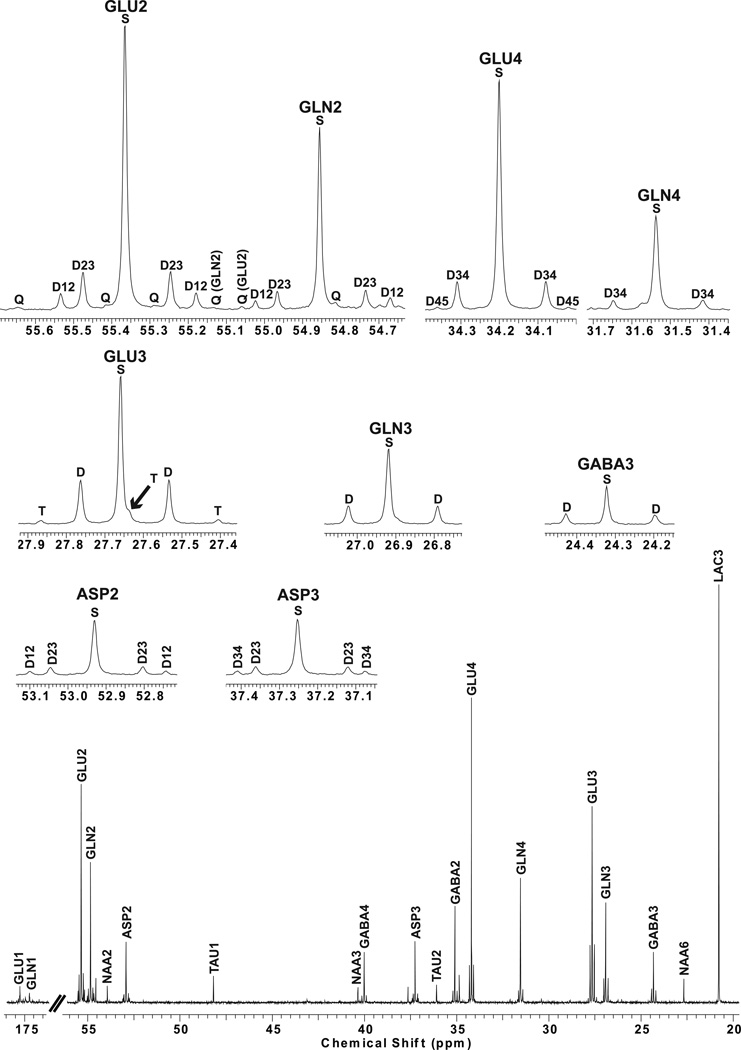
FIGURE 2. 13C-NMR spectrum of the mouse forebrain at 75 min of infusion.
Insets display the multiplets of some isotopomers detected in the 13C spectrum. GLU: glutamate, GLN: glutamine, ASP: aspartate, NAA: n-acetylaspartate, TAU: taurine. C#: carbon labeled in position #. Sx: singlet, Dxx: doublet, T: triplet, Q: quartet.
FIGURE 3. Evolution of 13C multiplets of GABA C2, glutamate C3, C4 and glutamine C4 over time.
The largest resonance achievable for each isotopomer is reached at 150 min of 13C-glucose infusion initiation.
