Abstract
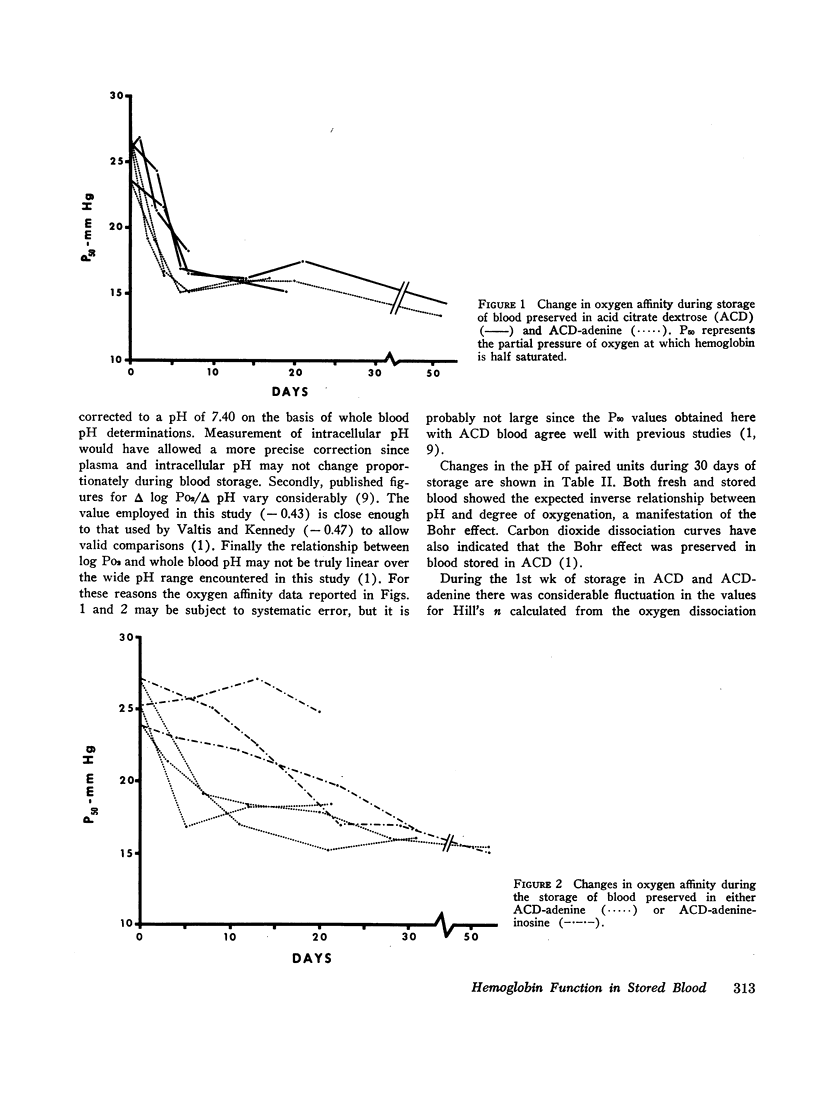
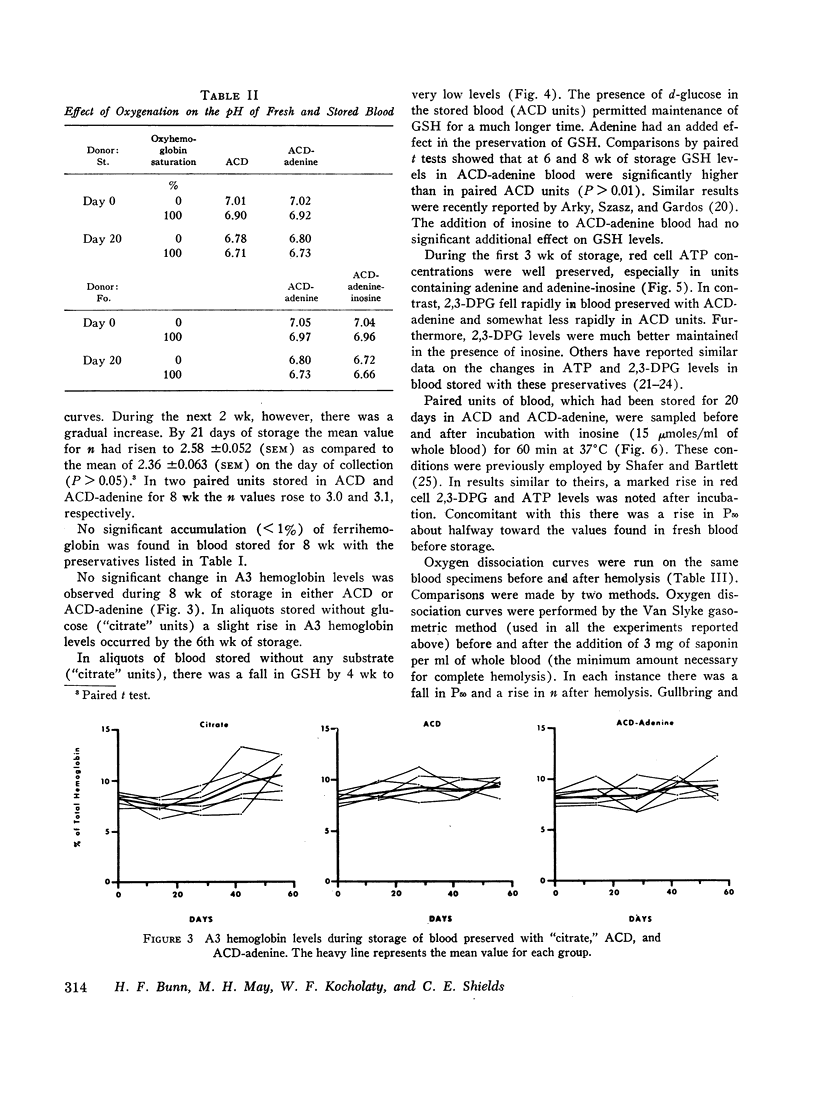
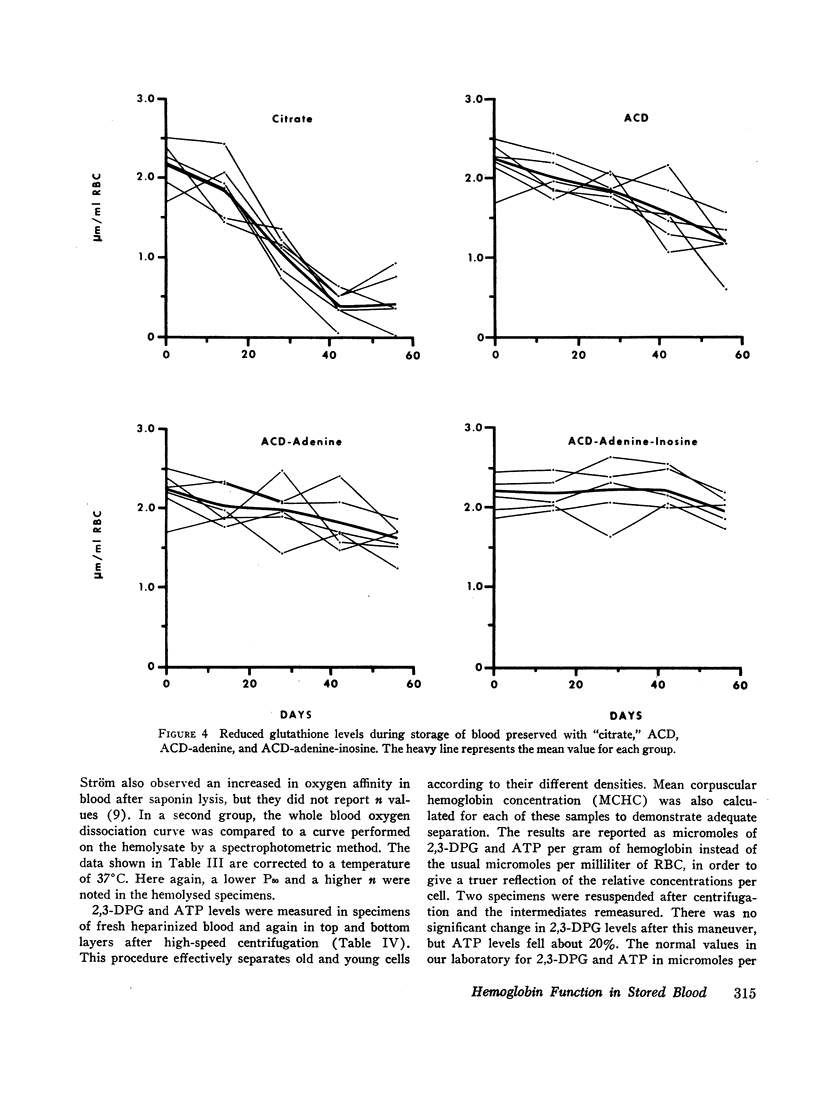
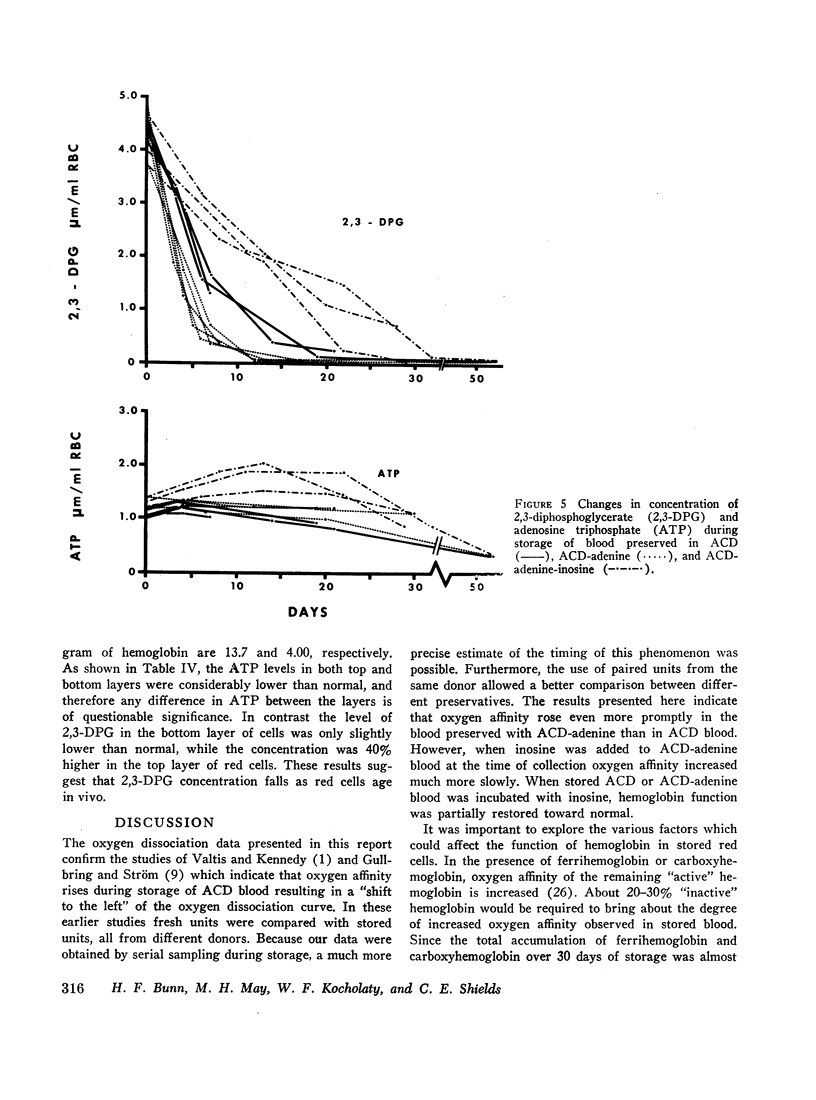
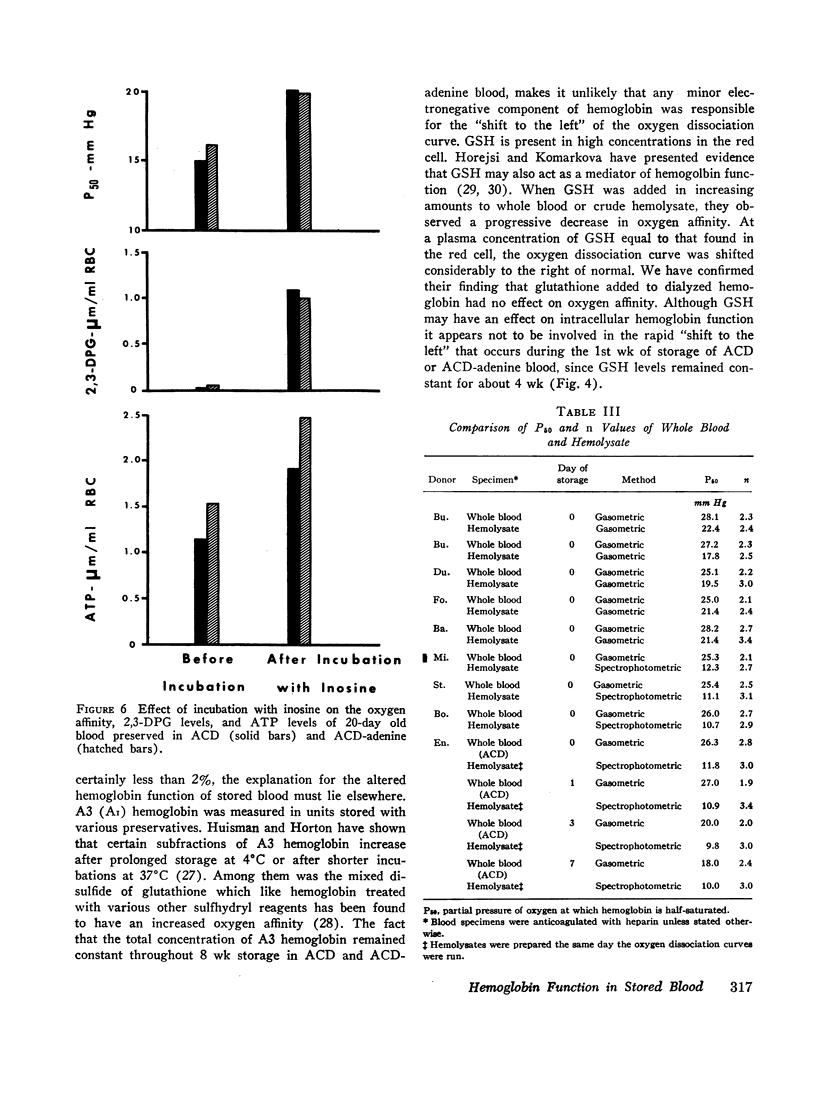
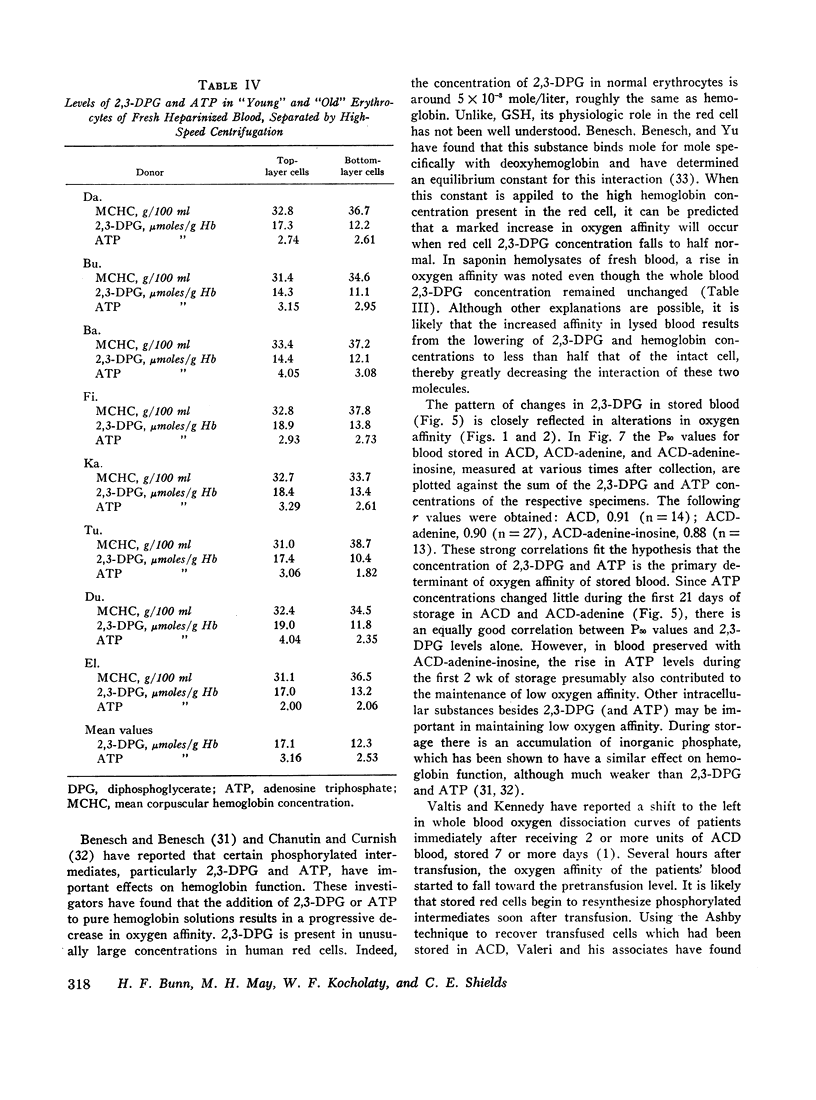
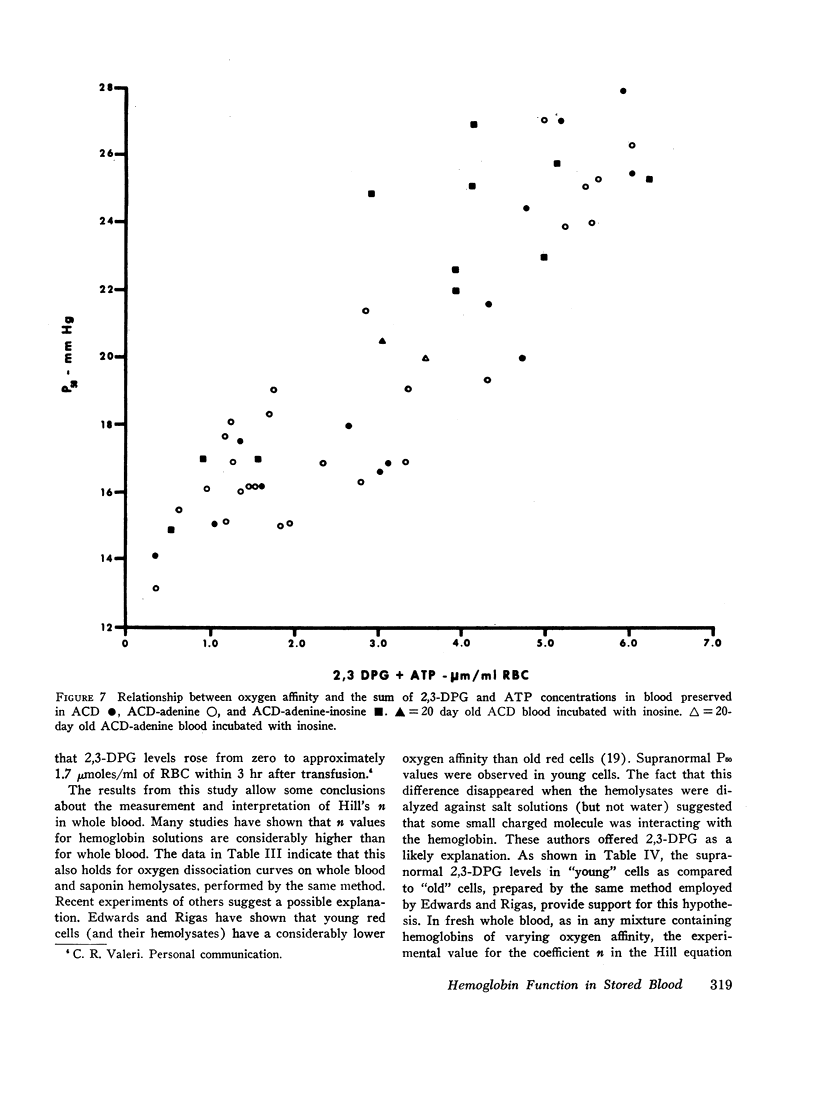
Serial oxygen dissociation curves were performed on blood units preserved in acid-citrate-dextrose (ACD), ACD-adenine, and ACD-adenine-inosine. Dividing blood from a single donor into two or more bags allowed direct comparison between preservatives. During the 1st wk of storage in ACD, a progressive increase in oxygen affinity was observed. Thereafter, little further change was noted. Oxygen affinity increased even more rapidly during initial storage in ACD-adenine. However, with the inclusion of inosine as a preservative, oxygen