Abstract
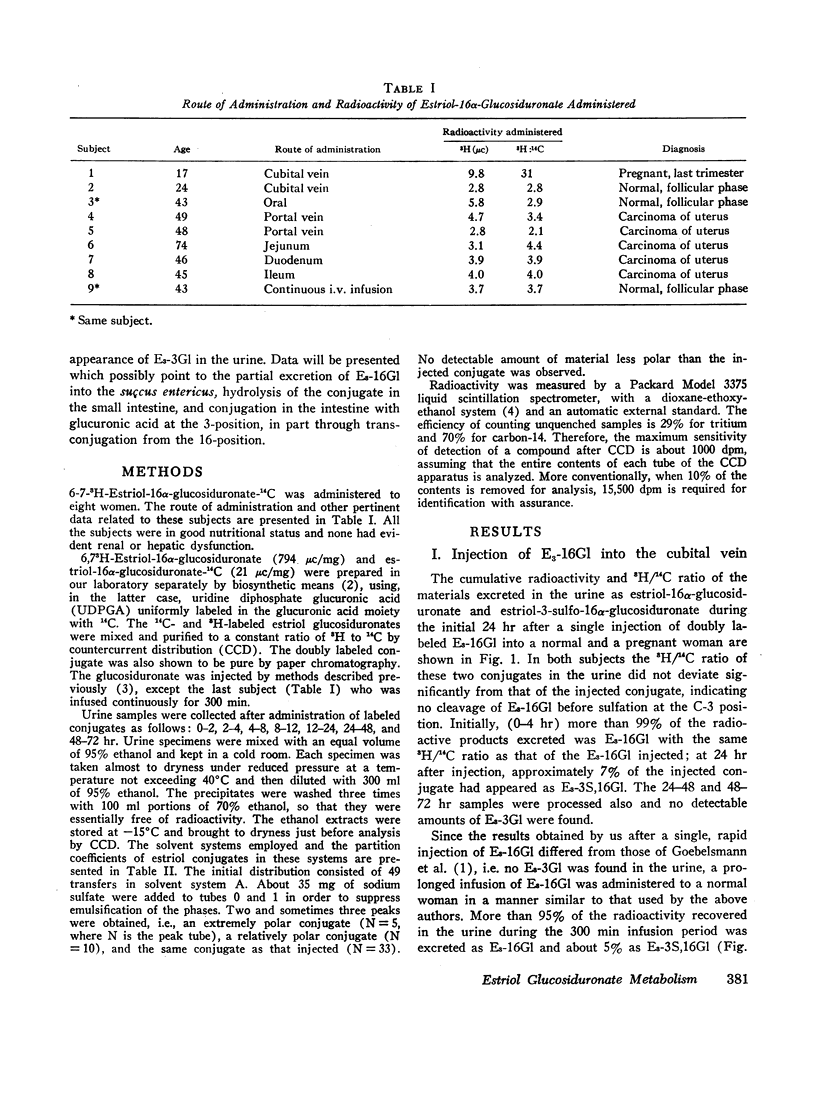
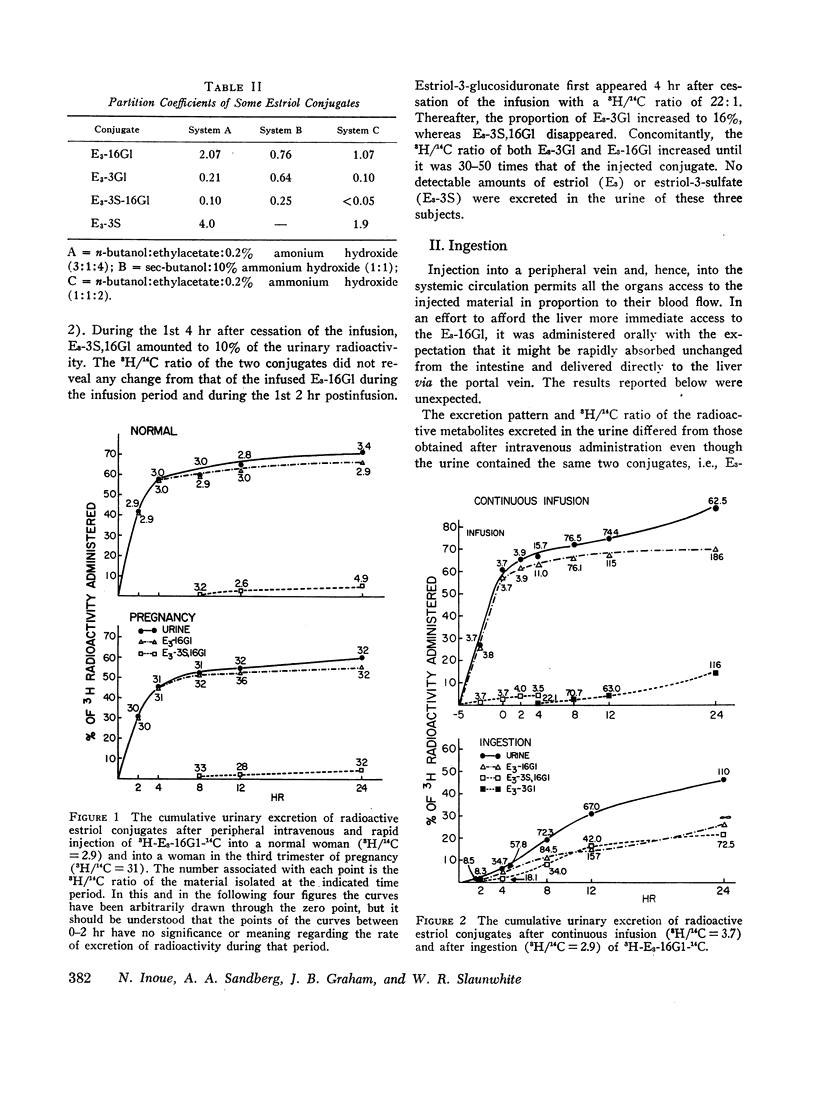
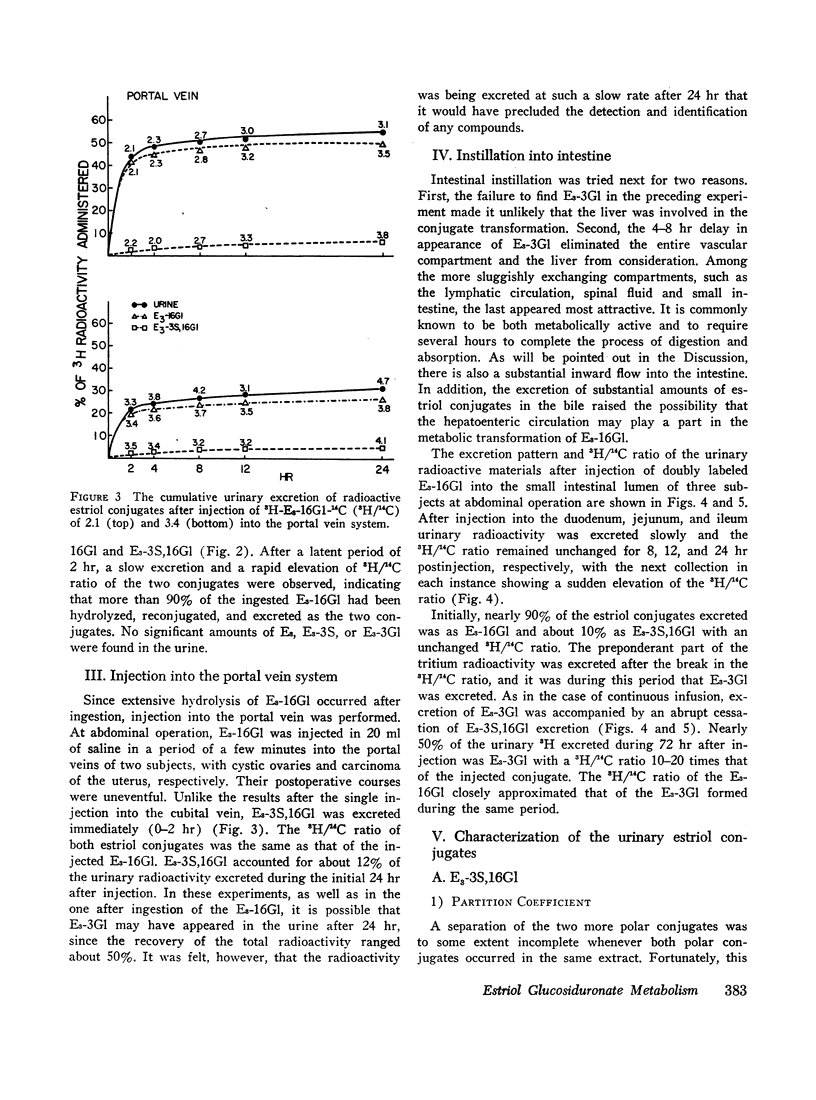
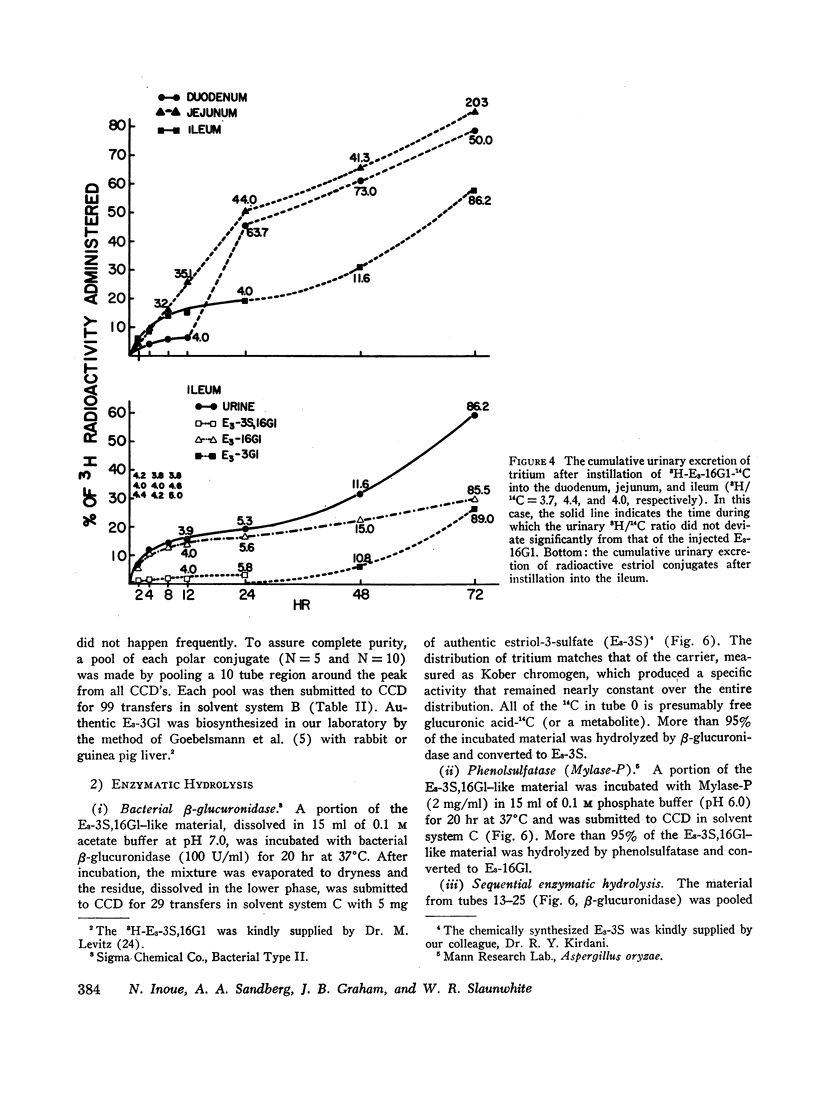
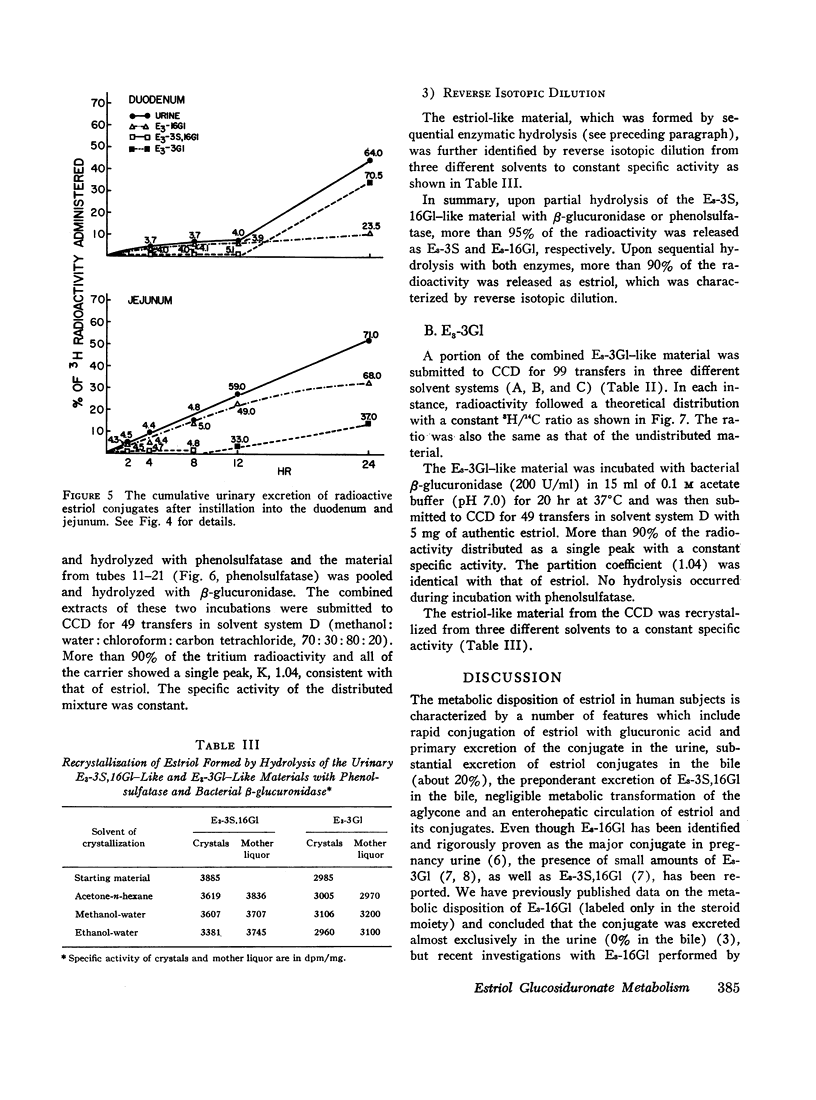
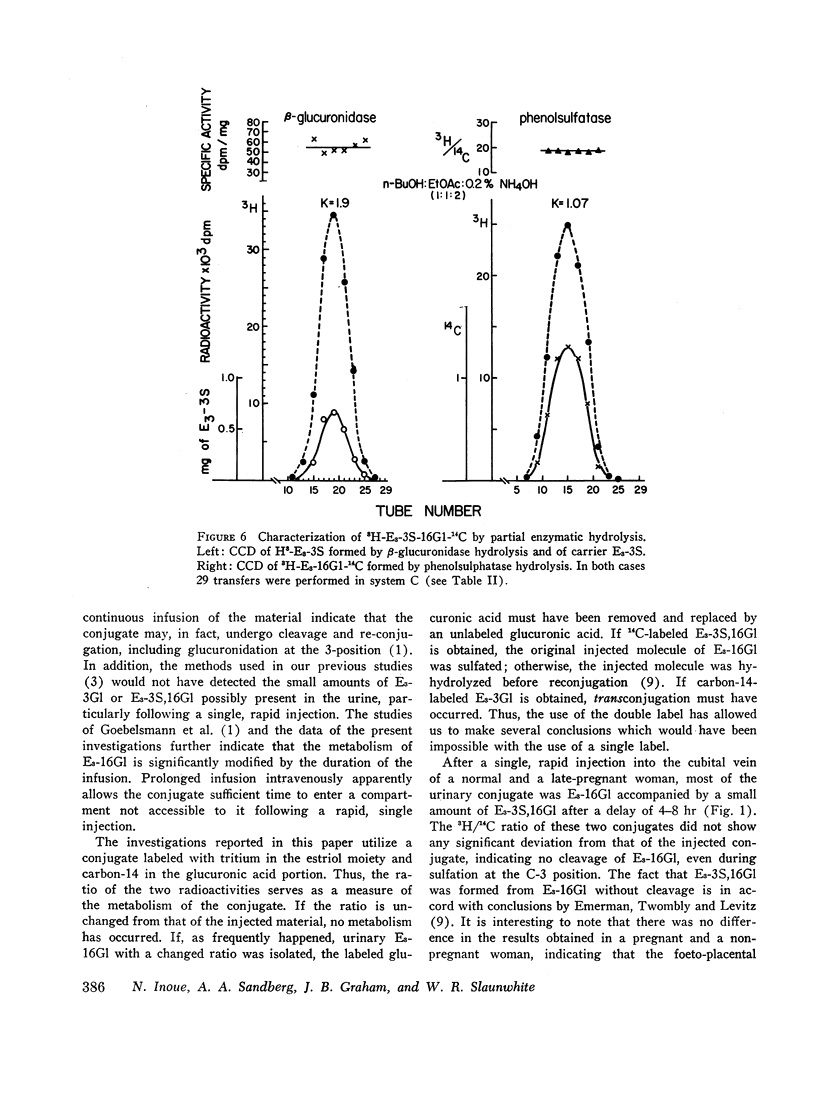
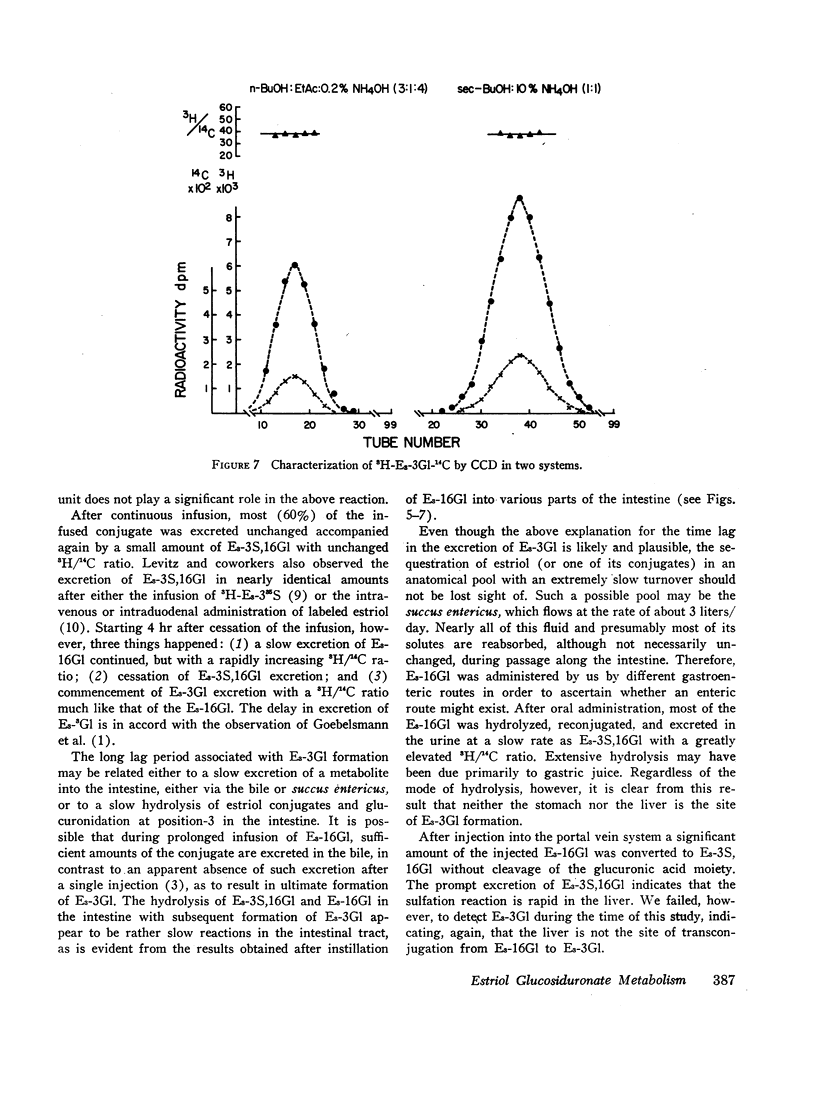
6,7-3H-Estriol-16α-glucosiduronate-14C was administered to eight women (nine studies) by several routes: both injection and infusion (300 min) into the cubital vein, injection into the portal vein system, ingestion and instillation into the duodenum, jejunum, and ileum. Urine, collected from 0-2, 2-4, 4-8, 8-12, and 12-24 hr, was analyzed by countercurrent distribution for its content of radioactive 3- and 16-glucosiduronate (E3-3Gl,E3-16Gl) and sulfoglucosiduronate (E3-3S,16Gl) of estriol as well as for 3H/14C ratio of each conjugate. After peripheral injection 50-60% of the injected E3-16Gl