Abstract
Adeno-associated viruses (AAV) are small, nonenveloped single-stranded DNA viruses which require helper viruses to facilitate efficient replication. These recombinant viruses are some of the most promising candidates for therapeutic gene transfer to treat many genetic and acquired diseases. Nevertheless, the presence of humoral responses to the wild-type AAV common among humans is one of the limitations of in vivo transduction efficacy in humans using cognate recombinant vector. In this study, based on the serum samples that we were able to collect from various clinical situations, we studied the impact of one to five plasmapheresis (PP), at 1–5 day intervals on neutralizing factor (NAF) titers specific for AAV types 1, 2, 6, and 8 in seropositive patients with diverse pathologies and immunosuppressor treatments. We show that frequent sessions of PP result in drastic reduction of NAF specific for AAV1, 2, 6, and 8 to undetectable levels or titers <1:5, mainly when initial titers, i.e., before the first PP were ≤1:20. Altogether, these results show that the use of PP and its possible association with pharmacological immunosuppressive treatments may help to design optimal management of seropositive patients for AAV gene therapy treatments.
Introduction
Adeno-associated viruses (AAV) is currently considered to be some of the most useful gene therapy vectors, in large part due to the lack of pathogenicity of the wild-type virus, the ability to establish long-term transgene expression, to transduce both dividing and nondividing cells over a broad host range (human, simian, murine, canine, and avian cells) and their low immunogenicity compared to other viruses. AAV is small, nonenveloped single-stranded DNA virus which belongs to the parvovirus family. AAV depends upon a helper virus such as adenovirus for active replication and in the absence of a helper establishes a latent state in which its genome is maintained essentially as chromatinized episomes and eventually as integrated into the host genome.1
Serotypes 1 and 2 recombinant AAV (rAAV) have met some success in phase I human trials.2,3,4,5,6 However, complications of treatment related to immune responses against the vector have emerged as serious obstacles for successful translations to humans. Indeed, in clinical trials, a detrimental cellular immune response against the AAV1 and 2 capsid, was observed using several routes of vector administration.7,8,9,10 Nevertheless, several other major observations showed that the host immune response to AAV capsid is primarily mediated by circulating antibodies, which may prevent repeated administrations.11 Data indicate that even low levels of neutralizing antibodies (1:5–1:10) completely abrogate AAV-mediated transduction.11,12,13
The exploitation of the distinct tissue tropism of the various AAV serotypes has provided an opportunity to improve the efficiency of gene delivery to specific target tissues. AAV1, 6, 8, and 9 vectors are among the most efficient for transduction of various tissues including muscle, liver, and heart.14,15,16,17,18,19,20
The presence of specific immunoglobulin and neutralizing factors (NAF) against the wild-type AAV, which commonly circulates in humans is one of the limitations of in vivo transduction efficacy using cognate recombinant vector.21,22,23
Plasmapheresis (PP), or therapeutic plasma exchange, is an extracorporeal technique that was established to remove large molecular weight substances from the plasma. Autoantibodies, immune complexes, cryoglobulins, and cholesterol-containing lipoproteins are examples of such substances. PP was also found to be effective for removing the pathogenic immunoglobulins.24 Approximately 75% of immunoglobulin M is intravascular with a short half-life. As a result, only one or two consecutive PPs are usually enough to rapidly reduce immunoglobulin M levels. In comparison, only 45% of immunoglobulin G (IgG) are intravascular with a longer half-life, and within 48 hours post-PP, the plasma IgG production returns to 40% of the preapheresis level. IgG serum level is also characterized by a “rebound” phenomenon, whereby effect of PP is transient and IgG level returns to pre-PP or even higher values, especially if the patient is not on immunosuppressive therapy. As a result, a more rigorous regimen involving more frequent PP sessions and the concomitant institution of an immunosuppressive therapy are both required to efficiently reduce IgG levels.25 Importantly, PP is generally a safe medical procedure performed worldwide in adult, but also in children, in pathological situations. Of note, procedures similar to PP are also performed each year on a large number of volunteer donors for plasma and platelets collection.