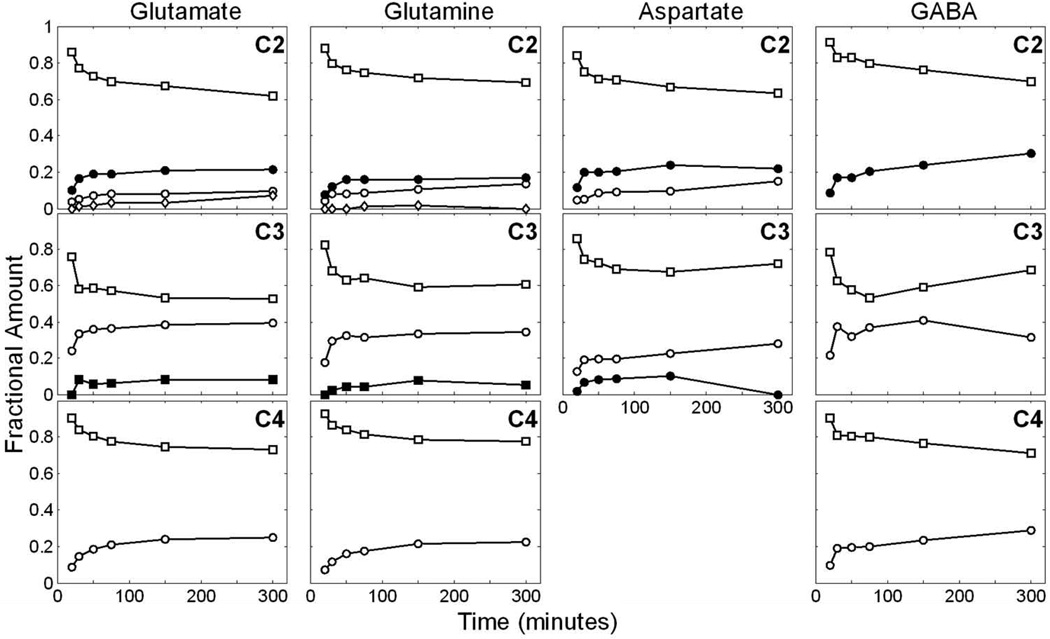
FIGURE 4. Time course of 13C multiplets of glutamate, glutamine, GABA and aspartate in all carbon positions as detected by 13C NMR spectroscopy.
The multiplet fractional amount is calculated by the area of each multiplet relative to the total resonance area of the isotopomer as described in the methods section. Overall, the fractional amount of each multiplet in each isotopomer reaches steady state at 150 min with the present infusion protocol, implying brain metabolic equilibrium. Open square: singlet; C2: open circle: doublet12, closed circle: doublet23, diamond: quartet; C3: open circle: doublet, closed square: triplet. C4: open circle: doublet34. For aspartate C3: open circle: doublet23, closed circle: doublet34.
Importantly, the relative areas of multiplet isotopomers were approximately constant after 150 min of infusion (Figure 3 and 4). At this point, the distribution of 13C among TCA cycle-derived intermediates reached steady state and the relative abundance of 13C multiplets no longer varied significantly. As stated earlier, there was a close correlation between a progressive decrease observed in the resonance areas attributed to singlet isotopomers and a simultaneous increase in the doublet, triplet, and quartet resonances detectable for each molecule. For example, Figure 2 illustrates a typical spectrum in which all isotopomers are identified after 75 min of infusion. Taurine labeled in carbons 1 and 2 manifested a modest signal in the NMR spectrum, probably deriving from naturally abundant 13C. Moreover, resonances from N-acetylaspartate in carbons 2, 3 and 6 were represented only as singlets, owing to the slow rate of 13C-label incorporation into both aspartyl (NAA C2 and C3) and acetyl (NAA C6) groups (Choi and Gruetter, 2004).
The mean blood glucose concentration at the end of the infusions was 7.5 ± 3.45 mM (averaging all infusion time courses) (normal range: 6.5–7 mM (Ayala et al., 2006)). The enrichment of blood 13C-glucose detected by 1H-NMR rose above natural abundance (1.1 %) to 21.2% at 20 min of infusion and progressively increased as follows: 40.88% at 30 min, 27.8% at 50 min, 33.6% at 75 min, 51.3% at 150 min, 37.4% at 300 min.
DISCUSSION
We have for the first time, illustrated the dynamic evolution of multiplet isotopomers in the awake mouse brain by ex vivo 13C NMR spectroscopy. Because of the superior resolution of the 13C spectra, the signals arising from glutamate, glutamine, aspartate and GABA were not only detected separately, but were also discerned as multiplets due to the coupling of adjacent 13C carbons within each molecule, which were precisely resolved using conventional NMR equipment. This method overcomes the common technical challenges posed by the mouse brain, namely the low sensitivity of 13C-NMR techniques to resolve 13C isotopomers and the notable effect of anesthesia on brain metabolism, while also allowing for the determination of isotopomer steady state commonly assumed in intraperitoneal injection approaches (Walls et al., 2011). We have shown that an accurate resolution of 13C multiplets in the awake mouse brain is feasible and potentially valuable to determine rates of substrate uptake and of major brain metabolic pathways with further assistance from metabolic modeling. Importantly, in contrast with other studies performed in rats (Patel et al., 2004; Sibson et al., 1998; Sibson et al., 2001), the concentration of 13C label infused avoided or minimized hyperglycemia (the maximum blood glucose level reached was 11 mM), preventing potential systemic metabolic confounders when excessive, supraphysiological glucose concentrations are reached.