affinity remained unaltered during the first 2 wk. Increases in oxygen affinity correlated well with falling levels of red cell 2,3-diphosphoglycerate (2,3-DPG) during storage. No significant changes in glutathione, reduced form (GSH), or A3 (AI) hemoglobin levels were noted during the first 3 wk of storage. No significant accumulation of ferrihemoglobin was detected. When blood stored 20 days in ACD or ACD-adenine was incubated with inosine for 60 min at 37°C, 2,3-DPG and adenosine triphosphate (ATP) were resynthesized, and oxygen affinity was decreased. The distribution of 2,3-DPG in fresh and stored red cells appeared to influence experimental values for Hill's n, a measure of heme-heme interaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres S. M., Giannelli S., Jr, Armstrong R. G. Carboxyhemoglobin: Hemodynamic and Respiratory Responses to Small Concentrations. Science. 1965 Jul 9;149(3680):193–194. doi: 10.1126/science.149.3680.193. [DOI] [PubMed] [Google Scholar]
- BARTELS H., BETKE K., HILPERT P., NIEMEYER G., RIEGEL K. [The so-called standard O2 dissociation curve in normal adults]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;272:372–383. [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Chanutin A. The effect of the addition of adenine and nucleosides at the beginning of storage on the concentrations of phosphates of human erythrocytes during storage in acid-citrate-dextrose and citrate-phosphate-dextrose. Transfusion. 1967 Mar-Apr;7(2):120–132. doi: 10.1111/j.1537-2995.1967.tb04852.x. [DOI] [PubMed] [Google Scholar]