was excreted unchanged along with about 5% as E3-3S,16Gl with an unchanged 3H/14C ratio, indicating direct sulfation of the injected E3-16Gl. During a 300 min infusion, urinary excretion closely resembled that following injection. But 2-4 hr after the end of the infusion excretion of E3-3S, 16Gl stopped, excretion of E3-3Gl (17%/24 hr) with an elevated 3H/14C ratio started, and excretion of E3-16Gl continued (70%/24 hr), but with a rapidly increasing 3H/14C ratio. This indicated sequestration in a sluggishly metabolizing compartment where two processes occurred: (a) extensive hydrolysis of E3-16Gl followed by reconjugation at either C3 or C16 with unlabeled uridine diphosphate glucuronic acid (UDPGA), thereby increasing the 3H/14C ratio; and (b) transconjugation from C16 to C3, thereby producing E3-3Gl with finite 3H/14C ratios. Instillation into various segments of the small intestine produced results qualitatively similar to those after intravenous infusion, whereas ingestion and intraportal injection resembled peripheral intravenous injection. Therefore, we have postulated the possibility of an enteric circulation (in addition to an enterohepatic circulation) in which the steroid or its conjugates are transported into the small intestine in the succus entericus, modified, and then reabsorbed and excreted in the urine-a process which requires several hours.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertinchamps A. J., Miller S. T., Cotzias G. C. Interdependence of routes excreting manganese. Am J Physiol. 1966 Jul;211(1):217–224. doi: 10.1152/ajplegacy.1966.211.1.217. [DOI] [PubMed] [Google Scholar]
- DICZFALUSY E., FRANKSSON C., MARTINSEN B. Oestrogen conjugation by the human intestinal tract. Acta Endocrinol (Copenh) 1961 Sep;38:59–72. doi: 10.1530/acta.0.0380059. [DOI] [PubMed] [Google Scholar]