Kinetic studies designed to measure rates of biochemical reactions within a metabolic network rely upon the analysis of multiple 13C spectra. Steady-state isotopomer analysis can also allow for the determination of relative flux and labeling patterns of metabolic pathways, such as anaplerosis (Malloy, 1998). For instance, in the heart (mostly considered as a single-type cell organ for the purposes of NMR analysis), a comprehensive isotopomer analysis of glutamate C4 under steady-state 13C enrichment conditions allowed the determination of carbon flux through the oxidative and anaplerotic pathways (Malloy et al., 1987). Moreover, a similar analysis of glutamate C4 – in this case not restricted to isotopic steady-state conditions- can be applied to estimate the enrichment of 13C-acetyl-CoA in the intact tissue, a valuable parameter that provides information about substrate oxidation and competition in cells (Malloy et al., 1990). In the brain, the same analysis can be developed to compute relative rates of anaplerosis and 13C enrichment of acetyl-CoA, with the caveats imposed by a multi-compartment system in which the rate of anaplerosis may differ between compartments, and in which the pool of 13Cacetyl-CoA may substantially diverge, depending of the availability and of substrate preference in each cell type considered.
As illustrated in Figure 2, multiplets were well resolved in all isotopomers, with glutamate exhibiting the highest resolvability of multiplets in all carbon positions relative to other isotopomers. Two potential reasons explain this finding: 1) glutamate is highly abundant in the brain (~ 12 µmol/g of wet tissue) relative to other 13C-labeled amino acids (Chang et al., 1981), 2) and glutamate and α-ketoglutarate are in rapid exchange relative to neuronal and glial TCA cycle flux, resulting in the early appearance of glutamate in the 13C spectrum relative to other isotopomers (Oz et al., 2004; Yang et al., 2009). On the other hand, the 13C label incorporation (detected as 13C multiplets) was found to be roughly proportional for interrelated molecules such as glutamate, glutamine and GABA. Overall, brain metabolic steady state was reached approximately at 150 min of infusion, which was significantly earlier than that previously observed in anesthetized mice (~250 min after infusion of larger amount of [1,6-13C2]glucose than the present protocol (bolus: 0.130 mg/g, infusion: 0.016 mg/g/min; (Nabuurs et al., 2008)). As noted in earlier studies of the rat brain, a likely explanation for this phenomenon is that anesthesia can limit the interpretation of metabolic fluxes as it significantly impacts the rate of certain key fluxes, such as the rate of the glutamate-glutamine cycle (Vnt), which is notably higher in conscious rats (0.57 ± 0.21 µmol/g/min) (Oz et al., 2004) relative to rats anesthetized with α-chloralose (0.2 ± 0.08 µmol/g/min) (Sibson et al., 2001) or pentobarbital (0.04 ± 0.01 µmol/g/min) (Choi et al., 2002).
