Abstract
Human respiratory syncytial virus (RSV) is a ubiquitous pathogen that infects everyone worldwide early in life and is a leading cause of severe lower respiratory tract disease in the pediatric population as well as in the elderly and in profoundly immunosuppressed individuals. RSV is an enveloped, nonsegmented negative-sense RNA virus that is classified in Family Paramyxoviridae and is one of its more complex members. Although the replicative cycle of RSV follows the general pattern of the Paramyxoviridae, it encodes additional proteins. Two of these (NS1 and NS2) inhibit the host type I and type III interferon (IFN) responses, among other functions, and another gene encodes two novel RNA synthesis factors (M2-1 and M2-2). The attachment (G) glycoprotein also exhibits unusual features, such as high sequence variability, extensive glycosylation, cytokine mimicry, and a shed form that helps the virus evade neutralizing antibodies. RSV is notable for being able to efficiently infect early in life, with the peak of hospitalization at 2–3 months of age. It also is notable for the ability to reinfect symptomatically throughout life without need for significant antigenic change, although immunity from prior infection reduces disease. It is widely thought that re-infection is due to an ability of RSV to inhibit or subvert the host immune response. Mechanisms of viral pathogenesis remain controversial. RSV is notable for a historic, tragic pediatric vaccine failure involving a formalin-inactivated virus preparation that was evaluated in the 1960’s and that was poorly protective and paradoxically primed for enhanced RSV disease. RSV also is notable for the development of a successful strategy for passive immunoprophylaxis of high-risk infants using RSV-neutralizing antibodies. Vaccines and new antiviral drugs are in pre-clinical and clinical development, but controlling RSV remains a formidable challenge.
1. History
Human respiratory syncytial virus (RSV) was first isolated in 1955 from a captive chimpanzee with upper respiratory tract illness (URI) (Morris et al., 1956). It was quickly identified as a human virus and shown to be a major pediatric respiratory pathogen (Chanock and Finberg, 1957; Chanock et al., 1957). RSV is now recognized as the most important viral agent of lower respiratory tract illness (LRI) in infants worldwide (Hall et al., 2009; Nair et al., 2010), as well as an important cause of LRI in older individuals with cardiopulmonary disease or in whom immune responses are impaired or reduced (Dowell et al., 1996; Falsey, 2007; Falsey et al., 2005; Hall et al., 1986; Whimbey and Ghosh, 2000). Infants and young children at high risk for severe RSV disease can be substantially protected by the passive administration of a commercially available RSV-neutralizing monoclonal antibody (MAb) (Mejias and Ramilo, 2008; Wu et al., 2008). The guanosine analog ribavirin is available for antiviral therapy; however, its efficacy is marginal and controversial and it is not recommended for routine use (2006). There remains a need for more effective antiviral drugs and for a vaccine for protection of the general population. This article will review notable features of the virus, new findings, and the prospects for control of this important pathogen.
2. Virus, RNAs, and proteins
RSV is an enveloped non-segmented negative-sense RNA virus. It replicates in the cytoplasm and buds at the plasma membrane (Fig. 1). RSV is classified in Family Paramyxoviridae, Order Mononegavirales. This family has two subfamilies (i) Pneumovirinae, which consists of RSV, human metapneumovirus (HMPV), and their animal relatives, and (ii) Paramyxovirinae, which includes Sendai virus, the human parainfluenza viruses (HPIVs), measles virus, and a number of other pathogens of humans and animals.
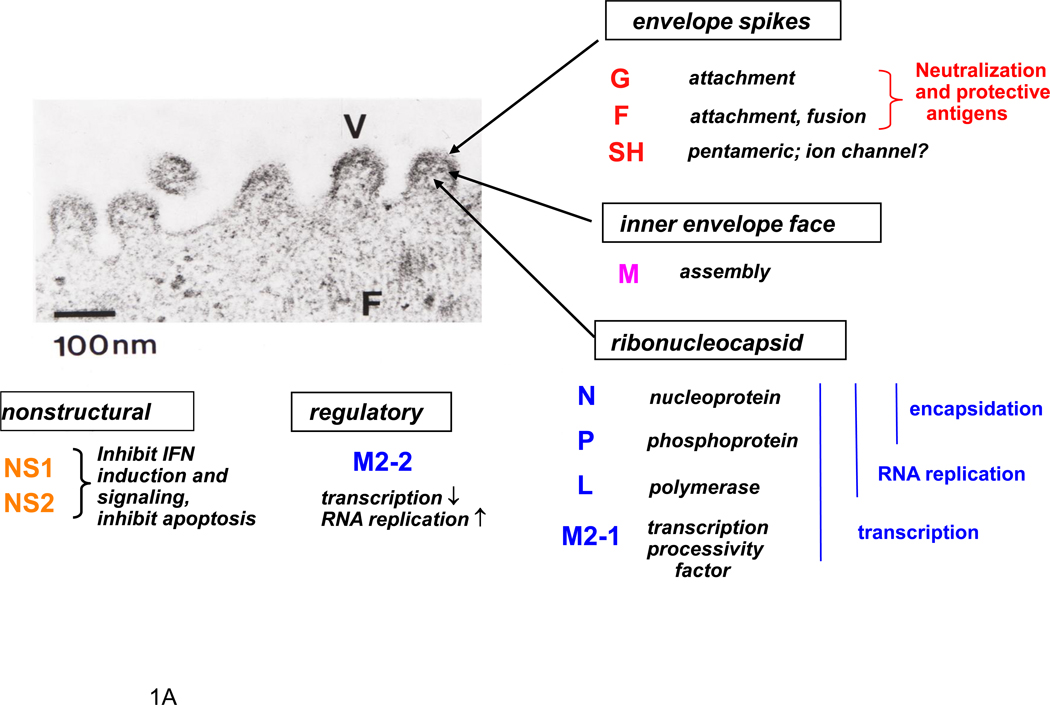
Figure 1.
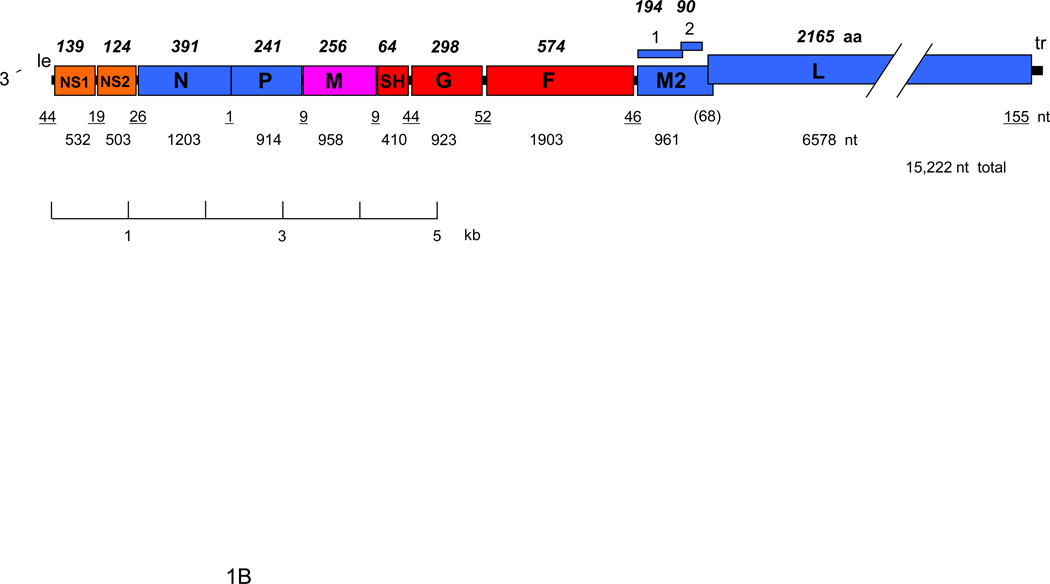
RSV proteins (A) and gene map (B). Panel A shows a negatively-stained electron micrograph of budding RSV virions: V indicates a budding virion and F indicates filamentous cytoplasmic structures thought to be nucleocapsids (courtesy of Dr. Robert M. Chanock) (Kalica et al., 1973). The locations of viral proteins in the virion, and their functions when known, are indicated. Panel B shows a map of the negative sense genome (RSV strain A2), approximately to scale. The overlapping M2-1 and M2-2 ORFs are shown over the gene. Numbers below the map indicate nucleotide (nt) lengths: those of the 3´ leader (le) and 5´ trailer (tr) and intergenic regions are underlined, and the length of the gene overlap in parentheses. Italicized, bold numbers over the map indicate the amino acid (aa) lengths of the unmodified proteins. The viral proteins are as follows: G, attachment glycoprotein; F, fusion glycoprotein; SH, small hydrophobic glycoprotein; M, matrix protein; N, nucleoprotein; P, phosphoprotein; L, large polymerase protein; M2-1, product of the first ORF in the M2 mRNA; M2-2; product of the second ORF in the M2 RNA.
Some of the notable features of RSV molecular biology are summarized in Table 1. RSV grown in cell culture includes pleomorphic spherical particles but predominantly consists of long filaments. Progeny particles mostly remain cell-associated, suggestive of incomplete budding. Release requires sonication, vortexing, or some other means, increasing the contamination by cellular material. Titer is modest, approximately 107 infectious units per ml. Infectivity is unstable and can readily be lost during handling, although losses can be substantially reduced by inclusion of sucrose or other stabilizers. These factors pose substantial obstacles to research and vaccine development.
Table 1.
Notable features of RSV genetics and biology
|
The RSV genome is 15.2 kb in length and has 10 genes in the order 3´ NS1-NS2-N-P-M-SH-F-G-M2-L (Fig. 1). These are transcribed into 10 separate mRNAs. Each encodes a single polypeptide except for the M2 mRNA, which has an upstream ORF encoding the M2-1 protein and a second, downstream ORF that briefly overlaps the upstream ORF and encodes the M2-2 protein. Translation of the M2-2 ORF depends on re-initiation by ribosomes as they exit the M2-1 ORF; surprisingly, re-initiation was found to be influenced by a RNA structure located ~150 nucleotides upstream of the M2-2 translational start site (Gould and Easton, 2005; Gould and Easton, 2007). With a total of 11 distinct viral proteins, RSV is one of the more complex members of Paramyxoviridae: compared to other members of the family, the RSV genome is similar in size but has several additional genes and proteins, namely NS1, NS2, SH, M2-1 and M2-2 (Fig. 1).
RSV transcription and RNA replication follow the general Mononegavirales model. The polymerase enters the genome at the 3´ end, which begins with a 44-nucleotide leader region (Fearns et al., 2002; McGivern et al., 2005). Genes are transcribed sequentially guided by short gene-start and gene-end signals to yield individual mRNAs (Kuo et al., 1996). During RNA replication, the polymerase ignores transcription signals and produces a complete positive sense replicative intermediate called the antigenome, which then serves as the template for producing progeny genomes. Somewhat unusually, two of the RSV genes (M2 and L) overlap by 68 nucleotides (Collins et al., 1987): studies with mini-replicons showed that, following transcription of the M2 gene, the viral polymerase scans in both the upstream and downstream directions to locate the L gene start site (Fearns and Collins, 1999a). Scanning may be a more general activity of the polymerase, and is speculated to occur at each gene junction during sequential transcription as well as during initiation of transcription and RNA replication. Other recent mini-replicon studies mapped a cis-acting signal necessary for both transcription and RNA replication to the first 11 nucleotides of the genome and identified additional leader sequences necessary for optimal transcription or encapsidation (Fearns et al., 2002; McGivern et al., 2005). These studies confirmed that chain elongation of replicative RNA depends on encapsidation (McGivern et al., 2005), and made the somewhat unusual observation that the first nucleotide of nascent mRNA and antigenome can be chosen by the polymerase independent of the template (Kuo et al., 1997; Noton et al., 2010).
RNA synthesis is carried out by the large L polymerase protein, which also performs mRNA capping (Liuzzi et al., 2005) and polyadenylation. The actual template is the viral ribonucleoprotein (RNP) or nucleocapsid, a complex of viral genomic or antigenomic RNA tightly and completely bound by N protein. The tight encapsidation of the viral genome (and antigenome) is characteristic of Mononegavirales. This likely prevents degradation of these RNAs, which lack the stabilizing features of a 5´ cap and 3´ poly A. It also is thought that encapsidation minimizes detection of these RNAs by pattern recognition receptors in the host cell, in particular (i) the cytoplasmic helicases RIG-I and MDA-5, which detect cytoplasmic tri-phosphorylated RNA and dsRNA and initiate signal transduction to activate the cellular transcription factors IRF3 and NFκB involved in inducing type I interferon (IFN) and pro-inflammatory cytokines, and (ii) dsRNA-activated protein kinase R (PKR), which also activates NFκB as well as phosphorylating eukaryotic elongation factor 2a (eIF-2a) to inhibit protein synthesis as part of antiviral defense.
A crystal structure was recently described for the RSV N protein expressed in bacteria and isolated as a decamer ring in association with bacterial RNA (Tawar et al., 2009). This ring likely mimics one turn of the helical RNP. Each N monomer was organized as a core region containing two domains, N- and C-terminal, which were connected through a hinge region that was associated with seven nucleotides of the bound RNA. The arrangement of the nucleotides relative to the protein suggested that passage of the polymerase induces a transient local conformational change that allows access to the RNA template. The N-terminal domain of N has a long β hairpin projecting away from the core that is not present in the 3D structure of other Mononegavirales N proteins and may be a site of contact with the L polymerase. The structure also indicated that the C-terminus of the N protein extends above the plane of the ring, thus being available for interaction with the P protein during RNA synthesis.
The P protein is a homotetramer which interacts with N (Garcia-Barreno et al., 1996), L (Khattar et al., 2001), and M2-1 (Asenjo et al., 2006) and which is an essential co-factor of the polymerase. Although much shorter than other Paramyxoviridae counterparts, RSV P has similar activities. The C-terminus of P interacts with the C-terminus of N to open the RNP structure so that the polymerase, tethered by P, can reach the bases in the viral RNA. In addition, P interacts with newly synthesized N (N°) to prevent illegitimate assembly of the latter and to deliver it to the nascent chain during genome replication (Castagne et al., 2004; Curran et al., 1995). Promoter clearance and chain elongation by the viral polymerase during transcription appears to be dependent on the P protein (Dupuy et al., 1999) and on capping of the nascent transcript (Liuzzi et al., 2005).
M2-1 and M2-2 are novel RNA synthesis factors. M2-1 is a transcription processivity factor: in its absence, transcription terminates prematurely and non-specifically within several hundred nucleotides (Collins et al., 1996; Fearns and Collins, 1999b). M2-1 also enhances read-through transcription at gene junctions to generate polycistronic RNAs (Hardy and Wertz, 1998), which may reflect the same processivity activity. M2-1 is a homotetramer that binds to the P protein and RNA in a competitive manner, suggesting that P associates with soluble M2-1 and delivers it to the RNA template (Tran et al., 2009). M2-1 contains a zinc finger motif that appears to be related to the cellular zinc finger protein tristetraprolin (Hardy and Wertz, 2000). Tristetraprolin binds cellular mRNAs and affects mRNA stability, but the significance of this similarity remains unclear. The other factor involved in RNA synthesis, the M2-2 protein, is a small, non-abundant species that accumulates during infection and appears to shift RNA synthesis from transcription to RNA replication (Bermingham and Collins, 1999).
The non-structural NS1 and NS2 accessory proteins also may affect RNA synthesis, since they inhibited transcription and RNA replication by a mini-replicon (Atreya et al., 1998). Other paramyxovirus accessory proteins also have been shown to down-regulate viral RNA synthesis, and recent studies with Sendai virus and HPIV1 indicate that preventing overly robust RNA synthesis avoids the accumulation of unencapsidated genomes and dsRNA that would otherwise be recognized by RIG-I, MDA-5, and PKR (Boonyaratanakornkit et al., 2011; Takeuchi et al., 2008). Therefore, part of the inhibition of IFN induction mediated by NS1 and NS2 may be related to this apparent capacity to down-regulate viral RNA synthesis.
The M protein is thought to line the inner surface of the viral envelope. M appears to play a central role in budding (Henderson et al., 2002; Teng and Collins, 1998) and may also silence viral RNA synthesis in preparation for packaging (Ghildyal et al., 2003). A crystal structure was recently determined for the RSV M protein and revealed a monomer that is organized into compact N-terminal and C-terminal domains joined by a short linker (Money et al., 2009). The surface of M contains a large positively charged region that extends across both of the domains and the linker and may mediate association with the plasma membrane and RNP.