- Dahn K., Brewer H. Biogenese von Ostriol-16alpha-monoglucuronid und Ostriol-17beta-monoglucuronid. Acta Endocrinol (Copenh) 1966 May;52(1):43–53. [PubMed] [Google Scholar]
- Emerman S., Twombly G. H., Levitz M. Biliary and urinary metabolites of estriol-15-3H-3-sulfate-35S in women. J Clin Endocrinol Metab. 1967 Apr;27(4):539–548. doi: 10.1210/jcem-27-4-539. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S. Enzymic catalysis of glucuronyl transfer. J Biol Chem. 1957 Mar;225(1):435–452. [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebelsmann U., Cooke I., Wiqvist N., Diczfalusy E. Comparison of the metabolism of oestriol-3-glucosiduronate and oestriol-16-glucosiduronate in pregnant women. Acta Endocrinol (Copenh) 1966 May;52(1):30–42. doi: 10.1530/acta.0.0520030. [DOI] [PubMed] [Google Scholar]
- Goebelsmann U., Diczfalusy E., Katz J., Levitz M. Biosynthesis of radioactive estriol-3-glucosiduronate by guinea pig liver homogenate. Steroids. 1965 Dec;6(6):859–864. doi: 10.1016/0039-128x(65)90106-6. [DOI] [PubMed] [Google Scholar]
- Goebelsmann U., Sjöberg K., Wiqvist N., Diczfalusy E. Oestriol-3-glucosiduronate, a major urinary metabolite of oestriol and oestriol-16(17?)-glucosiduronate. Acta Endocrinol (Copenh) 1965 Oct;50(2):261–272. doi: 10.1530/acta.0.0500261. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., NEEMAN M. Isolation and characterization of estriol 16 alpha-glucosiduronic acid from human pregnancy urine. J Biol Chem. 1963 Apr;238:1273–1282. [PubMed] [Google Scholar]
- Hähnel R. The quantitative relationship of oestrogen-3-glucosiduronates and oestrogen-16 (or 17)-glucosiduronates in human late pregnancy urine. J Endocrinol. 1967 Aug;38(4):417–422. doi: 10.1677/joe.0.0380417. [DOI] [PubMed] [Google Scholar]