Previous in vivo NMR approaches to mouse brain metabolism were limited because of the low 13C spectral resolution leading to a substantial overlap between adjacent isotopomers, i.e. glutamate and glutamine C3, and because animals were subjected to anesthesia that interferes, in all likelihood, with brain metabolism (Nabuurs et al., 2008; Peled-Kamar et al., 1998). The studies cited have analyzed the appearance of 13C in TCA cycle-related metabolites (for example, glutamate and glutamine) by measuring the 13C contents of individual carbons identified in the NMR spectra. However, we have shown that multiplets can provide additional information (superior to 13C contents alone) for the computation of metabolic flux rates and the 13C enrichment of TCA cycle substrates (Jeffrey et al., 1999). For instance, the estimation of the oxygen consumption in the rat heart is significantly improved when multiplets are added to the kinetic analysis of NMR data, detecting 60% changes in oxygen utilization depending upon experimental conditions (Jeffrey et al., 1999). This feature of the method can simplify the characterization of different physiological and pathological conditions associated with abnormal oxygen consumption and metabolic rate. On the other hand, the presented approach will potentially benefit from innovative 13C-enhancing techniques such as cryogenically cooled probes and hyperpolarization (Kovacs et al., 2005). Cryogenically cooled probes typically enable a 3–4-fold enhancement of the detection sensitivity in high-resolution NMR compared to conventional probes (Kovacs et al., 2005). Additionally, methods that strengthen the polarization of nuclear spins (termed hyperpolarization methods) such as dynamic nuclear polarization (DNP) can increase the 13C sensitivity by a factor of up to 10,000 (Ardenkjaer-Larsen et al., 2003). We anticipate that these techniques may potentially improve even more the quality of the ex vivo 13C spectra and will enhance the resolution of in vivo 13C multiplets in the mouse brain.
In summary, the detection and analysis of time-dependent ex vivo 13C NMR spectroscopy data arising from awake mouse brain metabolism can be enhanced to include information-rich multiple resonances. In this work, we have presented a method for the generation of high-quality 13C spectra from the mouse brain that permitted accurate resolution of multiplets in glutamate, glutamine, GABA and aspartate. The results demonstrate that, overall, all detected TCA cycle-derived isotopomers manifest a similar evolution of multiplets reaching steady state at 150 min with a quasi-physiological [1,6-13C2]glucose infusion. We anticipate that this method will be useful for the estimation of the rates of major metabolic pathways in the normal awake mouse brain and also in transgenic mouse models of human neurological diseases.
Highlights.
We have developed a method for the ex vivo resolution of NMR multiplets arising from the awake mouse brain after the infusion of [1,6-13C2]glucose. (Most NMR studies have been conducted in large animals (often under anesthesia) because the weight of the target organ is a limiting factor for NMR).
NMR spectra obtained by this method display favorable signal-to-noise ratios that lend themselves to precise analysis.
The method enables the accurate resolution of multiplets over time in the awake mouse brain.
We anticipate that this method will be broadly applicable to compute brain fluxes in normal and transgenic mouse models of neurological disorders.
ACKNOWLEDGMENTS
We would like to acknowledge the assistance of Fundación Caja Madrid (IMV) and NIH grants RR002584 and EB000461 (FMJ, CRM, JMP), F32NS065640 (LBG) and Dallas Women’s Foundation (Billingsley Fund) (JMP). We also acknowledge the experimental and technical assistance of Charles Storey and Hyeonman Baek, respectively.
Abbreviations
- NMR
nuclear magnetic resonance
- TCA
tricarboxylic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander JJ, Zwingmann C, Quigg R. MRL/lpr mice have alterations in brain metabolism as shown with [1H-13C] NMR spectroscopy. Neurochem Int. 2005;47:143–151. doi: 10.1016/j.neuint.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Chang JY, Martin P, Bernasconi R, Braun DG. High-sensitivity amino acid analysis: measurement of amino acid neurotransmitter in mouse brain. FEBS Lett. 1981;132:117–120. doi: 10.1016/0014-5793(81)80441-3. [DOI] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1-13C]glucose in the rat brain in vivo. J Neurochem. 2004;91:778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Dobbins RL, Malloy CR. Measuring in-vivo metabolism using nuclear magnetic resonance. Curr Opin Clin Nutr Metab Care. 2003;6:501–509. doi: 10.1097/00075197-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Escartin C, Valette J, Lebon V, Bonvento G. Neuron-astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem. 2006;99:393–401. doi: 10.1111/j.1471-4159.2006.04083.x. [DOI] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Hassel B, Westergaard N, Schousboe A, Fonnum F. Metabolic differences between primary cultures of astrocytes and neurons from cerebellum and cerebral cortex. Effects of fluorocitrate. Neurochem Res. 1995;20:413–420. doi: 10.1007/BF00973096. [DOI] [PubMed] [Google Scholar]