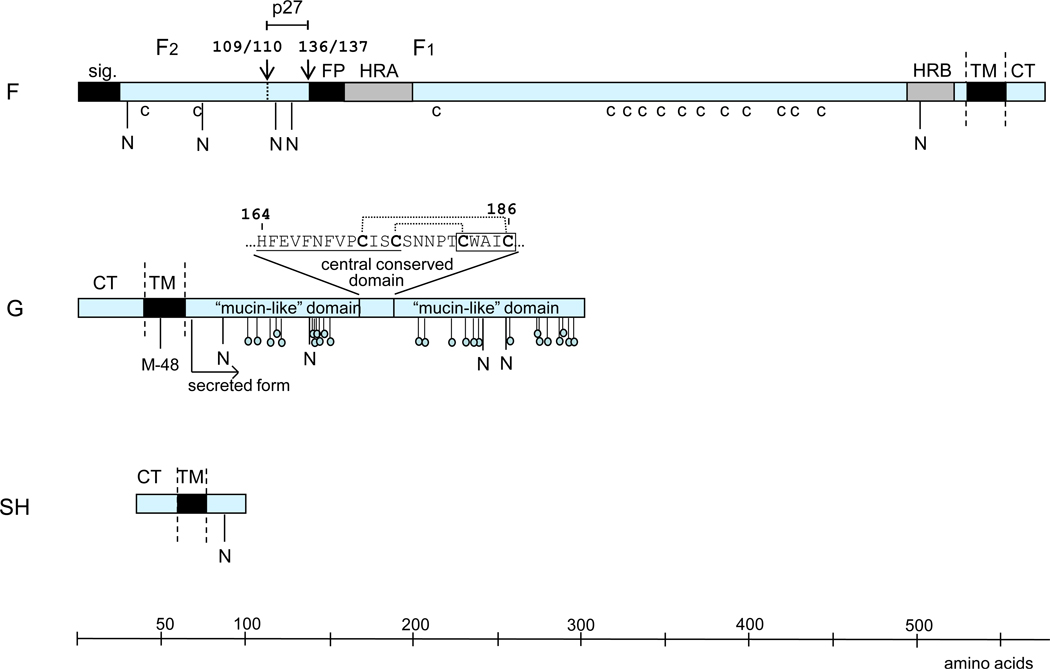
Progress also has been made in further characterizing the RSV glycoproteins (Fig. 2). The RSV F protein is generally similar in overall structure and function to its counterparts in Paramyxovirinae despite extensive sequence divergence. Like these counterparts, RSV F mediates viral penetration as well as fusion between infected cells and their neighbors. The RSV F protein also activates toll-like receptor (TLR)4 on human leukocytes independent of virus replication to initiate innate immune responses (Kurt-Jones et al., 2000). The RSV F0 precursor is cleaved at two closely spaced sites (instead of one for other paramyxoviruses) by a furin-like enzyme to generate the F1 and F2 chains (F2 N-terminal to F1) and become fusion competent (Gonzalez-Reyes et al., 2001; Zimmer et al., 2001). In addition, the RSV F protein efficiently mediates fusion on its own, whereas fusion by members of Paramyxovirinae usually requires cooperative interaction between F and the cognate attachment protein. Recently, insertion of the two RSV cleavage sites into the F protein of recombinant Sendai virus resulted in a hyperfusogenic phenotype with reduced thermostability of the virus, decreased dependence on the attachment (HN) protein for fusion, and decreased dependence on sialic acid for attachment (Rawling et al., 2011; Rawling et al., 2008). Thus, several notable properties of RSV may be linked to the unusual double-cleavage sequence of its F protein.
Figure 2.
The surface glycoproteins of RSV (strain A2), drawn approximately to scale. Hydrophobic domains are shown as black boxes (sig., signal peptide; FP, fusion peptide; TM, transmembrane anchor). Heptad repeats (HRA and HRB) in F are in grey. CT: cytoplasmic tail. The cleavage sites in F are indicated with downward facing arrows and identified by amino acid position. Cysteine residues in F that are conserved among human pneumoviruses are indicated (c). Potential acceptor sites for N-linked carbohydrate are indicated as downward facing stalks with N. The 25 potential acceptor sites for O-linked sugars in G that are predicted by NetOGlyc 2.0 to be the most likely to be utilized are indicated as downward facing stalks with small circles. The expanded sequence above G protein shows the conserved 13-amino acid segment (underlined) and cystine noose; cysteine residues are bold; the disulfide bonding pattern is indicated by dotted lines (Gorman et al., 1997); and the fractalkine CX3C motif is boxed. M-48 in the HRSV G protein is the translational start site for the secreted form, and the mature secreted form is indicated (Roberts et al., 1994).
As with other Paramyxoviridae, the RSV F protein is present in the virus particle in a metastable pre-fusion structure (Lamb, 1993). Binding with the target cell triggers a series of conformational changes in the F protein including the formation of a pre-hairpin intermediate, in which the hydrophobic fusion peptide at the N-terminus of the F1 chain is inserted into the target membrane. Refolding of this intermediate results in the assembly of a highly stable post-fusion structure dictated mostly by formation of a six-helix bundle (6HB) containing heptad repeats A and B (HRA and HRB) from each monomer (Zhao et al., 2000). Such an arrangement places the fusion peptide and the transmembrane region of the F1 chain at the same end of the F protein with the consequent merging and fusion of the viral and cell membranes. The free-energy released on 6HB formation drives the membrane fusion process (Russell et al., 2001).
Two different groups have solved recently the atomic structure of the RSV F protein ectodomain in its post-fusion conformation based on analysis of a version of the protein that was engineered to remove the fusion peptide and the transmembrane domain and cytoplasmic tail (McLellan et al., 2011; Swanson et al., 2011). As predicted, the structure is similar to those described for the HPIV3 (Yin et al., 2005) and Newcastle disease virus (Swanson et al., 2010) F proteins, with a cone shape that resembles the images obtained by electron microscopy of a soluble RSV F protein ectodomain preparation (Calder et al., 2000). Significantly, the local structures of epitopes recognized by neutralizing MAbs, such as have recently been visualized in complexes of Fab-peptides (McLellan et al., 2010a; McLellan et al., 2010b), are present in the post-fusion structure as well as in a predicted pre-fusion conformation that was modeled from the equivalent structure from PIV5 (Yin et al., 2006). In agreement with this hypothesis, it was recently shown that several MAbs could bind and immunoprecipitate a pre-fusion form of the RSV F protein (Chaiwatpongsakorn et al., 2011), including the antibody precursor of palivizumab that is also able to bind to post-fusion F (McLellan et al., 2011). Conservation of these epitopes in the two otherwise dissimilar structures can explain the recent finding that MAbs able to bind to the post-fusion form of RSV F can neutralize virus infectivity if pre-incubated with the virus before addition to cells; i.e., before the F protein is activated for fusion (Magro et al., 2010). It has been proposed that these MAbs bind to the pre-fusion conformation of RSV F and inhibit the subsequent conformational changes required for membrane fusion.
Recent findings indicate that the HRSV F protein also plays a major role in viral attachment, involving interaction with the cellular protein nucleolin (Tayyari et al., 2011). This interaction was shown to be necessary for efficient infection in cell culture and in the mouse model. Previously, the G protein had been identified as playing a major role in RSV attachment (Levine et al., 1987). G indeed is essential for efficient replication in vivo, although virus lacking the G gene can replicate efficiently in Vero cells in vitro (Karron et al., 1997a; Techaarpornkul et al., 2001; Techaarpornkul et al., 2002; Teng and Collins, 1998; Teng et al., 2001). Both G and F had been shown to bind to glycosaminoglycans (GAGs), which are long unbranched chains of repeating disaccharide subunits that are part of the host cell glycocalyx (Feldman et al., 1999), and binding to GAGs is necessary for efficient infection of cell lines by RSV. Taken together, these observations suggest that efficient attachment by RSV in vivo depends on both F and G, and may depend on two different binding events involving GAGs and nucleolin.
RSV G is an unusual protein. Its ectodomain consists mainly of two large “mucin-like” domains, so-named because, like cellular mucins, they are rich in proline, serine, and threonine residues, contain several N-linked and many O-linked sugar side chains, and may have extended, unfolded secondary structures (Johnson et al., 1987b; Wertz et al., 1985). The carbohydrate content of the G protein is considerable, shifting the apparent molecular weight of the polypeptide backbone from 32,000 to 90,000 (Wertz et al., 1989). More recently, analysis of G protein produced in an in vitro model of human airway epithelium (HAE) – a pseudostratified mucocilliary tissue that closely resembles the authentic epithelium (Zhang et al., 2002) – suggested that the carbohydrate content is much higher, yielding an apparent molecular weight of 180,000 (Kwilas et al., 2009). It is speculated that this sheath of host-specified carbohydrate helps shield the protein from immune recognition. Whether due to carbohydrate, or its unfolded structure, or some other reason, G is a less efficient neutralization and protective antigen compared to F (Olmsted et al., 1986), and most individual MAbs against G do not neutralize infectivity (Martinez et al., 1997). However, mixtures of MAbs directed against non-overlapping epitopes of G have a synergistic effect on virus neutralization, suggesting that steric hindrance of binding to the cell surface may be the basic mechanism by which serum polyclonal anti-G antibodies inhibit RSV infectivity (Martinez and Melero, 1998). Recently, high-affinity anti-G antibodies were obtained by cloning mRNAs obtained from rare human B cells (Collarini et al., 2009). These antibodies were highly neutralizing in vitro and may use a still-uncharacterized mechanism to inhibit virus infectivity different from those of other more common anti-G antibodies.
The two mucin-like domains in G are highly variable and contain multiple epitopes that are poorly conserved between strains. These two domains are separated by a central region to which conserved epitopes have been mapped (Martinez et al., 1997). This conserved region includes a segment of 13 highly conserved amino acids that overlaps with a segment containing four closely spaced, invariant cysteine residues that are disulfide-bonded to form a cystine noose (Gorman et al., 1997; Johnson et al., 1987b). The downstream pair of cysteine residues conform to a CX3C motif that is embedded in a region of limited sequence relatedness to the CXC3 chemokine fractalkine, and a peptide containing this sequence mimicked the leukocyte attractant activity of fractalkine in an in vitro assay (Tripp et al., 2001). Studies in the mouse model comparing wild-type RSV with a mutant lacking the CX3C motif showed that fractalkine mimicry reduces the pulmonary influx of immune cells involved in innate and adaptive responses to infection (Harcourt et al., 2006). In addition, the cysteine-rich domain of G was shown to inhibit activation of TLR2, 4, and 9 in human monocytes, thus suppressing innate immune responses (Polack et al., 2005). G also is expressed in an abundant secreted form due to use of a secondary translational initiation codon (Hendricks et al., 1988; Roberts et al., 1994). Recent findings showed that this secreted form functions as an antigen decoy to help the virus escape neutralizing antibodies, and also acts to reduce antibody-mediated clearance by immune cells (Bukreyev et al., 2008). A comparable secreted antigen is not found in other common respiratory viruses and may contribute to the ability of RSV to infect unusually early in life and re-infect throughout life despite the presence of antibodies from maternal transfer or from previous infection.
RSV encodes a third, short transmembrane glycoprotein, SH, that (like G) is anchored into the membrane by a hydrophobic signal-anchor sequence near the N-terminus, with the C-terminus oriented extracellularly (Collins and Mottet, 1993). SH accumulates in multiple forms due to the use of two translational start sites and the variable presence of N-linked sugar and polylactosaminoglycan, although the significance of the multiple forms is unknown (Anderson et al., 1992; Olmsted and Collins, 1989). The SH protein shares structural features with a class of small hydrophobic proteins, the viroporins (Gonzalez and Carrasco, 2003), that insert into the membrane of infected cells and induce permeability to ions and small molecules. In agreement with this hypothesis, SH increases membrane permeability when expressed in bacteria (Perez et al., 1997), and when incorporated into artificial membranes it forms pentameric and hexameric pore-like structures with cation channel-like activity (Carter et al., 2010; Gan et al., 2008). The SH protein was reported to reduce apoptosis and to inhibit tumor necrosis factor (TNF)-α expression and signaling, but the effects seemed small (Fuentes et al., 2007). Deletion of SH resulted in a virus that was slightly attenuated in mice and chimpanzees (Bukreyev et al., 1997; Whitehead et al., 1999), but deletion of SH from an experimental vaccine candidate did not increase its attenuation in seronegative children (Karron et al., 2005).
The NS1 and NS2 proteins interfere with both the induction of IFN-β and subsequent signaling from the IFN receptor. The NS1 protein had the greater effect on inhibiting IFN induction, although inhibition was greatest with both proteins together (Spann et al., 2004). NS1 and NS2 may form functional heteromers. These proteins impede the signal transduction pathway leading to up-regulation of IFN-β at multiple steps: NS2 binds to the cytoplasmic receptor RIG-I (Ling et al., 2009); either protein (and especially NS1) mediates a decrease in TRAF3 (Swedan et al., 2009); and NS1 mediates a decrease in IKKε (Swedan et al., 2009). The NS proteins also strongly suppress IFN-induced signal transduction from its receptor through the JAK/S TAT pathway (Ramaswamy et al., 2004). This suppression is mediated by proteosomal degradation of the STAT2 signal transduction factor (Ramaswamy et al., 2004). This is due primarily to NS2 (Lo et al., 2005; Ramaswamy et al., 2006; Swedan et al., 2009), although there also is evidence that NS1 can assemble ubiquitin ligase enzymes to target STAT2 for the proteosome (Elliott et al., 2007a). RSV also inhibits induction of type III IFN in human epithelial cells and macrophages (Spann et al., 2004), which is thought to involve the same signaling pathway (Donnelly and Kotenko, 2010). Type III IFN uses a different receptor, but the downstream signaling depends on STAT1 and STAT2 (Donnelly and Kotenko, 2010; Mordstein et al., 2010) and thus should similarly be suppressed by the RSV NS proteins. NS1 and NS2 also activate pro-survival pathways in the infected cell, prolonging its survival and increasing viral yield (Bitko et al., 2007).
Recent studies have revealed additional, novel ways that RSV uses to prevent activation of cellular defense in response to infection. For example, the RSV N protein was shown to bind to PKR, preventing it from phosphorylating eIF-2a, and presumably also from activating NFκB (Groskreutz et al., 2010). In another study, RSV was shown to block the formation of stress granules, which can otherwise restrict RSV replication, via expression of RNA derived from the trailer region (Hanley et al., 2010). RSV infection also was recently shown to result in degradation of the outer mitochondrial membrane-associated adaptor MAVS, which is in the signal transduction pathways leading to activation of IRF3 and NFκB (Yoboua et al., 2010). Thus, RSV has multiple means of inhibiting IFN induction and signaling, the induction of pro-inflammatory cytokines, the induction of apoptosis, and other aspects of cellular defense against infection. At least in the case of type I IFN, the inhibition exerted by RSV appears to be more effective than that of HMPV, HPIVs, and influenza virus (Guerrero-Plata et al., 2005a; Guerrero-Plata et al., 2005b; Hall et al., 1981; Hall et al., 1978; McIntosh, 1978). This likely contributes to the high infectivity of RSV, and may also reduce immune responses by precluding the adjuvant effects of IFN (Valarcher et al., 2003).
3. Epidemiology and Evolution
RSV is a highly contagious virus that can infect an individual multiple times throughout life. Human are the only natural host for RSV, although the virus can readily infect and sometimes cause severe disease in non-human primates (Kondgen et al., 2008; Morris et al., 1956). RSV infections occur mostly in yearly epidemic outbreaks during the winter months in temperature countries or during the rainy season in tropical countries. Most children are infected by RSV during the first year of life and virtually all are infected by the age of two (Glezen et al., 1986). Re-infection is frequent during the first few years of life: in one prospective study, of the children who had been infected during the first year of life, 47% and 45% were re-infected during the second and third years of life, respectively (Glezen et al., 1986). Re-infection does not depend on antigenic differences. LRI can occur during the first or second infections, but there was a considerable reduction in disease severity in subsequent infections that presumably reflects increasing protective immunity (Henderson et al., 1979). Hospitalization for severe HRSV disease is most frequent between 6 weeks and 6 months of life, with a peak incidence at 2–3 months of life.
A recently published large prospective study in the United States provided a detailed evaluation of the pediatric impact of RSV in an affluent country. This study estimated that 2.1 million children under the age of five require medical attention each year due to RSV, resulting annually in 1,534,064 (1 of 13 for that age group) office visits, 517,747 (1 of 38) visits to the emergency room, and 57,527 hospitalizations (Hall et al., 2009). Sixty-one percent of the office visits were in children of 2 to 5 years of age: this showed that, while the impact of RSV disease is greatest during the first year of life, RSV also causes a substantial disease burden in children beyond this first year (Hall et al., 2009).
Another new study estimated that, globally, RSV caused almost 34 million LRI in children under 5 years of age in 2005, ten percent of them requiring hospitalization (Nair et al., 2010). This resulted in an estimated 66,000–199,000 deaths, with 99% of these deaths occurring in developing countries, reflecting both a greater population of infants and young children and the unjust consequences of inadequate resources. These values for morbidity and mortality probably are under-estimates, since RSV testing was incomplete and community LRI undercounted.
In affluent countries, deaths due to RSV appear to have declined in recent years. A survey from the United Kingdom in the mid 1970s estimated the fatality rate at 0.5% to 2.5% of hospitalized children with RSV infection (Clarke, 1978), and in 1985 RSV was estimated to be responsible for 4,500 pediatric deaths annually in the United States (Meissner, 1994). In contrast, more recent estimates are as low as 0.3% of hospitalized children. In the United States, the pediatric mortality rate was recently estimated to be 5.3 and 0.9 per 100,000 per year for individuals < 1 year of age and 1–4 years of age, respectively, totaling approximately 300 deaths per year (Thompson et al., 2003). This likely reflects improvement in supportive care.