- DEVERDIER C. H., GARBY L., HJELM M., HOEGMAN C. ADENINE IN BLOOD PRESERVATION: POSTTRANSFUSION VIABILITY AND BIOCHEMICAL CHANGES. Transfusion. 1964 Sep-Oct;4:331–338. doi: 10.1111/j.1537-2995.1964.tb02883.x. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Rigas D. A. Electrolyte-labile increase of oxygen affinity during in vivo aging of hemoglobin. J Clin Invest. 1967 Oct;46(10):1579–1588. doi: 10.1172/JCI105649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLBRING B., STROM G. Changes in oxygen-carrying function of human hemoglobin during storage in cold acid-citrate-dextrose solution. Acta Med Scand. 1956 Nov 5;155(6):413–430. doi: 10.1111/j.0954-6820.1956.tb14390.x. [DOI] [PubMed] [Google Scholar]
- HOREJSI J., KOMARKOVA A. The effect of SH-groups on the affinity of haemoglobin to oxygen. Clin Chim Acta. 1958 Mar;3(2):131–136. doi: 10.1016/0009-8981(58)90070-6. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., HORTON B. F. STUDIES ON THE HETEROGENEITY OF HEMOGLOBIN. 8. CHROMATOGRAPHIC AND ELECTROPHORETIC INVESTIGATIONS OF VARIOUS MINOR HEMOGLOBIN FRACTIONS PRESENT IN NORMAL AND IN VITRO MODIFIED RED BLOOD CELL HEMOLYSATES. J Chromatogr. 1965 Apr;18:116–123. doi: 10.1016/s0021-9673(01)80326-5. [DOI] [PubMed] [Google Scholar]
- JOCELYN P. C. The reduction of oxidized glutathione in erythrocyte haemolysates in pernicious anaemia. Biochem J. 1960 Nov;77:363–368. doi: 10.1042/bj0770363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys. 1958 Oct;77(2):478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- RUBINSTEIN D., KASHKET S., DENSTEDT O. F. Studies on the preservation of blood. VI. The influence of adenosine and inosine on the metabolism of the erythrocyte. Can J Biochem Physiol. 1958 Dec;36(12):1269–1276. [PubMed] [Google Scholar]