- Jeffrey FM, Reshetov A, Storey CJ, Carvalho RA, Sherry AD, Malloy CR. Use of a single (13)C NMR resonance of glutamate for measuring oxygen consumption in tissue. Am J Physiol. 1999;277:E1111–E1121. doi: 10.1152/ajpendo.1999.277.6.E1111. [DOI] [PubMed] [Google Scholar]
- Kovacs H, Moskau D, Spraul M. Cryogenically cooled probes--a leap in NMR technology. Progress in Nuclear Magnetic Resonance Spectroscopy. 2005;46:131–155. [Google Scholar]
- Malloy ADSaCM. 13C Isotopomer analysis of glutamate. A NMR method to probe metabolic pathways intersecting in the citric acid cycle. In: Berliner P-MLR LJ, editor. Biological Magnetic Resonance. In Vivo Carbon-13 NMR. New York: Kluwer Academic / Plenum Publishers; 1998. pp. 59–97. [Google Scholar]
- Malloy CR, Sherry AD, Jeffrey FM. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS Lett. 1987;212:58–62. doi: 10.1016/0014-5793(87)81556-9. [DOI] [PubMed] [Google Scholar]
- Malloy CR, Thompson JR, Jeffrey FM, Sherry AD. Contribution of exogenous substrates to acetyl coenzyme A: measurement by 13C NMR under non-steady-state conditions. Biochemistry. 1990;29:6756–6761. doi: 10.1021/bi00481a002. [DOI] [PubMed] [Google Scholar]
- Nabuurs CI, Klomp DW, Veltien A, Kan HE, Heerschap A. Localized sensitivity enhanced in vivo 13C MRS to detect glucose metabolism in the mouse brain. Magn Reson Med. 2008;59:626–630. doi: 10.1002/mrm.21498. [DOI] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, Behar KL. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- Peled-Kamar M, Degani H, Bendel P, Margalit R, Groner Y. Altered brain glucose metabolism in transgenic-PFKL mice with elevated L-phosphofructokinase: in vivo NMR studies. Brain Res. 1998;810:138–145. doi: 10.1016/s0006-8993(98)00899-3. [DOI] [PubMed] [Google Scholar]
- Robitaille PM, Rath DP, Skinner TE, Abduljalil AM, Hamlin RL. Transaminase reaction rates, transport activities and TCA cycle analysis by post-steady state 13C NMR. Magn Reson Med. 1993;30:262–266. doi: 10.1002/mrm.1910300218. [DOI] [PubMed] [Google Scholar]
- Schurr A. Neuronal energy requirements. In: Walz W, editor. The neuronal environment: brain homeostasis in health and disease. New Jersey: Humana Press; 2002. pp. 25–54. [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Muller TB, Westergaard N, Unsgard G, Petersen SB, Schousboe A. NMR spectroscopic study of cell cultures of astrocytes and neurons exposed to hypoxia: compartmentation of astrocyte metabolism. Neurochem Int. 1994;24:473–483. doi: 10.1016/0197-0186(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Tansey FA, Farooq M, Cammer W. Glutamine synthetase in oligodendrocytes and astrocytes: new biochemical and immunocytochemical evidence. J Neurochem. 1991;56:266–272. doi: 10.1111/j.1471-4159.1991.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Walls AB, Eyjolfsson EM, Smeland OB, Nilsen LH, Schousboe I, Schousboe A, Sonnewald U, Waagepetersen HS. Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J Cereb Blood Flow Metab. 2011;31:494–503. doi: 10.1038/jcbfm.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Ebert D, Haller RG. Relative rates of anaplerotic flux in rested and contracted rat skeletal muscle measured by 13C NMR spectroscopy. J Physiol. 2003;548:541–548. doi: 10.1113/jphysiol.2002.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RG, Stern MD, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Consequences of altered aspartate aminotransferase activity on 13C-glutamate labelling by the tricarboxylic acid cycle in intact rat hearts. Biochim Biophys Acta. 1995;1243:543–548. doi: 10.1016/0304-4165(95)00031-6. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu S, Shen J. Fast isotopic exchange between mitochondria and cytosol in brain revealed by relayed 13C magnetization transfer spectroscopy. J Cereb Blood Flow Metab. 2009;29:661–669. doi: 10.1038/jcbfm.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]