A number of factors can increase the risk for severe RSV disease early in life, including prematurity, low titers of maternal antibodies or lack of previous RSV infection (Berkovich, 1964; Cunningham et al., 1991), underlying cardiopulmonary disease (Groothuis et al., 1988; MacDonald et al., 1982), and immunosuppression or immunodeficiency disorders (Fishaut et al., 1980; McIntosh et al., 1984). In one study, the estimated number of RSV hospitalizations per 1000 during the first year of life was 388 for infants with chronic lung disease, 92 for those with congenital heart disease, 66 for those born at 29 to <33 weeks, and 30 for term infants with no underlying disease (Boyce et al., 2000). However, it is important to note that more than half of RSV hospitalizations occur in previously healthy, full term individuals. A number of recent studies have described associations between genetic polymorphisms involving host defense genes and severe pediatric RSV disease (Miyairi and DeVincenzo, 2008). Among other findings, these studies have implicated polymorphisms in pulmonary surfactant proteins, or associated with reduced activation of TLR4, or associated with increased expression of the IL-4 or IL-8 gene, or associated with other innate immune response factors (El Saleeby et al., 2010; Hacking et al., 2004; Janssen et al., 2007; Lofgren et al., 2010; Miyairi and DeVincenzo, 2008; Puthothu et al., 2007; Schuurhof et al., 2010; Siezen et al., 2009). However, findings have often been inconsistent, probably because the studies have been insufficiently large to validate associations.
RSV re-infects healthy adults at a rate of approximately 5–10% per year, a rate that increases with increased exposure to the virus, such as with health care personnel (Falsey, 2007; Falsey et al., 2005). Hospitalization due to RSV in otherwise healthy non-elderly adults is rare, but RSV is considered to be second only to seasonal influenza as a cause of medically significant respiratory tract disease in adulthood. Morbidity and mortality due to RSV is substantially increased in the elderly, presumably due in part to immune senescence. RSV is estimated to cause on average 17,358 deaths annually in the United States, with 78% of these deaths in adults over age 65 (Thompson et al., 2003). Thus, in more affluent countries, deaths due to RSV are much more frequent in the elderly than in the pediatric population, whereas in less affluent countries the pediatric burden is likely to be greater.
Morbidity and mortality due to RSV also is substantially increased in adults with underlying pulmonary or cardiac disease or who are severely immunosuppressed, especially with T cell deficiencies (Falsey, 2007; Falsey et al., 2005; Whimbey and Ghosh, 2000). In particular, the mortality rate associated with severe RSV infection in adults with profound immunosuppression due to leukemia or hematopoietic stem cell transplant can be as high as 80–100% (Whimbey and Ghosh, 2000).
Human RSV isolates are classified into two antigenic groups (A and B) (Mufson et al., 1985) that represent separate genetic lineages who divergence was recently calculated to have occurred approximately 350 years ago (Zlateva et al., 2005). The highest sequence divergence between the two groups is found in the G protein ectodomain and particularly in the two mucin-like regions mentioned before (Johnson et al., 1987a; Johnson et al., 1987b). Multiple genotypes within subgroups A and B have been identified that can co-circulate within the same season and community, with one or two dominant genotypes being replaced in successive years (Cane, 2001). In addition, there can be shifts in the predominance of subgroup A versus B occurring in 1- or 2-year cycles (Waris, 1991). This reflects a modest advantage of the heterologous strain in evading previously-induced immunity (White et al., 2005), but re-infection by the same subgroup also is very frequent.
Accumulation of genetic and antigenic changes with time within the same evolutionary branch, as well as identification of sites of positive selection which coincide partially with epitopes recognized by anti-G MAbs are suggestive of immune driven RSV evolution (Botosso et al., 2009). However, the RSV evolutionary pattern does not resemble that of influenza A virus, in which a strong immune selection by prevailing antibodies dictates the fast replacement worldwide of dominant strains in a linear manner. Instead, RSV evolution resembles that of influenza B viruses, in which a less strong immune selection favors slower co-evolution of several branches (Rota et al., 1992). This is exemplified by the recently described BA genotype within the antigenic group B of human RSV. These viruses that contain a 60-nucleotide duplication in the G gene were first identified in 1998. Although they spread very quickly worldwide, it was not until 2005 that they replaced other prevailing genotypes of RSV group B (Trento et al., 2010).
In apparent contradiction to the weak selection exerted by anti-G antibodies, the G protein is one of the most variable gene products found in any human virus. It is possible that this variability simply reflects the extreme plasticity of G to accommodate not only amino acid substitutions but in addition other more drastic sequence changes, such as insertions, deletions, frame-shift mutations and premature stop codons (Melero et al., 1997). The extensive variability of the G protein contrasts with the high level of sequence conservation of the F protein, including epitopes that are recognized by highly efficient neutralizing antibodies. It may be that structural restrictions limit the antigenic diversity of F; consistent with this, a number of escape mutants selected with F MAbs exhibited reduced fitness, although one actually had an apparent increase in fitness (Zhao et al., 2006; Zhu et al., 2011).
4. Protective immunity
A wealth of evidence indicates that protection against RSV infection is conferred mainly by neutralizing antibodies. The F and G glycoproteins are the only viral antigens able to induce neutralizing antibodies as well as relatively long-lived protection in animal models (Connors et al., 1991; Stott et al., 1987). Passive transfer of these antibodies (Graham et al., 1993; Prince et al., 1985a) or MAbs directed against F or G (Taylor et al., 1984; Walsh et al., 1984) protects mice and cotton rats against RSV challenge. Likewise, infants at high risk of severe RSV disease can be substantially protected by the prophylactic administration of RSV-neutralizing polyclonal or monoclonal antibodies, as discussed later (see section 7. Immunoprophylaxis with antibodies). Furthermore, a positive correlation has been found between high titres of serum neutralizing antibodies and protection of human volunteers against RSV challenge (Hall et al., 1991), and there is an inverse correlation between the titres of RSV-neutralizing serum antibodies and the risk of infection in children (Glezen et al., 1986) and in the elderly (Falsey and Walsh, 1998). Local secretory antibodies are thought to be particularly efficient in restricting RSV replication but generally decrease with time. Serum antibody responses are more durable, at least outside of infancy. However, serum antibodies gain access to the respiratory tract primarily by the inefficient process of passive transudation. This results in a steep concentration gradient, especially in the upper respiratory tract. For example, in the cotton rat model, RSV-neutralizing serum antibody titers of 1:390 and 1:3500 were required for a 99% reduction in RSV replication in the lower and upper respiratory tract, respectively (Prince et al., 1985b; Siber et al., 1994). In humans, there was a 1:350 concentration gradient between nasal washes and sera for influenza A virus antibodies (Wagner et al., 1987), which likely is similar for RSV. As a consequence, relatively high titers of serum antibodies are needed to confer protection in the respiratory tract.
Following RSV infection, there is an initial influx of NK cells to the site of infection that produce IFNγ and are cytotoxic to virus-infected cells. This is followed by recruitment of helper CD4+ and cytotoxic CD8+ lymphocytes to the site of infection. IFNγ enhances the differentiation of CD8+ lymphocytes and influences the differentiation of CD4+ lymphocytes that contribute to the generation and amplification of the humoral and cellular immune responses. CD8+ T cells also help regulate T cell and inflammatory responses by mechanisms that initially were thought to involve IFNγ but now appear to be more complex and are incompletely understood (Olson et al., 2008; Olson and Varga, 2007; Stevens et al., 2009). Recent studies in mice showed that macrophages provide an immediate response of pro-inflammatory cytokines following RSV infection (Pribul et al., 2008), and are a major producer of type I IFN (Kumagai et al., 2007). Macrophages appear to be important both in restricting the virus and in clearing debris later in infection that otherwise can promote further damage and inflammation (Reed et al., 2008). Other features of innate immunity also may contribute to restricting RSV infection and replication, such as surfactant proteins (Glasser et al., 2009; LeVine et al., 2004) and potentially eosinophils (Phipps et al., 2007) and neutrophils (Lukens et al., 2010).
Although antibodies are important for resistance to infection, T cell responses are probably of greater importance for virus clearance. For example, individuals with compromised T cell immunity can shed virus for months (Hall et al., 1986), and prolonged virus shedding is also observed in nude or irradiated BALB/c mice (Cannon et al., 1987) and in mice depleted of both CD4+ and CD8+ cells (Graham et al., 1991b). In mice, RSV-specific CD8+ T cells also provide protection against infection, but the effect is short-lived (Connors et al., 1991; Kulkarni et al., 1995).
Immune responses usually are markedly reduced during infancy compared to older individuals, which is particularly relevant to RSV given its high rate of infection during this period. For example, the titers of RSV-specific serum antibodies induced by primary RSV infection were 8- to 10-fold lower in individuals of 4–8 months of age compared to those of 9–21 months of age (Murphy et al., 1986). Antibody responses in young infants often are poorly neutralizing and are short-lived compared to older individuals: post-infection increases in RSV-specific IgA and IgG responses in infants were found to be greatly reduced one year later (Welliver et al., 1980). One factor in the reduced immune responses early in life is immunologic immaturity, which limits innate, antibody, and cellular responses (Adkins et al., 2004; Levy, 2007). For example, the B cell response to RSV infection of young infants <3 months of age was found to have a biased antibody gene repertoire and a greatly reduced frequency of somatic mutations compared to older individuals, which likely contributes to the poor neutralizing activity characteristic of responses in young infants (Williams et al., 2009). A second factor is immunosuppression by RSV-specific maternal serum antibodies that typically are present in young infants. In experimental animals, passive serum antibodies were shown to suppress both serum and secretory antibody responses to RSV infection, but did not suppress the cell-mediated response or priming for a secondary antibody response (Crowe et al., 2001; Murphy et al., 1988). A recent study examined a cohort of infants of <6 months of age with very low titers of RSV-specific maternal antibodies and found that the titers of RSV-neutralizing serum antibodies induced by primary RSV infection were indistinguishable from those of individuals of 6–24 months of age (Shinoff et al., 2008). This showed that, when the immunosuppressive effect of maternal antibodies is minimal, wild type RSV can induce a substantial antibody response even in young infants.
The observation that RSV can reinfect symptomatically without need for significant antigenic change is widely interpreted as evidence that there are long-term deficiences in protective immunity to RSV beyond the reduced responses that are characteristic of infancy. One possibility is that exposure to RSV antigen in the context of immunologic immaturity and maternal antibody-mediated immunosuppression may have long-term deleterious effects on protective responses. There indeed is evidence from the mouse model that exposure to RSV very early in life results in skewed responses during primary and secondary infection (Culley et al., 2002; Tasker et al., 2008). In addition, viral mechanisms might substantially inhibit the induction of protective immunity. For example, as already noted, the CX3C fractalkine motif in the G protein was shown to inhibit cellular responses to RSV infection in the mouse model (Harcourt et al., 2006), and the cysteine-rich domain of G was shown to reduce activation of TLR2, TLR4, and TLR9 in human cells (Polack et al., 2005). Other studies showed that the RSV NS1 protein partly suppresses human dendritic cell (DC) maturation and skews CD4+ and CD8+ T cell activation (Munir et al., 2011; Munir et al., 2008), and that RSV infection of human DC does not efficiently induce up-regulation of the chemokine receptor CCR7 necessary for DC migration to secondary lymphatic tissue (Le Nouen et al., 2011). RSV-infected DC also have been reported to direct reduced activation and altered polarization of CD4+ T lymphocytes in vitro (de Graaff et al., 2005; Guerrero-Plata et al., 2006; Rothoeft et al., 2007), although other results suggest that RSV does not differ significantly from HMPV, HPIV3, and influenza A virus in this regard (Le Nouen et al., 2010; Le Nouen et al., 2009). HRSV also can suppress T cell proliferative responses in vitro by direct contact mediated by the F protein (Schlender et al., 2002). Also, as noted, RSV appears to inhibit the host IFN system more efficiently than influenza and parainfluenza viruses.
However, these various mechanisms notwithstanding, it is not clear that protective immunity to RSV indeed is markedly deficient or inhibited – apart from the reduced responses in infancy as already noted. Primary infection of mice and cotton rats with HRSV induces robust antibody and cellular immune responses and long-lived protective immunity (Graham et al., 1991a; Prince et al., 1983). Infection of seronegative chimpanzees with wild-type HRSV or attenuated live vaccine candidates induced very robust serum antibody titers and protection (Crowe et al., 1994; Teng et al., 2000; Whitehead et al., 1999; Whitehead et al., 1998b). The titers of RSV-neutralizing antibodies in human adults also are quite high, giving no evidence of a deficient response (Falsey et al., 2006). As noted, RSV infection of young infants with very low maternal antibody titers resulted in very robust responses (Shinoff et al., 2008). Most importantly, re-infection with RSV usually is associated with substantially reduced disease, indicating that prior infections induce substantial protection.
Re-infection may be aided by features that help RSV evade, rather than directly inhibit, host defences. Several factors that allow RSV to evade host defense have already been noted, including the secreted form of the G protein (Bukreyev et al., 2008), the glycoprotein sheath of the G protein, and the presence of two antigenic subgroups (Waris, 1991). Other factors in evasion include the tropism of the virus for the superficial layer of the respiratory epithelium and its relatively non-invasive nature (described in the next section). This may delay and reduce the exposure of viral antigen to the host immune system. As noted, the lumen of the respiratory tract is poorly accessed by serum antibodies, sharply reducing their ability to restrict viral replication. In addition, recent studies in mice indicate that CD8+ cytotoxic T lymphocytes are functionally down-regulated in the lung, which may be a host mechanism to reduce tissue damage but would reduce immune protection (DiNapoli et al., 2008; Vallbracht et al., 2006). Thus, host and viral factors contribute to reduce the efficiency of immune control of RSV.
5. Pathogenesis
Disease manifestations during primary RSV infection vary widely among individuals, including URI, fever, otitis media, LRI that can vary widely from mild manifestations to life-threatening bronchiolitis and pneumonia, death in rare cases, post infection abnormalities in respiratory function that can persist through adolescence, and possible sensitization to asthma. Numerous host and viral factors have been suggested to be involved in RSV disease, but their roles remain controversial and likely vary in different individuals (Collins and Graham, 2008).
Some reports have suggested that RSV subgroup A or a particular genotype of subgroup A may be associated with greater clinical disease, but other studies have not found such a link (Brandenburg et al., 2000b; Martinello et al., 2002; Smyth et al., 2002). However, a subgroup A strain called line 19 recently was shown to induce enhanced production of IL-13 and mucus in mice compared to other strains, an effect that mapped to the F gene (Moore et al., 2009). It is possible that further studies will identify clinically relevant differences in RSV strains.
It is thought that RSV disease arises from both direct viral damage and the host immune response, but the relative contributions remain controversial. It generally is thought that there is a positive correlation between the level of virus replication and disease severity (DeVincenzo et al., 2010; DeVincenzo et al., 2005; Karron et al., 1997b; Martin et al., 2008), although this was not observed in some studies (Bennett et al., 2007; Hall et al., 1976; Wright et al., 2002). However, the extent to which this reflects increased viral damage versus an increased immune response is unclear.
Studies using in vitro models of adenoid or HAE epithelium showed that RSV infection is limited to the superficial ciliated cells and did not induce syncytia or invade underlying cells (Tristram et al., 1998; Wright et al., 2005; Zhang et al., 2002), which is consistent with histopathologic findings (Aherne et al., 1970; Johnson et al., 2007; Welliver et al., 2007). In the in vitro models, during an infection of several weeks, there was little visible damage to the tissue except that ciliary beating usually was impaired, in contrast to the rapid tissue destruction observed with influenza A virus (Wright et al., 2005; Zhang et al., 2002). This suggests that RSV is not inherently a highly cytopathic virus, although effects on ciliary function would facilitate the airway obstruction that is characteristic of RSV disease. In RSV-infected cotton rats, antiviral therapy that rapidly reduced virus replication had little effect on lung histopathology, whereas treatment with anti-inflammatory glucocorticoids reduced disease even though it interfered with clearance of the virus (Prince et al., 2000). This implied that disease was largely immune-mediated. However, the situation in humans is less clear. Treatment of RSV-infected humans with antiviral antibodies or drugs that reduce virus replication provided no improvement or limited improvement (Lagos et al., 2009; Malley et al., 1998; Rodriguez et al., 1994). However, treatment with anti-inflammatory corticosteroids alone also does not significantly reduce RSV disease (Krilov, 2011; Patel et al., 2004).
There is abundant evidence of inflammatory cytokines and activated granulocytes in the airways of infants and children with severe RSV disease, with neutrophils being by far the most abundant immune cell (Abu-Harb et al., 1999; Everard et al., 1994; Garofalo et al., 1992; McNamara et al., 2004; McNamara et al., 2003; Rosenberg and Domachowske, 2001; Smyth et al., 2002). A recent study of infants hospitalized for severe RSV disease showed that the appearance of neutrophil precursors in the peripheral blood, which precedes their influx into the lungs, closely followed the peak of virus shedding and was coincident with clinical symptoms, implying possible roles both in protection and disease (Lukens et al., 2010). However, while RSV may indeed induce a strong inflammatory response, the extent to which this is pathogenic versus protective remains unclear (Bennett et al., 2007; Laham et al., 2004; Sheeran et al., 1999).