- Levitz M., Katz J. Enterohepatic metabolism of estriol-3-sulfate-16-glucosiduronate in women. J Clin Endocrinol Metab. 1968 Jun;28(6):862–868. doi: 10.1210/jcem-28-6-862. [DOI] [PubMed] [Google Scholar]
- Levitz M., Katz J., Twombly G. H. The biosynthesis of labeled estriol-3-sulfate-16-glucosiduronate. Steroids. 1965 Nov;6(5):553–559. doi: 10.1016/0039-128x(65)90018-8. [DOI] [PubMed] [Google Scholar]
- PLAGER J. E., KNOPP R., SLAUNWHITE W. R., Jr, SANDBERG A. A. CORTISOL BINDING BY DOG PLASMA. Endocrinology. 1963 Sep;73:353–358. doi: 10.1210/endo-73-3-353. [DOI] [PubMed] [Google Scholar]
- SANDBERG A. A., SLAUNWHITE W. R., Jr STUDIES ON PHENOLIC STEROIDS IN HUMAN SUBJECTS. VII. METABOLIC FATE OF ESTRIOL AND ITS GLUCURONIDE. J Clin Invest. 1965 Apr;44:694–702. doi: 10.1172/JCI105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAUNWHITE W. R., Jr, LICHTMAN M. A., SANDBERG A. A. STUDIES ON PHENOLIC STEROIDS IN HUMAN SUBJECTS. VI. BIOSYNTHESIS OF ESTRIOLGLUCOSIDURONIC ACID-16-C-14 BY HUMAN LIVER. J Clin Endocrinol Metab. 1964 Jul;24:638–643. doi: 10.1210/jcem-24-7-638. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Pierce C. E., Rider A., Clifton J. A. Absorption of L-methionine from the human small intestine. J Clin Invest. 1968 Feb;47(2):417–425. doi: 10.1172/JCI105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. R., Kellie A. E. Oestrogen conjugates of human late-pregnancy urine. Biochem J. 1967 Jul;104(1):83–94. doi: 10.1042/bj1040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoa K. F., Levitz M. Comparison of the conjugated metabolites of intravenously and intraduodenally administered oestriol. Acta Endocrinol (Copenh) 1968 Apr;57(4):657–668. doi: 10.1530/acta.0.0570657. [DOI] [PubMed] [Google Scholar]