- SEVERINGHAUS J. W. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol. 1958 May;12(3):485–486. doi: 10.1152/jappl.1958.12.3.485. [DOI] [PubMed] [Google Scholar]
- SHAFER A. W., BARTLETT G. R. Phosphorylated carbohydrate intermediates of the human erythrocyte during storage in acid citrate dextrose. III. Effect of incubation at 37 degrees C. with inosine, inosine plus adenine, and adenosine after storage for 6, 10, 14 and 18 weeks. J Clin Invest. 1962 Apr;41:690–695. doi: 10.1172/JCI104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFER A. W., TROMBOLD J. S. PHOSPHORYLATED CARBOHYDRATE INTERMEDIATES OF THE HUMAN ERYTHROCYTE DURING STORAGE IN ACID CITRATE DEXTROSE. IV. EFFECT OF ADDITION OF ADENINE, INOSINE, INOSINE-ADENINE AND ADENOSINE AT THE BEGINNING OF STORAGE. Transfusion. 1964 Mar-Apr;4:120–123. doi: 10.1111/j.1537-2995.1964.tb02842.x. [DOI] [PubMed] [Google Scholar]
- SIMON E. R., CHAPMAN R. G., FINCH C. A. Adenine in red cell preservation. J Clin Invest. 1962 Feb;41:351–359. doi: 10.1172/JCI104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGITA Y., SIMON E. R. THE MECHANISM OF ACTION OF ADENINE IN RED CELL PRESERVATION. J Clin Invest. 1965 Apr;44:629–642. doi: 10.1172/JCI105176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. F., Antonini E., Brunori M., Wyman J. Studies on human hemoglobin treated with various sulfhydryl reagents. J Biol Chem. 1966 Jan 10;241(1):241–248. [PubMed] [Google Scholar]
- VALTIS D. J. Defective gas-transport function of stored red blood-cells. Lancet. 1954 Jan 16;266(6803):119–124. doi: 10.1016/s0140-6736(54)90978-2. [DOI] [PubMed] [Google Scholar]