Depletion studies in the mouse model of RSV infection indicated that the CD4+ and CD8+ T lymphocyte subsets contribute individually to clearing the infection, but that both also contribute to disease, especially CD8+ T cells (Graham et al., 1991b). This suggested that RSV disease might involve an overly robust CD8+ T cell response. Infants and children indeed have increases in CD8+ T cells in response to RSV infection, and a greater proportion of CD8+ T cells compared to individuals infected with rhinovirus (Everard et al., 1994; Heidema et al., 2007; Heidema et al., 2008; McNamara et al., 2003). However, recent studies indicated that the timing the CD8+ T cell response in hospitalized RSV-infected infants occurs substantially after that of peak clinical symptoms (Heidema et al., 2007; Lukens et al., 2010), and that the magnitude of the CD8+ T cell response did not correlate with disease severity (Heidema et al., 2007). Thus, CD8+ T lymphocytes may contribute to disease in some individuals, but do not seem to be a major determinant.
Th2 biased stimulation of CD4+ T lymphocytes also has been suggested to mediate RSV disease. The excessive production of mucus, airway plugging, wheezing, and long-lasting effects on lung function that are common manifestations of RSV disease have some similarity with asthma, which involves a Th2-bias. Also, immune responses in young infants can have a Th2 bias remaining from the prenatal period (Adkins et al., 2004). A positive association was found between RSV disease and a genetic polymorphism in the IL-4 gene that increases gene expression (high IL-4 levels are normally associated with a Th2-bias) (Miyairi and DeVincenzo, 2008). However, while some studies provided evidence of a Th-2 biased response in infants with severe RSV disease (Bendelja et al., 2000; Roman et al., 1997), other studies documented Th1 responses (Brandenburg et al., 2000a), or identified infants with either Th1 or Th2 responses (Lee et al., 2007; Mobbs et al., 2002), or found Th2-biased responses primarily in individuals with a history of asthma or allergy (Kim et al., 2003; Legg et al., 2003). Another study documented Th2 responses in infants ≤3 months of age in response to RSV, influenza virus, and HPIVs, suggesting that this is specific to young age rather than RSV (Kristjansson et al., 2005). Early studies suggested that infants who developed wheezing in response to RSV infection had increases in RSV-specific IgE, which is promoted by the Th2 cytokines IL-4 and IL-13 and causes the release of inflammatory mediators from mast cells (Welliver et al., 1981), but this was not observed by others (De Alarcon et al., 2001). Thus, Th2 responses may contribute to RSV disease in some individuals, but may not be a predominant determinant.
The high rate of RSV infection and disease before six months of age implies that young age is important in pathogenesis. Reduced immune responses in young infants, as already noted, reduce the ability to control infection. The Th2 bias of young infants may play a role, as noted. The small diameter of bronchioles in infants makes them particularly susceptible to obstruction by edema, secretions, and immune and exfoliated cells (Hogg et al., 1970). Infancy is a time of lung growth and development, which may increase susceptibility to viral disease and possible long-term effects on lung function (Gern et al., 2005). For example, infants with severe RSV infection express increased levels of neutrotropic factors and receptors (Tortorolo et al., 2005), which may be associated with a higher index of obstructive sleep apnea (Snow et al., 2009). Thus, the ability of RSV to infect and cause disease somewhat earlier in life than other respiratory viruses may be a factor in its greater contribution to human disease.
A number of studies have shown that RSV bronchiolitis or pneumonia early in life is frequently followed by lingering abnormalities in airway function, including recurrent wheezing, reduced oxygenation, and deficits in pulmonary function tests suggestive of peripheral airway obstruction (Hall et al., 1984; Long et al., 1995; Noble et al., 1997; Sigurs et al., 2010; Stein et al., 1999). These effects are rarely severe but can persist through adolescence and early adulthood. It is not clear to what extent these sequelae are caused by the viral infection per se or reflect underlying anatomical or functional abnormalities that existed before the infection and indeed may have contributed to its severity. A recent study provided evidence that prevention of severe RSV disease by the use of RSV-neutralizing antibody substantially reduced the risk of recurrent wheezing in children who lacked a predisposition to asthma, suggesting that RSV infection plays a causal role (Simoes et al., 2010). Conversely, other studies provided evidence for a role of pre-existing vulnerability (Castro-Rodriguez et al., 1999; Thomsen et al., 2009). Finally, some studies have suggested that pediatric RSV infection can result in allergic sensitization and the development of asthma (Sigurs et al., 2010; Sigurs et al., 2005), but other studies have not observed this link and it remains controversial.
6. Antivirals
Ribavirin, a nucleoside analogue that interferes with the replication of a number of RNA and DNA viruses, was the first drug licensed for treatment of RSV infection in humans. Despite exhibiting potent activity against RSV in tissue culture and experimental animals (Hruska et al., 1982), ribavirin use in the clinic is now very limited due to the lack of proven efficacy (Rodriguez et al., 1994), the difficulty of administration (i.e. usually by aerosol), and concerns for toxicity. Currently, ribavirin is not recommended for the routine treatment of RSV infection (2006). It does find use in treating RSV infection in high-risk individuals, sometimes in combination with intravenous RSV-neutralizing immunoglobulin and corticosteroids, but efficacy remains controversial.
A number of small molecule a nti-RSV compounds have been described over the last 15–20 years. Most of them have been discovered by screening of chemical libraries of natural products using classic virologic assays, modified for high throughput testing. Time-of-addition experiments have been used to disclose the step of the replication cycle blocked by the drugs, and selection of escape mutants followed by sequencing has uncovered the viral targets of some of these compounds. Several drugs have been tested in animal models, and a few of them are in the early stages of clinical trials.
Remarkably, many of the small molecule RSV inhibitors turned out to target the F protein. One of the reasons for this predominance may be that fusion of the viral and cell membranes occurs outside the cell and therefore inhibitors do not need to cross the membrane barrier. A series of benzimidazoles derivatives have been reported to interact with the F protein and, although dissimilar in structure, they all seem to bind to a hydrophobic pocket of the trimeric coiled-coil made by HRA (amino acids 153–209) of the F1 chain (Zhao et al., 2000). Binding to this pocket would interfere with the proper interaction of HRA with HRB (amino acids 482–520) and the formation of the 6HB that is required for completion of membrane fusion (Melikyan et al., 2000).
For example, it was shown that a radiolabelled analogue of the benzimidazole BMS-433771 containing a photoreactive diazirine group was covalently linked to Tyr198 of the F protein when photoactivated with UV light in the presence of virus (Cianci et al., 2004). Recently, the crystal structure of another benzimidazole derivative (TMC353121) bound to the 6HB formed by isolated sequences of HRA and HRB was solved (Roymans et al., 2010). TMC353121 was seen to interact with key residues of the hydrophobic cavity, including Tyr198, resulting in a stable but distorted 6HB. An important difference between TMC353121 and BMS-433771 was that the former required both HRA and HRB for binding whereas the latter could bind to peptides of HRA structured as trimeric coiled-coils in the absence of HRB. This difference may be due to the specific interactions that each drug makes with HRA and HRB residues.
A diverse array of other benzimidazoles (e.g., JNJ-2408068) and chemically unrelated compounds (e.g., VP14637) with subnanomolar activities have been described. Interestingly they also seem to target the hydrophobic pocket of the 6HB (Douglas et al., 2005; Lundin et al., 2010). Consequently, many of these compounds select escape mutants with similar or identical amino acid changes and which exhibit cross-resistance. Some of the selected mutations alter residues at or near the hydrophobic pocket that may interact with drugs. This has been shown directly for TMC353121. In other mutants, however the selected mutations lie outside the expected site in the F molecule, and the actual mechanism by which they mediate resistance is not understood at present.
Other RSV inhibitors have been described besides those targeting the F protein. For instance, NMSO3, a sulphated sialyl lipid, has been reported to inhibit virus binding, and mutants resistant to this compound had mutations in the G protein, identifying it as the presumptive target (Kimura et al., 2004). Many negatively charged polymers, including heparin and dextran sulphate, also interfere with RSV binding, but their inhibitory concentrations are too high to find application in the clinic.
Sudo et al. (Sudo et al., 2005) discovered a benzazepine derivative (YM-53404) that worked relatively late in the replication cycle and that selected mutations in the L gene. Another benzodiazepine compound (RSV604) that worked late in the replication cycle was shown to select mutations in the N gene, pointing to this molecule as the drug target (Chapman et al., 2007). Interestingly, these mutations were located in the long β hairpin of the RSV N structure noted above that may mediate interaction between N protein in the RNP and the polymerase complex (Tawar et al., 2009).
Besides chemical compounds, other types of molecules have been shown to inhibit RSV infectivity. For instance, HRB-derived peptides, analogous to those used to treat human immunodeficiency virus (HIV) infections, were demonstrated to block RSV induced membrane fusion (Lambert et al., 1996), but no further progress has been reported on these inhibitors. Small interfering RNAs (siRNAs) targeting the NS1 (Zhang et al., 2005) or P (Bitko et al., 2005) gene were shown to inhibit RSV replication in tissue culture and in the mouse model. More recently, a randomized clinical trial with intranasal siRNA targeting the N gene (ALN-RSV01) was reported to have protective activity in human volunteers challenged with RSV (DeVincenzo et al., 2010), and the same drug had some beneficial effect on long-term allograft function in lung transplant recipients with RSV infection (Zamora et al., 2011).
As described in the following section (7. Immunoprophylaxis with antibodies), prophylaxis with RSV-neutralizing antibodies has been effective in reducing RSV disease in young infants; therefore, these preparations also have been evaluated as therapy of established RSV infection. For example, the RSV-neutralizing MAbs palivizumab or motavizumab have been administered intravenously to infants and children hospitalized for RSV disease. Treatment was associated with a ~10-fold reduction in shedding, but with no clear effect on clinical outcome (Lagos et al., 2009; Malley et al., 1998). A phase 2 study to evaluate motavizumab for treatment of children hospitalized with RSV disease has been completed (clinicaltrials.govNCT00421304), although results have not been reported. Antibodies also have been used on an individual basis in treatment of severely immunocompromised individuals, in whom shedding can be much more prolonged (Lazar et al., 2006). A preparation of purified serum antibodies from adults selected for high RSV-neutralizing activity (RI-001, ADMA Biologics) was recently evaluated in a phase 2 study for safety and the ability to prevent the development of LRI in RSV-infected immunocompromised adults (clinicaltrials.govNCT00632463) and also has been used in treatment of several immunocompromised patients (Falsey et al., 2009).
Several difficulties remain for the therapeutic use of antivirals against RSV. One of them is the narrow window for intervention. RSV infects and causes symptoms in the upper respiratory tract before reaching the lungs and there is a delay of ~ 4 days between the first symptoms and hospitalization. Rapid diagnostic tests are therefore vitally important for rapid identification and proper treatment of naturally occurring infections. Another problem is the idea that host immune responses contribute to viral disease, as noted. Therefore, combined anti-viral and anti-inflammatory therapy with more specific and less toxic drugs may be a promising alternative. Finally, the emergence of resistant viruses that are easily selected in vitro with various drugs challenges the widespread use of anti-RSV compounds. As with other viruses (e.g. HIV), combination therapies with drugs targeted to different RSV gene products should avoid selection of resistant viruses.
With the lack of available effective antiviral therapy, treatment of acute RSV LRI mainly involves supportive care, including administration of intravenous fluids, humidified oxygen, and mechanical ventilation in more severe cases. Systemic or inhaled corticosteroids – used on the premise that inflammation contributes to RSV disease - provide no clear improvement and are not recommended for routine use (2006; Krilov, 2011; Patel et al., 2004). The leukotriene receptor antagonist montelukast – another anti-inflammatory agent - was not effective in acute disease (Amirav et al., 2008), and whether it has an effect on post-RSV wheezing remains unclear (Kim et al., 2010; Krilov, 2011; Proesmans et al., 2009). In the United States, infants with severe RSV disease are often treated with inhaled bronchodilators in an attempt to relieve airway constriction, but this provides only modest short-term benefit (2006; Levin et al., 2008).
7. Immmunoprophylaxis with antibodies
Systemic immunoprophylaxis with RSV-neutralizing antibodies provides substantial protection against severe RSV disease in high-risk infants and children (2009; Mejias and Ramilo, 2008; Wu et al., 2008). The first product for human use, RSV Immune Globulin Intravenous (RS-IGIV, Respigam™, produced by MedImmune), consisted of purified serum antibodies from donors screened for high RSV-neutralizing activity and was licensed in 1996 (this product is similar to, and preceded, RI-001 mentioned above). Monthly administration during RSV season reduced the frequency of hospitalization for RSV disease by 55% or more and days spent in intensive care by 97% (1997; Groothuis et al., 1993; Mejias and Ramilo, 2008). However, this product had the disadvantage of involving intravenous infusion, the theoretical risk of adventitious agents, and possible interference with live pediatric vaccines due to its polyvalent nature (Wu et al., 2008). RSV-IVIG was superseded by development of the MAb palivizumab and has not been commercially available since 2004.
Palivizumab (Synagis™; MedImmune) is an RSV-neutralizing, F-specific MAb that was developed by humanizing a murine MAb (Beeler and van Wyke Coelingh, 1989; Johnson et al., 1997; Wu et al., 2008). Licensed in 1998, this MAb is 50- to 100-fold more effective on a weight basis than RSV-IVIG, allowing administration in a much smaller volume by monthly intramuscular injection. Its clinical efficacy is similar to that of RSV-IGIV (1998). Whereas RSV-IVIG was not recommended for use in infants with cyanotic heart disease due to the large dose volume (Groothuis et al., 1993), palivizumab is safe and effective in this population (Feltes et al., 2003). Palivizumab is widely used for prophylaxis of infants and young children at high risk for severe RSV disease due to prematurity and underlying disease, and also can be used in hematopoietic stem cell recipients (Boeckh et al., 2001). Antibody prophylaxis thus represents the first and only means available to specifically reduce the incidence of severe RSV disease. These products do not necessarily prevent RSV infection, but can restrict replication sufficient to reduce disease. Antibody-resistant mutants have not been a significant problem: they are detected in ~5% of treated individuals who nonetheless developed RSV LRI, and they usually appear to have a modest reduction in growth fitness in vitro (Zhu et al., 2011).
Palivizumab was modified by in vitro affinity maturation to create a more potent derivative, called motavizumab (MEDI-524 or Numax, MedImmune)(Wu et al., 2008), This resulted in a 70-fold increase in antigen binding, a 20-fold increase in neutralization activity in vitro, and increased protection in cotton rats including in the upper respiratory tract (Mejias and Ramilo, 2008; Wu et al., 2007). In a clinical study involving 6635 preterm infants, the group that received motavizumab had 26% and 55% reductions in HRSV hospitalization and medically-attended HRSV LRI (MALRI), respectively, compared to palivizumab (Carbonell-Estrany et al., 2010; Gill and Welliver, 2009). However, this product was associated with a slight increase in the incidence of hypersensitivity reactions and anti-drug antibodies. In a June 2, 2010 meeting, the US FDA Antiviral Drugs Advisory Committee declined to support this drug application, and its further development for RSV prophylaxis is not being pursued, but this antibody might find use in anti-viral therapy. In addition, motavizumab was engineered further to increase its serum half-life by increasing its affinity for a receptor that recycles antibodies taken up intracellularly for degradation (Dall'Acqua et al., 2006). This antibody (MEDI-557) has been evaluated for tolerability and pharmacokinetics in health adults (clinicaltrials.govNCT00578682). However, given the issues with its motavizumab parent, it seems unlikely that this derivative will be developed further for prophylaxis.
As noted, fully human G-specific MAbs have been developed from human B cell clones and were shown to compare favorably with palivizumab in neutralizing activity in vitro and in rodents (Collarini et al., 2009). In another development, llama-derived heavy chain antibody fragments (nanobodies) directed against the F protein were reported (Hultberg et al., 2011). Multimeric nanobody constructs (either multiparatopic or multivalent) showed improved neutralizing potencies that compare favorably with palivizumab.
8. Pediatric vaccines
Given the early incidence of RSV hospitalization, vaccination should be initiated during the first weeks of life. The reduced immune responses characteristic of infancy probably will necessitate multiple vaccine doses, which might be given as part of the routine vaccination schedule at 2, 4, and 6 months of age.
Development of a pediatric RSV vaccine has been complicated by the experience with a formalin-inactivated RSV vaccine (FI-RSV) evaluated in the 1960s in infants and young children. This vaccine consisted of FI-RSV that was concentrated with alum adjuvant and administered intramuscularly. The vaccine was well tolerated and appeared at the time to be moderately immunogenic, but it proved to be poorly protective against natural RSV infection. Furthermore, vaccinees that were subsequently infected by natural exposure experienced immune-mediated enhancement of disease: nearly 80% of infected vaccinees required hospitalization. Subsequent analysis indicated that the immune response to FI-RSV in the vaccinees was markedly different from that to natural RSV infection, including poor induction of virus-neutralizing serum antibodies (Murphy and Walsh, 1988) and an exaggerated CD4+ T lymphocyte response (Kim et al., 1976). These observations were confirmed and extended in animal models. The poor neutralizing response likely involved denaturation of neutralization antigens as well as deficient antibody affinity maturation (Delgado et al., 2009). These antibodies were unable to efficiently restrict viral replication, but they bound to antigen and created antibody-antigen complexes that activated complement and contributed to disease (Melendi et al., 2007). The exaggerated CD4+ T cell response was found to involve the Th2 subset (Connors et al., 1994; de Swart et al., 2007; Waris et al., 1996), which was directly confirmed to be involved in disease enhancement in the mouse model (Connors et al., 1992). The Th2 biased response appeared to result from poor stimulation of NK cells and CD8+ CTL, which otherwise down-regulate Th2 and inflammatory responses to RSV antigens, as already noted. In addition, the formalin fixation creates carbonyl groups that enhance the Th2 response (Moghaddam et al., 2006). In summary, the aberrant responses to FI-RSV included induction of antibodies that bound viral antigen but did not neutralize infectivity, resulted in poor stimulation of cells and intracellular signaling pathways important in immunoregulation, and increased stimulation of Th2 cells.
This phenomenon of vaccine-primed disease enhancement also has been observed following RSV infection of experimental animals that were immunized with purified RSV F and G glycoprotein preparations as experimental vaccines (Murphy et al., 1990). In contrast, it has not been observed with natural RSV infection and re-infection, or in most cases with viral or DNA vectors expressing RSV antigens. These different outcomes for killed-virus/subunit RSV vaccines versus live/vectored RSV vaccines likely reflect a greater efficiency of the latter in inducing a broad, regulated immune response that down-regulates Th2 lymphocytes and generates antibodies that efficiently neutralize infectivity. Thus, inactivated RSV vaccines are generally considered unsuitable for pediatric use. It may be that the use of improved adjuvants (Boukhvalova et al., 2006) or improved antigen constructs (Swanson et al., 2011) will yield inactivated vaccines that are safe in RSV-naïve infants. However, it may be hard to establish safety for any new inactivated vaccine, since disease enhancement sometimes only becomes evident with time (Murphy et al., 1990), and because it is unclear how accurately experimental animals model the human response.
Efforts to develop a pediatric RSV vaccine have focused on live-attenuated vaccines, which were free of disease enhancement in experimental animals and in clinical trials (Wright et al., 2007). Intranasal administration of a live vaccine stimulates local as well as systemic immunity. The topical route of administration also was found to partly reduce the immunosuppressive effects of passive serum antibodies (Crowe et al., 1995). Beginning in the 1960’s a number of live attenuated RSV strains were developed by serial passage at suboptimal temperatures (cold-passage, cp) (Friedewald et al., 1968) and growth in the presence of mutagens followed by identification of temperature sensitive (ts) mutants (Crowe et al., 1994). Among the biologically-derived viruses that were evaluated clinically, the most promising one (called cpts248/404) was well-tolerated and immunogenic in seronegative infants and children older than 6 months of age, but caused mild congestion in younger infants and thus was insufficiently attenuated (Wright et al., 2000).
All subsequent live attenuated viruses have been developed by reverse genetics, which provides the means to introduce attenuating mutations into RSV in desired combinations to make “designer” vaccines (Collins and Murphy, 2005). Attenuating mutations were identified by analysis of existing attenuating viruses (Whitehead et al., 1998a) or were designed using new strategies such as deleting accessory proteins, or introducing host-range restriction elements (Bermingham and Collins, 1999; Buchholz et al., 2000; Teng et al., 2000; Whitehead et al., 1999). Novel mutations also may have desirable properties such as improved immunogenicity and increased genetic stability, as noted below. Reverse genetics also provides the possibility to increase the genetic stability of amino acid substitutions (McAuliffe et al., 2004) and to produce virus with short, well-defined passage histories, important for safety (Surman et al., 2007).
Several of these new attenuated RSV strains have been evaluated in clinical trials (Karron et al., 2005; Wright et al., 2006). The most promising candidate, called rA2cp248/404/1030ΔSH or MEDI-559, contains a number of introduced mutations, including two amino acid substitutions in the L polymerase, a nucleotide substitution in the M2 gene-start signal, and deletion of the SH gene. The MEDI-559 virus is strongly temperature-sensitive, which would restrict replication in vivo, especially in the warmer lower respiratory tract. In 1- to 2-month old, RSV-naive infants, the virus was well tolerated, which represents an important advance. Furthermore, a single dose provided substantial restriction of a second vaccine dose administered two months later, indicative of protective immunity. Despite this observed protection, the majority of vaccinees did not have detectable rises in serum antibodies. This reflects the general difficulty in detecting correlates of immunity to RSV in young infants, due to weak responses and limitations on sampling. Importantly, it remains to be determined whether this lead vaccine candidate will provide protection against natural infection. MEDI-559 presently is in expanded studies in young infants to further monitor tolerability and immunogenicity (clinicalstudies.govNCT00767416), and subsequently will be evaluated in expanded studies for the ability to induce protection against medically attended RSV disease from natural exposure in the community. Of note, this vaccine candidate exhibited genetic instability involving the loss of one of the two amino acid substitutions in the L protein (Karron et al., 2005). These partial revertants remained highly attenuated and were not associated with disease, but it would be preferable to have a more stable vaccine candidate, and efforts to stabilize this virus are underway (Luongo et al., 2009).
Other candidates also are entering clinical trials. One has the deletion of most of the M2-2 coding sequence (Teng et al., 2000). As noted, the lack of M2-2 results in increased transcription and antigen synthesis (Bermingham and Collins, 1999) and thus may be more immunogenic, and a gene deletion would be expected to be stable. Another potential vaccine candidate contains deletion of the major IFN antagonist NS1 gene (Teng et al., 2000). This would result in increased IFN production and signaling during infection and might thereby increase immunogenicity, which was observed with BRSV lacking the major NS IFN antagonist protein (Valarcher et al., 2003). Thus, attenuation can involve various mechanisms: point mutations in the L polymerase or gene-start signal likely reduce viral RNA synthesis; deletion of M2-2 increases transcription at the expense of RNA replication, and deletion of NS1 reduces the ability of the virus to inhibit the host IFN system. It will be interesting to characterize the properties of these vaccines in humans.
Another live attenuated RSV vaccine strategy uses an attenuated PIV as a vector to express the RSV F and/or G protein from one or two added genes. This provides a pediatric vaccine against both the PIV vector and RSV, and has the advantage of the improved in vitro growth (important for vaccine manufacture) and stability of PIV compared to RSV. In one example, bovine PIV3 (which is attenuated in humans due to the host range difference) was modified so that its F and HN major protective antigen genes were replaced with those of HPIV3 in order to provide homologous protection against HPIV3. This attenuated B/HPIV3 virus was further modified by the insertion of RSV F and/or G, creating a bivalent vaccine against HPIV3 and RSV (Schmidt et al., 2002; Tang et al., 2008). One such construct, in which B/HPIV3 expresses RSV F from a gene added between the N and P genes (MEDI-534), is presently in clinical trials in children >6 mo of age who are seronegative to both RSV and HPIV3 (clinicaltrials.govNCT00686075). Another related approach uses Sendai virus (murine PIV1), which has substantial antigenic cross-reactivity with HPIV1 and may be attenuated in humans, as the vector to express RSV protective antigens (Jones et al., 2009). This provides a potential bivalent vaccine against HPIV1 and RSV. Other vectored approaches, such as using replication-defective adenoviruses (Shao et al., 2009) or alphaviruses (Elliott et al., 2007b), also are being developed for possible pediatric use.
There also is a need for an RSV vaccine in older children and adults who are at risk for severe RSV infection due to underlying disease or old age. Life attenuated RSV vaccines appear to be too restricted in replication in these RSV-experienced individuals to be immunogenic (Gonzalez et al., 2000). Vectored vaccines might be feasible, but would need to be based on a vector that is not a common human pathogen in order to avoid immune restriction. Also, the development of vector-specific immunity would interfere with subsequent doses. Another alternative would be protein vaccines, for which suppression by existing immunity can be partly overcome by increased dose (Murphy et al., 1991). Observations from the original FI-RSV vaccine trials and studies in experimental animals (Waris et al., 1997) indicated that disease enhancement is observed only in RSV-naïve recipients. This indicated that RSV protein vaccines do not prime for disease enhancement in RSV-experienced individuals, which was confirmed in clinical trials outlined below.
Subunit vaccines based on the RSV F protein isolated from infected cell culture have been evaluated in healthy adults, in children over 12 months of age who were healthy or who had chronic lung disease from prematurity or cystic fibrosis, in the elderly, and in pregnant women in whom the goal was to increase the titer of maternal antibodies in the newborn (Belshe et al., 1993; Falsey and Walsh, 1997; Groothuis et al., 1998; Munoz et al., 2003; Paradiso et al., 1994; Piedra et al., 1996; Tristram et al., 1993). In these studies, the vaccines were well tolerated but were not very immunogenic. More recently, a vaccine consisting of the RSV F, G, and M proteins from RSV-infected cells was evaluated in elderly individuals in conjunction with the inactivated seasonal influenza virus vaccine, and was well tolerated and moderately immunogenic (Falsey et al., 2008). However, development has been suspended. Another protein based vaccine, called BBG2Na, consisted of a fragment of the G protein containing the central conserved domain that was fused to the albumin-binding domain of the streptococcal G protein and was expressed in bacteria. However, this vaccine may have induced an increased Th2 response in infant macaques (de Waal et al., 2004) and in clinical trials in adults it was not very immunogenic (Power et al., 2001) and was associated with hypersensitivity reactions in some recipients. More recently, as already noted, a post-fusion form of the F protein was produced with deletion of the major hydrophobic regions (McLellan et al., 2011; Swanson et al., 2011). Importantly, this expressed protein forms stable trimers that were recognized by a number of neutralizing MAbs. In rodents, this antigen induced high titers of neutralizing serum antibodies and protection against RSV challenge. This may represent an improved RSV subunit vaccine.
Virus-like particle (VLP) vaccines represent another approach (Jennings and Bachmann, 2008). VLP vaccines for hepatitis B virus and papillomavirus are in wide use. In addition, a VLP vaccine against influenza virus has been in use in a number of countries since 1997 and compares favorably with the standard inactivated influenza vaccine (Herzog et al., 2009; Kanra et al., 2004). VLPs may mimic some aspects of the superior immunogenicity of virus compared to purified protein vaccines (Jennings and Bachmann, 2008). In the case of RSV, a single formulation is presently reported to be in early clinical trials (clinicaltrials.govNCT01290419). This vaccine is called an “RSV-F Particle Vaccine” but is not otherwise described. In other work, the production of RSV VLPs was enhanced by co-expression of the NDV N and P proteins, which stimulated particle formation (McGinnes et al., 2011). Whether a VLP-based RSV vaccine would be free from disease enhancement in RSV-naïve infants is unknown.
Numerous additional approaches to an HRSV vaccine continue to be evaluated in pre-clinical studies, including synthetic peptides, engineered multi-epitope vaccines, antigen expressed by recombinant baculoviruses or produced in plants, antigen fused to carrier proteins such as cholera toxin B subunit, immune-stimulating complexes containing RSV antigens, the use of adjuvants such as CpG oligonucleotides or biopolymer nanoparticles, and vectors including vesicular stomatitis virus, vaccinia virus (Modified Vaccinia Ankara), rhinovirus, Newcastle disease virus, Mycobacterium bovis BCG, and Salmonella typhimurium. Whether any of these offers advantages remains to be seen.
Can successful RSV vaccines be developed? In the case of older children and adults, it seems likely that improved methods of producing RSV antigen, or alternatively the use of a VLP, will result in an effective subunit vaccine that could be given periodically, possibly in conjunction with the yearly influenza virus vaccine. In the case of RSV-naïve infants and children, it remains to be seen whether vaccine candidates will be sufficiently immunogenic in infancy to provide effective protection against RSV disease. It seems unlikely that complete protection against infection will be achieved, but a substantial reduction in RSV replication should be effective in controlling severe disease. The recent successful development of live-attenuated pediatric vaccines against rotavirus, which faced many of the same obstacles, gives hope for success.
9. Future prospects and thanks
Since the first isolation of RSV in the 1950’s, research has come a long way in understanding this important pathogen, but major challenges remain. Beginning in the 1980’s, cloning and reverse genetics technologies and improved immunological methods have augmented classical virological approaches, and have uncovered a number of intricacies of RSV replication, biology, and the host response that have been summarized here. More recently, this has been further augmented by the beginning of descriptions of viral structures at the atomic level. We anticipate that this will lead to a fuller understanding of virus replication and viral antigens, and will help develop improved antiviral drugs and vaccines for control of RSV infections.
For basic science, major challenges include (i) understanding the basis of the pathology associated with RSV infections, and (ii) understanding the effects that RSV has on the host immune response. As with many human pathogens, these goals are impeded by the lack of a convenient, available animal model that faithfully reproduces RSV infection and disease. Another complication is that the first exposure to RSV typically occurs in young infants with immature immune systems and lungs, and in the presence of immunosuppressive maternal antibodies, and thus multiple factors are in play. A number of additional approaches are helping to fill the gaps, including genomic and proteomic technologies, increased study of innate immune responses, genetic susceptibility studies, challenge studies in adults, more detailed analysis of hospitalized infants, improved in vitro models such as airway epithelium and immune cells, and other approaches. These have begun to yield data on isolated aspects, but an integrated picture is still missing.
Despite this incomplete understanding, research led to the previous development of palivizumab for prophylaxis of high-risk infants, and at the present time a number of vaccine candidates and antiviral drugs seem at the doorstep of clinical application. It seems likely that, in the upcoming years, new medicines will be on the market for the prevention and/or therapy of RSV disease. But, it should not be forgotten that much of the burden of RSV disease is in developing countries, which must be able to benefit from any progress made to control RSV.
Finally, we would like to thank Brian Mahy for inviting us to write this review. Brian has been a mentor – directly or indirectly - for many of us over the years in many areas of virology. His authoritative and wide-ranging editorial work has contributed to fundamental books and journals, like this one. But for us, we are particularly grateful for Brian’s (and Penny’s) major contributions to the organization of the Negative Strand RNA Virus Meetings that since the 1970’s have been the most enjoyable and rewarding event for virologists in this field, for the excellent quality of science, for the enjoyable social events that have been a very important part of those meetings, and for the spirit of collegiality that Brian helped infuse into this research community.
Table 2.
Notable features of RSV pathology and epidemiology
|
Acknowledgments
Research in Madrid lab is partially funded by grant SAF2009-11632 (to J.A.M.) from the Ministerio de Ciencia e Innovación (Spain). P.L.C. was supported by the Intramural Program of NIAID, NIH (USA).
Abbreviations
- 6HB
six-helix bundle
- CCR
chemokine receptor
- DC
dendritic cell
- eIF-2a
eukaryotic translation initiation factor 2a
- FI-RSV
formalin-inactivated RSV
- GAG
glycosaminoglycan
- HAE
human airway epithelium
- HIV
human immunodeficiency virus
- HMPV
human metapneumovirus
- HPIV
human parainfluenza virus
- HR
heptad repeat
- IFN
interferon
- IKKε
inhibitor of NFκB kinase ε
- IL
interleukin
- IRF3
interferon regulatory factor 3
- JAK
Janus kinase
- LRI
lower respiratory tract illness
- MAb
monoclonal antibody
- MALRI
medically-attended LRI
- MAVS
mitochondrial antiviral-signaling protein
- MDA-5
melanoma differentiation-associated gene 5
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PKR
dsRNA-regulated protein kinase
- RIG-I
retinoic acid inducible gene I
- RNP
ribonucleoprotein
- RSV
respiratory syncytial virus
- RSV-IVIG
intravenous immunoglobulin selected for increased RSV-neutralizing activity
- STAT
signal transducer and activator of transcription
- Th
T helper cell
- TNF
tumor necrosis factor
- TLR
toll-like receptor
- TRAF3
TNF receptor-associated factor 3
- URI
upper respiratory tract illness
- VLP
virus-like particle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. The IMpact-RSV study group. Pediatrics. 1998;102(3):531–537. [PubMed] [Google Scholar]
- Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- From the American Academy of Pediatrics: Policy statements--Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124(6):1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- Abu-Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. 1999;14(1):139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Aherne W, Bird T, Court SD, Gardner PS, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23(1):7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirav I, Luder AS, Kruger N, Borovitch Y, Babai I, Miron D, Zuker M, Tal G, Mandelberg A. A double-blind, placebo-controlled, randomized trial of montelukast for acute bronchiolitis. Pediatrics. 2008;122(6):e1249–e1255. doi: 10.1542/peds.2008-1744. [DOI] [PubMed] [Google Scholar]
- Anderson K, King AM, Lerch RA, Wertz GW. Polylactosaminoglycan modification of the respiratory syncytial virus small hydrophobic (SH) protein: a conserved feature among human and bovine respiratory syncytial viruses. Virology. 1992;191(1):417–430. doi: 10.1016/0042-6822(92)90203-2. [DOI] [PubMed] [Google Scholar]
- Asenjo A, Calvo E, Villanueva N. Phosphorylation of human respiratory syncytial virus P protein at threonine 108 controls its interaction with the M2-1 protein in the viral RNA polymerase complex. J Gen Virol. 2006;87(Pt 12):3637–3642. doi: 10.1099/vir.0.82165-0. [DOI] [PubMed] [Google Scholar]
- Atreya PL, Peeples ME, Collins PL. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J Virol. 1998;72(2):1452–1461. doi: 10.1128/jvi.72.2.1452-1461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. Journal of Virology. 1989;63(7):2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Anderson EL, Walsh EE. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis. 1993;168(4):1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- Bendelja K, Gagro A, Bace A, Lokar-Kolbas R, Krsulovic-Hresic V, Drazenovic V, Mlinaric-Galinovic G, Rabatic S. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121(2):332–338. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Garofalo RP, Cron SG, Hosakote YM, Atmar RL, Macias CG, Piedra PA. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195(10):1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- Berkovich S. Acute respiratory illness in the premature nursery associated with respiratory syncytial virus infections. Pediatrics. 1964;34:753–760. [PubMed] [Google Scholar]
- Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A. 1999;96(20):11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11(1):50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Bitko V, Shulyayeva O, Mazumder B, Musiyenko A, Ramaswamy M, Look DC, Barik S. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J Virol. 2007;81(4):1786–1795. doi: 10.1128/JVI.01420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184(3):350–354. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit J, Bartlett E, Schomacker H, Surman S, Akira S, Bae YS, Collins P, Murphy B, Schmidt A. The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J Virol. 2011;85(4):1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botosso VF, Zanotto PM, Ueda M, Arruda E, Gilio AE, Vieira SE, Stewien KE, Peret TC, Jamal LF, Pardini MI, Pinho JR, Massad E, Sant'anna OA, Holmes EC, Durigon EL. Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog. 2009;5(1) doi: 10.1371/journal.ppat.1000254. e1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24(23):5027–5035. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- Brandenburg AH, Kleinjan A, van Het Land B, Moll HA, Timmerman HH, de Swart RL, Neijens HJ, Fokkens W, Osterhaus AD. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol. 2000a;62(2):267–277. [PubMed] [Google Scholar]
- Brandenburg AH, van Beek R, Moll HA, Osterhaus AD, Claas EC. G protein variation in respiratory syncytial virus group A does not correlate with clinical severity. J Clin Microbiol. 2000b;38(10):3849–3852. doi: 10.1128/jcm.38.10.3849-3852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz UJ, Granzow H, Schuldt K, Whitehead SS, Murphy BR, Collins PL. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): Effects on host range and evaluation as a live-attenuated HRSV vaccine. J Virol. 2000;74(3):1187–1199. doi: 10.1128/jvi.74.3.1187-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71(12):8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Yang L, Fricke J, Cheng L, Ward JM, Murphy BR, Collins PL. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82(24):12191–12204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder LJ, Gonzalez-Reyes L, Garcia-Barreno B, Wharton SA, Skehel JJ, Wiley DC, Melero JA. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271(1):122–131. doi: 10.1006/viro.2000.0279. [DOI] [PubMed] [Google Scholar]
- Cane PA. Molecular epidemiology of respiratory syncytial virus. Rev Med Virol. 2001;11(2):103–116. doi: 10.1002/rmv.305. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Stott EJ, Taylor G, Askonas BA. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987;62(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Estrany X, Simoes EA, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- Carter SD, Dent KC, Atkins E, Foster TL, Verow M, Gorny P, Harris M, Hiscox JA, Ranson NA, Griffin S, Barr JN. Direct visualization of the small hydrophobic protein of human respiratory syncytial virus reveals the structural basis for membrane permeability. FEBS Lett. 2010;584(13):2786–2790. doi: 10.1016/j.febslet.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne N, Barbier A, Bernard J, Rezaei H, Huet JC, Henry C, Da Costa B, Eleouet JF. Biochemical characterization of the respiratory syncytial virus P-P and P-N protein complexes and localization of the P protein oligomerization domain. J Gen Virol. 2004;85(Pt 6):1643–1653. doi: 10.1099/vir.0.79830-0. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez JA, Holberg CJ, Wright AL, Halonen M, Taussig LM, Morgan WJ, Martinez FD. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159(6):1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- Chaiwatpongsakorn S, Epand RF, Collins PL, Epand RM, Peeples ME. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85(8):3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock RM, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. epidemiological aspects of infection in infants and young children. American Journal of Hygiene. 1957;66:291–300. doi: 10.1093/oxfordjournals.aje.a119902. [DOI] [PubMed] [Google Scholar]
- Chanock RM, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent. I. Isolation, properties and characterization. American Journal of Hygiene. 1957;66:281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- Chapman J, Abbott E, Alber DG, Baxter RC, Bithell SK, Henderson EA, Carter MC, Chambers P, Chubb A, Cockerill GS, Collins PL, Dowdell VC, Keegan SJ, Kelsey RD, Lockyer MJ, Luongo C, Najarro P, Pickles RJ, Simmonds M, Taylor D, Tyms S, Wilson LJ, Powell KL. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother. 2007;51(9):3346–3353. doi: 10.1128/AAC.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci C, Langley DR, Dischino DD, Sun Y, Yu KL, Stanley A, Roach J, Li Z, Dalterio R, Colonno R, Meanwell NA, Krystal M. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc Natl Acad Sci U S A. 2004;101(42):15046–15051. doi: 10.1073/pnas.0406696101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SKR, Gardner PS, Poole PM, et al. Respiratory syncytial virus infection: admissions to hospital in industrial, urban, and rural areas. Report to the Medical Research Council Subcommittee on Respiratory Syncytial Virus Vaccines. Br Med J. 1978;2(6140):796–798. doi: 10.1136/bmj.2.6140.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, Tripp RA, Walsh EE, Keyt BA, Kauvar LM. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. 2009;183(10):6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Hill MG, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci U S A. 1996;93(1):81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Mottet G. Membrane orientation and oligomerization of the small hydrophobic protein of human respiratory syncytial virus. J Gen Virol. 1993;74(Pt 7):1445–1450. doi: 10.1099/0022-1317-74-7-1445. [DOI] [PubMed] [Google Scholar]
- Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–173. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Olmsted RA, Spriggs MK, Johnson PR, Buckler-White AJ. Gene overlap and site-specific attenuation of transcription of the viral polymerase L gene of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987;84(15):5134–5138. doi: 10.1073/pnas.84.15.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65(3):1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HCr, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68(8):5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HCd, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66(12):7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JE, Jr, Bui PT, Davis AR, Chanock RM, Murphy BR. A further attenuated derivative of a cold-passaged temperature-sensitive mutant of human respiratory syncytial virus retains immunogenicity and protective efficacy against wild-type challenge in seronegative chimpanzees. Vaccine. 1994;12(9):783–-790. doi: 10.1016/0264-410x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Crowe JE, Jr, Bui PT, Siber GR, Elkins WR, Chanock RM, Murphy BR. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine. 1995;13(9):847–855. doi: 10.1016/0264-410x(94)00074-w. [DOI] [PubMed] [Google Scholar]
- Crowe JE, Jr, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910–3918. doi: 10.4049/jimmunol.167.7.3910. [DOI] [PubMed] [Google Scholar]
- Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CK, McMillan JA, Gross SJ. Rehospitalization for respiratory illness in infants of less than 32 weeks' gestation. Pediatrics. 1991;88(3):527–532. [PubMed] [Google Scholar]
- Curran J, Marq JB, Kolakofsky D. An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during the nascent chain assembly step of genome replication. J Virol. 1995;69(2):849–855. doi: 10.1128/jvi.69.2.849-855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281(33):23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- De Alarcon A, Walsh EE, Carper HT, La Russa JB, Evans BA, Rakes GP, Platts-Mills TA, Heymann PW. Detection of IgA and IgG but not IgE antibody to respiratory syncytial virus in nasal washes and sera from infants with wheezing. J Pediatr. 2001;138(3):311–317. doi: 10.1067/mpd.2001.111277. [DOI] [PubMed] [Google Scholar]
- de Graaff PM, de Jong EC, van Capel TM, van Dijk ME, Roholl PJ, Boes J, Luytjes W, Kimpen JL, van Bleek GM. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175(9):5904–5911. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- de Swart RL, van den Hoogen BG, Kuiken T, Herfst S, van Amerongen G, Yuksel S, Sprong L, Osterhaus AD. Immunization of macaques with formalin-inactivated human metapneumovirus induces hypersensitivity to hMPV infection. Vaccine. 2007;25(51):8518–8528. doi: 10.1016/j.vaccine.2007.10.022. [DOI] [PubMed] [Google Scholar]
- de Waal L, Power UF, Yuksel S, van Amerongen G, Nguyen TN, Niesters HG, de Swart RL, Osterhaus AD. Evaluation of BBG2Na in infant macaques: specific immune responses after vaccination and RSV challenge. Vaccine. 2004;22(8):915–922. doi: 10.1016/j.vaccine.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15(1):34–-41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107(19):8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191(11):1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- DiNapoli JM, Murphy BR, Collins PL, Bukreyev A. Impairment of the CD8+ T cell response in lungs following infection with human respiratory syncytial virus is specific to the anatomical site rather than the virus, antigen, or route of infection. Virol J. 2008;5:105. doi: 10.1186/1743-422X-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30(8):555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JL, Panis ML, Ho E, Lin KY, Krawczyk SH, Grant DM, Cai R, Swaminathan S, Chen X, Cihlar T. Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncytial virus fusion by similar mechanisms. Antimicrob Agents Chemother. 2005;49(6):2460–2466. doi: 10.1128/AAC.49.6.2460-2466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell SF, Anderson LJ, Gary HE, Jr, Erdman DD, Plouffe JF, File TM, Jr, Marston BJ, Breiman RF. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174(3):456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- Dupuy LC, Dobson S, Bitko V, Barik S. Casein kinase 2-mediated phosphorylation of respiratory syncytial virus phosphoprotein P is essential for the transcription elongation activity of the viral polymerase; phosphorylation by casein kinase 1 occurs mainly at Ser(215) and is without effect. J Virol. 1999;73(10):8384–8392. doi: 10.1128/jvi.73.10.8384-8392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Saleeby CM, Li R, Somes GW, Dahmer MK, Quasney MW, DeVincenzo JP. Surfactant protein A2 polymorphisms and disease severity in a respiratory syncytial virus-infected population. J Pediatr. 2010;156(3):409–414. doi: 10.1016/j.jpeds.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M, Power UF, Johnston JA. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol. 2007a;81(7):3428–3436. doi: 10.1128/JVI.02303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MB, Chen T, Terio NB, Chong SY, Abdullah R, Luckay A, Egan MA, Boutilier LA, Melville K, Lerch RA, Long D, Eldridge JH, Parks CL, Udem SA, Hancock GE. Alphavirus replicon particles encoding the fusion or attachment glycoproteins of respiratory syncytial virus elicit protective immune responses in BALB/c mice and functional serum antibodies in rhesus macaques. Vaccine. 2007b;25(41):7132–7144. doi: 10.1016/j.vaccine.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71(5):428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28(2):171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Koval C, Khorana M, Walsh EE, Sorrentino MS, Grossman A, Betts RF. Use of high titer RSV immunoglobulin (RI-001-RSV-IVIG) in immunocompromised adults. Infectious Disease Society of America 47th Annual Meeting, Presentation 607.2009. [Google Scholar]
- Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78(11):1493–1497. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15(10):1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis. 1998;177(2):463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, Nichol KL, Simoes EA, Wright PF, Sales VM. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (rsv) vaccines--nonadjuvanted vaccine or vaccine adjuvanted with alum--given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis. 2008;198(9):1317–1326. doi: 10.1086/592168. [DOI] [PubMed] [Google Scholar]
- Fearns R, Collins PL. Model for polymerase access to the overlapped L gene of respiratory syncytial virus. J Virol. 1999a;73(1):388–397. doi: 10.1128/jvi.73.1.388-397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearns R, Collins PL. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol. 1999b;73(7):5852–5864. doi: 10.1128/jvi.73.7.5852-5864.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearns R, Peeples ME, Collins PL. Mapping the transcription and replication promoters of respiratory syncytial virus. J Virol. 2002;76(4):1663–1672. doi: 10.1128/JVI.76.4.1663-1672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman SA, Hendry RM, Beeler JA. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73(8):6610–6617. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr, Connor EM, Sondheimer HM. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96(2):179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Forsyth BR, Smith CB, Gharpure MA, Chanock RM. Low-temperature-grown RS virus in adult volunteers. JAMA. 1968;203(8):690–694. [PubMed] [Google Scholar]
- Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol. 2007;81(15):8361–8366. doi: 10.1128/JVI.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SW, Ng L, Lin X, Gong X, Torres J. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci. 2008;17(5):813–820. doi: 10.1110/ps.073366208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barreno B, Delgado T, Melero JA. Identification of protein regions involved in the interaction of human respiratory syncytial virus phosphoprotein and nucleoprotein: significance for nucleocapsid assembly and formation of cytoplasmic inclusions. J Virol. 1996;70(2):801–808. doi: 10.1128/jvi.70.2.801-808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R, Kimpen JL, Welliver RC, Ogra PL. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120(1):28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115(4):668–674. doi: 10.1016/j.jaci.2005.01.058. quiz 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal R, Baulch-Brown C, Mills J, Meanger J. The matrix protein of Human respiratory syncytial virus localises to the nucleus of infected cells and inhibits transcription. Arch Virol. 2003;148(7):1419–1429. doi: 10.1007/s00705-003-0112-y. [DOI] [PubMed] [Google Scholar]
- Gill MA, Welliver RC. Motavizumab for the prevention of respiratory syncytial virus infection in infants. Expert Opin Biol Ther. 2009;9(10):1335–1345. doi: 10.1517/14712590903287499. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Witt TL, Senft AP, Baatz JE, Folger D, Maxfield MD, Akinbi HT, Newton DA, Prows DR, Korfhagen TR. Surfactant protein C-deficient mice are susceptible to respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L64–L72. doi: 10.1152/ajplung.90640.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Gonzalez IM, Karron RA, Eichelberger M, Walsh EE, Delagarza VW, Bennett R, Chanock RM, Murphy BR, Clements-Mann ML, Falsey AR. Evaluation of the live attenuated cpts 248/404 RSV vaccine in combination with a subunit RSV vaccine (PFP-2) in healthy young and older adults. Vaccine. 2000;18:1763–1772. doi: 10.1016/s0264-410x(99)00527-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Carrasco L. Viroporins. FEBS Lett. 2003;552(1):28–34. doi: 10.1016/s0014-5793(03)00780-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes L, Ruiz-Arguello MB, Garcia-Barreno B, Calder L, Lopez JA, Albar JP, Skehel JJ, Wiley DC, Melero JA. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci U S A. 2001;98(17):9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JJ, Ferguson BL, Speelman D, Mills J. Determination of the disulfide bond arrangement of human respiratory syncytial virus attachment (G) protein by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Protein Sci. 1997;6(6):1308–1315. doi: 10.1002/pro.5560060619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould PS, Easton AJ. Coupled translation of the respiratory syncytial virus m2 open reading frames requires upstream sequences. J Biol Chem. 2005;280(23):21972–21980. doi: 10.1074/jbc.M502276200. [DOI] [PubMed] [Google Scholar]
- Gould PS, Easton AJ. Coupled translation of the second open reading frame of M2 mRNA is sequence dependent and differs significantly within the subfamily Pneumovirinae. J Virol. 2007;81(16):8488–8496. doi: 10.1128/JVI.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Bunton LA, Wright PF, Karzon DT. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991a;34(1):7–13. doi: 10.1002/jmv.1890340103. [published erratum appears in J Med Virol 1991 Dec;35(4):307]. [DOI] [PubMed] [Google Scholar]
- Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991b;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Davis TH, Tang YW, Gruber WC. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr Res. 1993;34(2):167–172. doi: 10.1203/00006450-199308000-00013. [DOI] [PubMed] [Google Scholar]
- Groothuis JR, Gutierrez KM, Lauer BA. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics. 1988;82(2):199–203. [PubMed] [Google Scholar]
- Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EA. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177(2):467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329(21):1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Groskreutz DJ, Babor EC, Monick MM, Varga SM, Hunninghake GW. Respiratory syncytial virus limits alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) phosphorylation to maintain translation and viral replication. J Biol Chem. 2010;285(31):24023–24031. doi: 10.1074/jbc.M109.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005a;79(16):10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005b;79(23):14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34(3):320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking D, Knight JC, Rockett K, Brown H, Frampton J, Kwiatkowski DP, Hull J, Udalova IA. Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibility. Genes Immun. 2004;5(4):274–282. doi: 10.1038/sj.gene.6364067. [DOI] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr. 1976;89(1):11–15. doi: 10.1016/s0022-3476(76)80918-3. [DOI] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Simons RL. Interferon production in adults with respiratory syncytial viral infection. Ann Intern Med. 1981;94(1):53–55. doi: 10.7326/0003-4819-94-1-53. [DOI] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Simons RL, Geiman JM. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978;93(1):28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr. 1984;105(3):358–364. doi: 10.1016/s0022-3476(84)80005-0. [DOI] [PubMed] [Google Scholar]
- Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 1986;315(2):77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley LL, McGivern DR, Teng MN, Djang R, Collins PL, Fearns R. Roles of the respiratory syncytial virus trailer region: Effects of mutations on genome production and stress granule formation. Virology. 2010 doi: 10.1016/j.virol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol. 2006;176(3):1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Wertz GW. The product of the respiratory syncytial virus M2 gene ORF1 enhances readthrough of intergenic junctions during viral transcription. J Virol. 1998;72(1):520–526. doi: 10.1128/jvi.72.1.520-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RW, Wertz GW. The Cys(3)-His(1) motif of the respiratory syncytial virus M2-1 protein is essential for protein function. J Virol. 2000;74(13):5880–5885. doi: 10.1128/jvi.74.13.5880-5885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ, Semple MG, Smyth RL, Kimpen JL, van Bleek GM. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179(12):8410–8417. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- Heidema J, Rossen JW, Lukens MV, Ketel MS, Scheltens E, Kranendonk ME, van Maren WW, van Loon AM, Otten HG, Kimpen JL, van Bleek GM. Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J Immunol. 2008;181(8):5551–5559. doi: 10.4049/jimmunol.181.8.5551. [DOI] [PubMed] [Google Scholar]
- Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Henderson G, Murray J, Yeo RP. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology. 2002;300(2):244–254. doi: 10.1006/viro.2002.1540. [DOI] [PubMed] [Google Scholar]
- Hendricks DA, McIntosh K, Patterson JL. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol. 1988;62(7):2228–2233. doi: 10.1128/jvi.62.7.2228-2233.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Hartmann K, Kunzi V, Kursteiner O, Mischler R, Lazar H, Gluck R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27(33):4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Williams J, Richardson JB, Macklem PT, Thurlbeck WM. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282(23):1283–1287. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- Hruska JF, Morrow PE, Suffin SC, Douglas RG., Jr In vivo inhibition of respiratory syncytial virus by ribavirin. Antimicrob Agents Chemother. 1982;21(1):125–130. doi: 10.1128/aac.21.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultberg A, Temperton NJ, Rosseels V, Koenders M, Gonzalez-Pajuelo M, Schepens B, Ibanez LI, Vanlandschoot P, Schillemans J, Saunders M, Weiss RA, Saelens X, Melero JA, Verrips CT, Van Gucht S, de Haard HJ. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One. 2011;6(4):e17665. doi: 10.1371/journal.pone.0017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, van 't Slot R, Wijmenga C, Goeman JJ, Kimpen JL, van Houwelingen HC, Kimman TG, Hoebee B. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196(6):826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389(5):521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Jr, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987a;61(10):3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987b;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27(12):1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica AR, Wright PF, Hetrick FM, Chanock RM. Electron microscopic studies of respiratory syncytial temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):248–258. doi: 10.1007/BF01252772. [DOI] [PubMed] [Google Scholar]
- Kanra G, Marchisio P, Feiterna-Sperling C, Gaedicke G, Lazar H, Durrer P, Kursteiner O, Herzog C, Kara A, Principi N. Comparison of immunogenicity and tolerability of a virosome-adjuvanted and a split influenza vaccine in children. Pediatr Infect Dis J. 2004;23(4):300–306. doi: 10.1097/00006454-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, Harris DO, Randolph VB, Udem SA, Murphy BR, Sidhu MS. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A. 1997a;94(25):13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, Murphy BR, Collins PL. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191(7):1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- Karron RA, Wright PF, Crowe JE, Jr, Clements ML, Thompson J, Makhene M, Casey R, Murphy BR. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus (RSV) vaccines in chimpanzees, adults, infants and children. J. Infect. Dis. 1997b;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- Khattar SK, Yunus AS, Collins PL, Samal SK. Deletion and substitution analysis defines regions and residues within the phosphoprotein of bovine respiratory syncytial virus that affect transcription, RNA replication, and interaction with the nucleoprotein. Virology. 2001;285(2):253–269. doi: 10.1006/viro.2001.0960. [DOI] [PubMed] [Google Scholar]
- Kim CK, Choi J, Kim HB, Callaway Z, Shin BM, Kim JT, Fujisawa T, Koh YY. A randomized intervention of montelukast for post-bronchiolitis: effect on eosinophil degranulation. J Pediatr. 2010;156(5):749–754. doi: 10.1016/j.jpeds.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kim SW, Park CS, Kim BI, Kang H, Koh YY. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J Allergy Clin Immunol. 2003;112(1):64–71. doi: 10.1067/mai.2003.1618. [DOI] [PubMed] [Google Scholar]
- Kim HW, Leikin SL, Arrobio J, Brandt CD, Chanock RM, Parrott RH. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ishioka K, Hashimoto K, Mori S, Suzutani T, Bowlin TL, Shigeta S. Isolation and characterization of NMSO3-resistant mutants of respiratory syncytial virus. Antiviral Res. 2004;61(3):165–171. doi: 10.1016/j.antiviral.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kondgen S, Kuhl H, N'Goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Matz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin WI, Pauli G, Boesch C, Leendertz FH. Pandemic human viruses cause decline of endangered great apes. Curr Biol. 2008;18(4):260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Krilov LR. Respiratory syncytial virus disease: update on treatment and prevention. Expert Rev Anti Infect Ther. 2011;9(1):27–32. doi: 10.1586/eri.10.140. [DOI] [PubMed] [Google Scholar]
- Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, Jonsdottir I. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116(4):805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Collins PL, Bacik I, Yewdell JW, Bennink JR, Crowe JE, Jr, Murphy BR. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol. 1995;69(2):1261–1264. doi: 10.1128/jvi.69.2.1261-1264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27(2):240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Kuo L, Fearns R, Collins PL. Analysis of the gene start and gene end signals of human respiratory syncytial virus: quasi-templated initiation at position 1 of the encoded mRNA. J. Virol. 1997;71(7):4944–4953. doi: 10.1128/jvi.71.7.4944-4953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L, Grosfeld H, Cristina J, Hill MG, Collins PL. Effect of mutations in the gene-start and gene-end sequence motifs on transcription of monocistronic and dicistronic minigenomes of respiratory syncytial virus. J Virol. 1996;70(10):6892–6901. doi: 10.1128/jvi.70.10.6892-6901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83(20):10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos R, DeVincenzo JP, Munoz A, Hultquist M, Suzich J, Connor EM, Losonsky GA. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)-specific humanized monoclonal antibody, when administered to RSV-infected children. Pediatr Infect Dis J. 2009;28(9):835–837. doi: 10.1097/INF.0b013e3181a165e4. [DOI] [PubMed] [Google Scholar]
- Laham FR, Israele V, Casellas JM, Garcia AM, Lac Prugent CM, Hoffman SJ, Hauer D, Thumar B, Name MI, Pascual A, Taratutto N, Ishida MT, Balduzzi M, Maccarone M, Jackli S, Passarino R, Gaivironsky RA, Karron RA, Polack NR, Polack FP. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189(11):2047–2056. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- Lamb RA. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197(1):1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Barney S, Lambert AL, Guthrie K, Medinas R, Davis DE, Bucy T, Erickson J, Merutka G, Petteway SR., Jr Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci U S A. 1996;93(5):2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar I, Canaan A, Weibel C, Kahn JS. Novel mutations in the respiratory syncytial virus G gene identified in viral isolates from a girl with severe combined immune deficiency treated with intravenous immune globulin. J Clin Virol. 2006;37(3):168–173. doi: 10.1016/j.jcv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Le Nouen C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, Collins PL, Rabin RL, Buchholz UJ. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One. 2010;5(11):e15017. doi: 10.1371/journal.pone.0015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Nouen C, Hillyer P, Winter CC, McCarty T, Rabin RL, Collins PL, Buchholz UJ. Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human respiratory syncytial virus (HRSV) and metapneumovirus (HMPV) PLoS Path. 2011;7:e1002105. doi: 10.1371/journal.ppat.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Nouen C, Munir S, Losq S, Winter CC, McCarty T, Stephany DA, Holmes KL, Bukreyev A, Rabin RL, Collins PL, Buchholz UJ. Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology. 2009;385(1):169–182. doi: 10.1016/j.virol.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FE, Walsh EE, Falsey AR, Lumb ME, Okam NV, Liu N, Divekar AA, Hall CB, Mosmann TR. Human infant respiratory syncytial virus (RSV)-specific type 1 and 2 cytokine responses ex vivo during primary RSV infection. J Infect Dis. 2007;195(12):1779–1788. doi: 10.1086/518249. [DOI] [PubMed] [Google Scholar]
- Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168(6):633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- Levin DL, Garg A, Hall LJ, Slogic S, Jarvis JD, Leiter JC. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatr Crit Care Med. 2008;9(6):598–604. doi: 10.1097/PCC.0b013e31818c82b4. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, Korfhagen T. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31(2):193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68(Pt 9):2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Ling Z, Tran KC, Teng MN. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol. 2009;83(8):3734–3742. doi: 10.1128/JVI.02434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi M, Mason SW, Cartier M, Lawetz C, McCollum RS, Dansereau N, Bolger G, Lapeyre N, Gaudette Y, Lagace L, Massariol MJ, Do F, Whitehead P, Lamarre L, Scouten E, Bordeleau J, Landry S, Rancourt J, Fazal G, Simoneau B. Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase. J Virol. 2005;79(20):13105–13115. doi: 10.1128/JVI.79.20.13105-13115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol. 2005;79:9315–9319. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren J, Marttila R, Renko M, Ramet M, Hallman M. Toll-like receptor 4 Asp299Gly polymorphism in respiratory syncytial virus epidemics. Pediatr Pulmonol. 2010;45(7):687–692. doi: 10.1002/ppul.21248. [DOI] [PubMed] [Google Scholar]
- Long CE, McBride JT, Hall CB. Sequelae of respiratory syncytial virus infections. A role for intervention studies. Am J Respir Crit Care Med. 1995;151(5):1678–1680. doi: 10.1164/ajrccm/151.5_Pt_1.1678. discussion 1680-1. [DOI] [PubMed] [Google Scholar]
- Ludwig K, Schade B, Bottcher C, Korte T, Ohlwein N, Baljinnyam B, Veit M, Herrmann A. Electron cryomicroscopy reveals different F1+F2 protein States in intact parainfluenza virions. J Virol. 2008;82(7):3775–3781. doi: 10.1128/JVI.02154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, Koenderman L, Wolfs TF, van Bleek GM. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84(5):2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A, Bergstrom T, Bendrioua L, Kann N, Adamiak B, Trybala E. Two novel fusion inhibitors of human respiratory syncytial virus. Antiviral Res. 2010;88(3):317–324. doi: 10.1016/j.antiviral.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Luongo C, Yang L, Winter CC, Spann KM, Murphy BR, Collins PL, Buchholz UJ. Codon stabilization analysis of the "248" temperature sensitive mutation for increased phenotypic stability of respiratory syncytial virus vaccine candidates. Vaccine. 2009;27(41):5667–5676. doi: 10.1016/j.vaccine.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald NE, Hall CB, Suffin SC, Alexson C, Harris PJ, Manning JA. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307(7):397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- Magro M, Andreu D, Gomez-Puertas P, Melero JA, Palomo C. Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol. 2010;84(16):7970–7982. doi: 10.1128/JVI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, Buckingham S, Vernacchio L, Ambrosino DM. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178(6):1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62(4):382–388. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello RA, Chen MD, Weibel C, Kahn JS. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186(6):839–842. doi: 10.1086/342414. [DOI] [PubMed] [Google Scholar]
- Martinez I, Dopazo J, Melero JA. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J Gen Virol. 1997;78(Pt 10):2419–2429. doi: 10.1099/0022-1317-78-10-2419. [DOI] [PubMed] [Google Scholar]
- Martinez I, Melero JA. Enhanced neutralization of human respiratory syncytial virus by mixtures of monoclonal antibodies to the attachment (G) glycoprotein. J Gen Virol. 1998;79(Pt 9):2215–2220. doi: 10.1099/0022-1317-79-9-2215. [DOI] [PubMed] [Google Scholar]
- McAuliffe JM, Surman SR, Newman JT, Riggs JM, Collins PL, Murphy BR, Skiadopoulos MH. Codon substitution mutations at two positions in the L polymerase protein of human parainfluenza virus type 1 yield viruses with a spectrum of attenuation in vivo and increased phenotypic stability in vitro. J Virol. 2004;78(4):2029–2036. doi: 10.1128/JVI.78.4.2029-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011;85(1):366–377. doi: 10.1128/JVI.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern DR, Collins PL, Fearns R. Identification of internal sequences in the 3' leader region of human respiratory syncytial virus that enhance transcription and confer replication processivity. J Virol. 2005;79(4):2449–2460. doi: 10.1128/JVI.79.4.2449-2460.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K. Interferon in nasal secretions from infants with viral respiratory tract infections. J Pediatr. 1978;93(1):33–36. doi: 10.1016/s0022-3476(78)80595-2. [DOI] [PubMed] [Google Scholar]
- McIntosh K, Kurachek SC, Cairns LM, Burns JC, Goodspeed B. Treatment of respiratory viral infection in an immunodeficient infant with ribavirin aerosol. Am J Dis Child. 1984;138(3):305–308. doi: 10.1001/archpedi.1984.02140410083024. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Chang JS, Yang Y, Kim A, Graham BS, Kwong PD. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J Virol. 2010a;84(23):12236–12244. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010b;17(2):248–250. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of the Respiratory Syncytial Virus Fusion Glycoprotein in the Post-fusion Conformation Reveals Preservation of Neutralizing Epitopes. J Virol. 2011;85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara PS, Flanagan BF, Baldwin LM, Newland P, Hart CA, Smyth RL. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet. 2004;363(9414):1031–1037. doi: 10.1016/S0140-6736(04)15838-8. [DOI] [PubMed] [Google Scholar]
- McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88(10):922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner HC. Economic impact of viral respiratory disease in children. J Pediatr. 1994;124(5 Pt 2):S17–S21. doi: 10.1016/S0022-3476(94)70186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias A, Ramilo O. Review of palivizumab in the prophylaxis of respiratory syncytial virus (RSV) in high-risk infants. Biologics. 2008;2(3):433–439. doi: 10.2147/btt.s3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendi GA, Hoffman SJ, Karron RA, Irusta PM, Laham FR, Humbles A, Schofield B, Pan CH, Rabold R, Thumar B, Thumar A, Gerard NP, Mitzner W, Barnum SR, Gerard C, Kleeberger SR, Polack FP. C5 modulates airway hyperreactivity and pulmonary eosinophilia during enhanced respiratory syncytial virus disease by decreasing C3a receptor expression. J Virol. 2007;81(2):991–999. doi: 10.1128/JVI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero JA, Garcia-Barreno B, Martinez I, Pringle CR, Cane PA. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol. 1997;78(Pt 10):2411–2418. doi: 10.1099/0022-1317-78-10-2411. [DOI] [PubMed] [Google Scholar]
- Melikyan GB, Markosyan RM, Hemmati H, Delmedico MK, Lambert DM, Cohen FS. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151(2):413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21(4):686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs KJ, Smyth RL, O'Hea U, Ashby D, Ritson P, Hart CA. Cytokines in severe respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2002;33(6):449–452. doi: 10.1002/ppul.10101. [DOI] [PubMed] [Google Scholar]
- Moghaddam A, Olszewska W, Wang B, Tregoning JS, Helson R, Sattentau QJ, Openshaw PJ. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- Money VA, McPhee HK, Mosely JA, Sanderson JM, Yeo RP. Surface features of a Mononegavirales matrix protein indicate sites of membrane interaction. Proc Natl Acad Sci U S A. 2009;106(11):4441–4446. doi: 10.1073/pnas.0805740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE, Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS., Jr A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol. 2009;83(9):4185–4194. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84(11):5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Blount RE, Savage RE. Recovery of cytopathic agent from chimpanzees with coryza. Proceedings of the Society for Experimental Biology and Medicine. 1956;92:544–550. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- Mufson MA, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Munir S, Hillyer P, Le Nouen C, Buchholz UJ, Rabin RL, Collins PL, Bukreyev A. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Path. 2011;7:1001336. doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol. 2008;82(17):8780–8796. doi: 10.1128/JVI.00630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21(24):3465–3467. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Graham BS, Prince GA, Walsh EE, Chanock RM, Karzon DT, Wright PF. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986;23(6):1009–1014. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol. 1988;62(10):3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Prince GA, Collins PL, Hildreth SW, Paradiso PR. Effect of passive antibody on the immune response of cotton rats to purified F and G glycoproteins of respiratory syncytial virus (RSV) Vaccine. 1991;9(3):185–189. doi: 10.1016/0264-410x(91)90151-u. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8(5):497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26(8):1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble V, Murray M, Webb MS, Alexander J, Swarbrick AS, Milner AD. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997;76(4):315–319. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noton SL, Cowton VM, Zack CR, McGivern DR, Fearns R. Evidence that the polymerase of respiratory syncytial virus initiates RNA replication in a nontemplated fashion. Proc Natl Acad Sci U S A. 2010;107(22):10226–10231. doi: 10.1073/pnas.0913065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted RA, Collins PL. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989;63(5):2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted RA, Elango N, Prince GA, Murphy BR, Johnson PR, Moss B, Chanock RM, Collins PL. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Hartwig SM, Varga SM. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol. 2008;181(11):7958–7968. doi: 10.4049/jimmunol.181.11.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179(8):5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, Smith DH. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatr Infect Dis J. 1994;13(9):792–798. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- Patel H, Platt R, Lozano JM, Wang EE. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2004;(3):CD004878. doi: 10.1002/14651858.CD004878. [DOI] [PubMed] [Google Scholar]
- Perez M, Garcia-Barreno B, Melero JA, Carrasco L, Guinea R. Membrane permeability changes induced in Escherichia coli by the SH protein of human respiratory syncytial virus. Virology. 1997;235(2):342–351. doi: 10.1006/viro.1997.8696. [DOI] [PubMed] [Google Scholar]
- Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110(5):1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman DA, Malinoski F, Hiatt PW. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996;15(1):23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero JA, Karron RA, Collins PL, Kleeberger SR. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci U S A. 2005;102(25):8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus AD, de Groot R, Corvaia N, Beck A, Bouveret-Le-Cam N, Bonnefoy JY. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis. 2001;184(11):1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- Pribul PK, Harker J, Wang B, Wang H, Tregoning JS, Schwarze J, Openshaw PJ. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J Virol. 2008;82(9):4441–4448. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Hemming VG, Horswood RL, Baron PA, Chanock RM. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. 1987;61(6):1851–1854. doi: 10.1128/jvi.61.6.1851-1854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Hemming VG, Horswood RL, Chanock RM. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985a;3(3):193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983;42(1):81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985b;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Mathews A, Curtis SJ, Porter DD. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model wit systemically administered monoclonal antibody (palivizumab) and glucocorticosteroid. J Infect Dis. 2000;182:1326–1330. [Google Scholar]
- Proesmans M, Sauer K, Govaere E, Raes M, De Bilderling G, De Boeck K. Montelukast does not prevent reactive airway disease in young children hospitalized for RSV bronchiolitis. Acta Paediatr. 2009;98(11):1830–1834. doi: 10.1111/j.1651-2227.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- Puthothu B, Forster J, Heinze J, Heinzmann A, Krueger M. Surfactant protein B polymorphisms are associated with severe respiratory syncytial virus infection, but not with asthma. BMC Pulm Med. 2007;7:6. doi: 10.1186/1471-2466-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy M, Shi L, Monick MM, Hunninghake GW, Look DC. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;30(6):893–900. doi: 10.1165/rcmb.2003-0410OC. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology. 2006;344(2):328–339. doi: 10.1016/j.virol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Rawling J, Cano O, Garcin D, Kolakofsky D, Melero JA. Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus. J Virol. 2011;85(6):2771–2780. doi: 10.1128/JVI.02065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling J, Garcia-Barreno B, Melero JA. Insertion of the two cleavage sites of the respiratory syncytial virus fusion protein in Sendai virus fusion protein leads to enhanced cell-cell fusion and a decreased dependency on the HN attachment protein for activity. J Virol. 2008;82(12):5986–5998. doi: 10.1128/JVI.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Brewah YA, Delaney T, Welliver T, Burwell T, Benjamin E, Kuta E, Kozhich A, McKinney L, Suzich J, Kiener PA, Avendano L, Velozo L, Humbles A, Welliver RC, Sr, Coyle AJ. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J Infect Dis. 2008;198(12):1783–1793. doi: 10.1086/593173. [DOI] [PubMed] [Google Scholar]
- Roberts SR, Lichtenstein D, Ball LA, Wertz GW. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68(7):4538–4546. doi: 10.1128/jvi.68.7.4538-4546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez WJ, Hall CB, Welliver R, Simoes EA, Ryan ME, Stutman H, Johnson G, Van Dyke R, Groothuis JR, Arrobio J, et al. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: a double-blind, multicenter, placebo-controlled trial. J Pediatr. 1994;125(1):129–135. doi: 10.1016/s0022-3476(94)70139-3. [DOI] [PubMed] [Google Scholar]
- Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156(1):190–195. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70(5):691–698. [PubMed] [Google Scholar]
- Rota PA, Hemphill ML, Whistler T, Regnery HL, Kendal AP. Antigenic and genetic characterization of the haemagglutinins of recent cocirculating strains of influenza B virus. J Gen Virol. 1992;73(Pt 10):2737–2742. doi: 10.1099/0022-1317-73-10-2737. [DOI] [PubMed] [Google Scholar]
- Rothoeft T, Fischer K, Zawatzki S, Schulz V, Schauer U, Korner Rettberg C. Differential response of human naive and memory/effector T cells to dendritic cells infected by respiratory syncytial virus. Clin Exp Immunol. 2007;150(2):263–273. doi: 10.1111/j.1365-2249.2007.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roymans D, De Bondt HL, Arnoult E, Geluykens P, Gevers T, Van Ginderen M, Verheyen N, Kim H, Willebrords R, Bonfanti JF, Bruinzeel W, Cummings MD, van Vlijmen H, Andries K. Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc Natl Acad Sci U S A. 2010;107(1):308–313. doi: 10.1073/pnas.0910108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CJ, Jardetzky TS, Lamb RA. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. Embo J. 2001;20(15):4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J Virol. 2002;76(3):1163–1170. doi: 10.1128/JVI.76.3.1163-1170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AC, Wenzke DR, McAuliffe JM, St, Clair M, Elkins WR, Murphy BR, Collins PL. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J Virol. 2002;76:1089–1099. doi: 10.1128/JVI.76.3.1089-1099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhof A, Bont L, Siezen CL, Hodemaekers H, van Houwelingen HC, Kimman TG, Hoebee B, Kimpen JL, Janssen R. Interleukin-9 polymorphism in infants with respiratory syncytial virus infection: an opposite effect in boys and girls. Pediatr Pulmonol. 2010;45(6):608–613. doi: 10.1002/ppul.21229. [DOI] [PubMed] [Google Scholar]
- Shao HY, Yu SL, Sia C, Chen Y, Chitra E, Chen IH, Venkatesan N, Leng CH, Chong P, Chow YH. Immunogenic properties of RSV-B1 fusion (F) protein gene-encoding recombinant adenoviruses. Vaccine. 2009;27(40):5460–5471. doi: 10.1016/j.vaccine.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sanchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18(2):115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Shinoff JJ, O'Brien KL, Thumar B, Shaw JB, Reid R, Hua W, Santosham M, Karron RA. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis. 2008;198(7):1007–1015. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson CM, Walsh EE, Piedra PA, Hemming VG, et al. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169(6):1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- Siezen CL, Bont L, Hodemaekers HM, Ermers MJ, Doornbos G, Van't Slot R, Wijmenga C, Houwelingen HC, Kimpen JL, Kimman TG, Hoebee B, Janssen R. Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes. Pediatr Infect Dis J. 2009;28(4):333–335. doi: 10.1097/INF.0b013e31818e2aa9. [DOI] [PubMed] [Google Scholar]
- Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126(2):256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth RL, Mobbs KJ, O'Hea U, Ashby D, Hart CA. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr Pulmonol. 2002;33(5):339–346. doi: 10.1002/ppul.10080. [DOI] [PubMed] [Google Scholar]
- Snow A, Dayyat E, Montgomery-Downs HE, Kheirandish-Gozal L, Gozal D. Pediatric obstructive sleep apnea: a potential late consequence of respiratory syncitial virus bronchiolitis. Pediatr Pulmonol. 2009;44(12):1186–1191. doi: 10.1002/ppul.21109. [DOI] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78(8):4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- Stevens WW, Sun J, Castillo JP, Braciale TJ. Pulmonary eosinophilia is attenuated by early responding CD8(+) memory T cells in a murine model of RSV vaccine-enhanced disease. Viral Immunol. 2009;22(4):243–251. doi: 10.1089/vim.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott EJ, Taylor G, Ball LA, Anderson K, Young KK, King AM, Wertz GW. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Miyazaki Y, Kojima N, Kobayashi M, Suzuki H, Shintani M, Shimizu Y. YM-53403, a unique anti-respiratory syncytial virus agent with a novel mechanism of action. Antiviral Res. 2005;65(2):125–131. doi: 10.1016/j.antiviral.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Surman SR, Collins PL, Murphy BR, Skiadopoulos MH. An improved method for the recovery of recombinant paramyxovirus vaccine candidates suitable for use in human clinical trials. J Virol Methods. 2007;141(1):30–33. doi: 10.1016/j.jviromet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Swanson K, Wen X, Leser GP, Paterson RG, Lamb RA, Jardetzky TS. Structure of the Newcastle disease virus F protein in the post-fusion conformation. Virology. 2010;402(2):372–379. doi: 10.1016/j.virol.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. Structural basis for immunization with post-fusion RSV F to elicit high neutralizing antibody titers. Proc. Natl. Acad. Sci. USA. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedan S, Musiyenko A, Barik S. Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J Virol. 2009;83(19):9682–9693. doi: 10.1128/JVI.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Komatsu T, Kitagawa Y, Sada K, Gotoh B. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J Virol. 2008;82(20):10102–10110. doi: 10.1128/JVI.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, Reisinger K, Chandler P, Hilty M, Walker RE, Gomez MM, Losonsky GA. Development of a PIV-vectored RSV vaccine: preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine. 2008;26(50):6373–6382. doi: 10.1016/j.vaccine.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Tasker L, Lindsay RW, Clarke BT, Cochrane DW, Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153(2):277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagne N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, Eleouet JF, Rey FA. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326(5957):1279–1283. doi: 10.1126/science.1177634. [DOI] [PubMed] [Google Scholar]
- Taylor G, Stott EJ, Bew M, Fernie BF, Cote PJ, Collins AP, Hughes M, Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011 doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- Techaarpornkul S, Barretto N, Peeples ME. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J Virol. 2001;75(15):6825–6834. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techaarpornkul S, Collins PL, Peeples ME. Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus. Virology. 2002;294(2):296–304. doi: 10.1006/viro.2001.1340. [DOI] [PubMed] [Google Scholar]
- Teng MN, Collins PL. Identification of the respiratory syncytial virus proteins required for formation and passage of helper-dependent infectious particles. J Virol. 1998;72(7):5707–5716. doi: 10.1128/jvi.72.7.5707-5716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MN, Whitehead SS, Bermingham A, Clair MS, Elkins WR, Murphy BR, Collins PL. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol. 2000;74:9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MN, Whitehead SS, Collins PL. Contribution of the respiratory syncytial virus g glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology. 2001;289(2):283–296. doi: 10.1006/viro.2001.1138. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, Duffy DL, Backer V, Bisgaard H. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179(12):1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172(2):233–237. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- Tran TL, Castagne N, Dubosclard V, Noinville S, Koch E, Moudjou M, Henry C, Bernard J, Yeo RP, Eleouet JF. The respiratory syncytial virus M2-1 protein forms tetramers and interacts with RNA and P in a competitive manner. J Virol. 2009;83(13):6363–6374. doi: 10.1128/JVI.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trento A, Casas I, Calderon A, Garcia-Garcia ML, Calvo C, Perez-Brena P, Melero JA. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010;84(15):7500–7512. doi: 10.1128/JVI.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2(8):732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- Tristram DA, Hicks W, Jr, Hard R. Respiratory syncytial virus and human bronchial epithelium. Arch Otolaryngol Head Neck Surg. 1998;124(7):777–783. doi: 10.1001/archotol.124.7.777. [DOI] [PubMed] [Google Scholar]
- Tristram DA, Welliver RC, Mohar CK, Hogerman DA, Hildreth SW, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18–36 months old. J Infect Dis. 1993;167(1):191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- Valarcher JF, Furze J, Wyld S, Cook R, Conzelmann KK, Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J Virol. 2003;77(15):8426–8439. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht S, Unsold H, Ehl S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur J Immunol. 2006;36(6):1434–1442. doi: 10.1002/eji.200535642. [DOI] [PubMed] [Google Scholar]
- Wagner DK, Clements ML, Reimer CB, Snyder M, Nelson DL, Murphy BR. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J Clin Microbiol. 1987;25(3):559–562. doi: 10.1128/jcm.25.3.559-562.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Schlesinger JJ, Brandriss MW. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris M. Pattern of respiratory syncytial virus epidemics in Finland: two-year cycles with alternating prevalence of groups A and B. J Infect Dis. 1991;163(3):464–469. doi: 10.1093/infdis/163.3.464. [DOI] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Day DB, Anderson LJ. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol. 1997;71(9):6935–6939. doi: 10.1128/jvi.71.9.6935-6939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70(5):2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver RC, Kaul TN, Putnam TI, Sun M, Riddlesberger K, Ogra PL. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980;96(5):808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]
- Welliver RC, Wong DT, Sun M, Middleton E, Jr, Vaughan RS, Ogra PL. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305(15):841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195(8):1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz GW, Collins PL, Huang Y, Gruber C, Levine S, Ball LA. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz GW, Krieger M, Ball LA. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989;63(11):4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whimbey E, Ghosh S. Respiratory syncytial virus infections in immunocompromised adults. Curr Clin Top Infect Dis. 2000;20:232–255. [PubMed] [Google Scholar]
- White LJ, Waris M, Cane PA, Nokes DJ, Medley GF. The transmission dynamics of groups A and B human respiratory syncytial virus (hRSV) in England & Wales and Finland: seasonality and cross-protection. Epidemiol Infect. 2005;133(2):279–289. doi: 10.1017/s0950268804003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Bukreyev A, Teng MN, Firestone CY, St Claire M, Elkins WR, Collins PL, Murphy BR. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73(4):3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Firestone CY, Collins PL, Murphy BR. A single nucleotide substitution in the transcription start signal of the M2 gene of respiratory syncytial virus vaccine candidate cpts248/404 is the major determinant of the temperature-sensitive and attenuation phenotypes. Virology. 1998a;247(2):232–239. doi: 10.1006/viro.1998.9248. [DOI] [PubMed] [Google Scholar]
- Whitehead SS, Juhasz K, Firestone CY, Collins PL, Murphy BR. Recombinant respiratory syncytial virus (RSV) bearing a set of mutations from cold-passaged RSV is attenuated in chimpanzees. J. Virol. 1998b;72(5):4467–4471. doi: 10.1128/jvi.72.5.4467-4471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JV, Weitkamp JH, Blum DL, LaFleur BJ, Crowe JE., Jr The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol Immunol. 2009;47(2–3):407–414. doi: 10.1016/j.molimm.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Gruber WC, Peters M, Reed G, Zhu Y, Robinson F, Coleman-Dockery S, Graham BS. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002;185(8):1011–1018. doi: 10.1086/339822. [DOI] [PubMed] [Google Scholar]
- Wright PF, Ikizler MR, Gonzales RA, Carroll KN, Johnson JE, Werkhaven JA. Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol. 2005;79(13):8651–8654. doi: 10.1128/JVI.79.13.8651-8654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O'Shea AF, Gruber WC, Murphy BR. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25(42):7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, Casey R, Eichelberger M, Thumar B, Randolph VB, Udem SA, Chanock RM, Murphy BR. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O'Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, Randolph VB, Deatly AM, Hackell JG, Gruber WC, Murphy BR. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis. 2006;193(4):573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol. 2008;317:103–123. doi: 10.1007/978-3-540-72146-8_4. [DOI] [PubMed] [Google Scholar]
- Yin HS, Paterson RG, Wen X, Lamb RA, Jardetzky TS. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci U S A. 2005;102(26):9288–9293. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439(7072):38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol. 2010;84(14):7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183(4):531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76(11):5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang H, Kong X, Mohapatra S, San Juan-Vergara H, Hellermann G, Behera S, Singam R, Lockey RF, Mohapatra SS. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11(1):56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- Zhao X, Liu E, Chen FP, Sullender WM. In vitro and in vivo fitness of respiratory syncytial virus monoclonal antibody escape mutants. J Virol. 2006;80(23):11651–11657. doi: 10.1128/JVI.01387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Singh M, Malashkevich VN, Kim PS. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc Natl Acad Sci U S A. 2000;97(26):14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, McAuliffe JM, Patel NK, Palmer-Hill FJ, Yang CF, Liang B, Su L, Zhu W, Wachter L, Wilson S, MacGill RS, Krishnan S, McCarthy MP, Losonsky GA, Suzich JA. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis. 2011;203(5):674–682. doi: 10.1093/infdis/jiq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Budz L, Herrler G. Proteolytic activation of respiratory syncytial virus fusion protein. Cleavage at two furin consensus sequences. J Biol Chem. 2001;276(34):31642–31650. doi: 10.1074/jbc.M102633200. [DOI] [PubMed] [Google Scholar]
- Zlateva KT, Lemey P, Moes E, Vandamme AM, Van Ranst M. Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein. J Virol. 2005;79(14):9157–9167. doi: 10.1128/JVI.79.14.9157-9167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]