In this study, we evaluated the effect of one to five consecutive PPs performed at 1–5 day intervals on the titer of NAF specific for AAV types 1, 2, 6, and 8 in seropositive patients with diverse pathologies and immunomodulator treatments. One major difficulty to establish the experimental cohort was the ability to have patients primarily undergoing successive PPs for other medical reason(s) other than gene therapy without receiving intravenous immunoglobulins (IVIg) or heterologous plasma during the last 4 weeks before the first PP, nor during the following successive PPs, because these compounds are rich in NAF. We found that despite the post-PP “rebound” profiles observed, the overall NAF titer fold decrease varied between 1 and 64 from the first to the fifth PP. Interestingly, NAF specific for AAV1, 2, 6, and 8 became undetectable (<1:2), respectively for 1/9, 1/10, 3/8, and 4/10 patients or low (≤1:5–1:2), respectively for 4/9, 1/10, 4/8, and 2/10 patients, after 2–5 consecutive PPs. Altogether, these results suggest that, in some preimmunized patients, the use of PP associated with classical pharmacological immunosuppressive treatments may allow the use of AAV vectors for gene therapy in seropositive patients.
Results
Patient/serum selection criteria
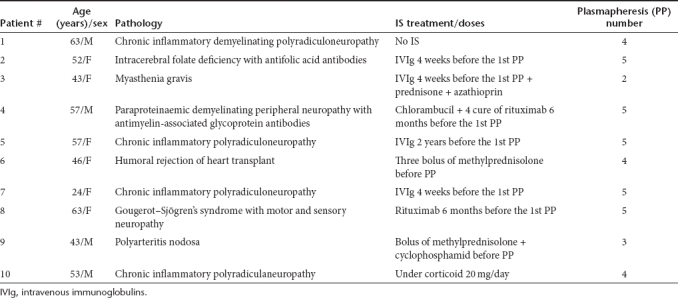
Selected patients were analyzed for the prevalence of NAF against rAAV types 1, 2, 6, and 8 before the first PP. Selection was a major hurdle since it was driven by: (i) the requirement of patients undergoing PPs for different diseases who did not receive IVIg or heterologous plasma during the last 4 weeks before the first PP, nor during the following successive courses of PPs; (ii) short intervals (≤5 days) (Table 2) between PP sessions. Other important factors that we could not control were: (i) the primary disease and (ii) the associated immunosuppressive treatment that varied in nature and dosing (Table 1). During the period of time of the study (November 2008 to September 2010), 58 patients that underwent repeated PPs were screened for the presence of NAF specific to AAV. Among them, 10 were eligible and enrolled.
Table 1. Characteristics of patients.
AAV NAF profiles after 3–5 successive PPs
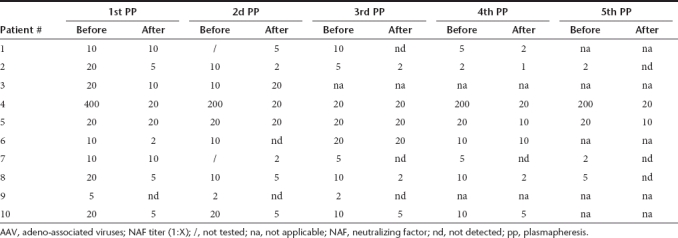
Selected sera were considered positive for neutralizing capacity when a minimum of 1:2 dilution of the serum inhibited vector transduction by 50% or more. Prior PP, all 10 patients were found positive at various degrees for all AAV serotypes studied except for patient #6 who had no detectable NAF against serotype 6 and patient #9 who had no detectable NAF against serotypes 1 and 6 (Tables 2,3,4, and 5). NAF titers at the screening step, i.e., prior PP, were (AAV2) 1:5–1:12,800, (AAV1) 1:20–1:800, (AAV6) 1:5–1:200, and (AAV8) 1:5–1:400. Serum samples were analyzed for the presence of AAV NAF before and after up to five successive PPs performed with a maximal interval of 5 days between them.
Table 2. Plasmaphereses schedule for each patient.
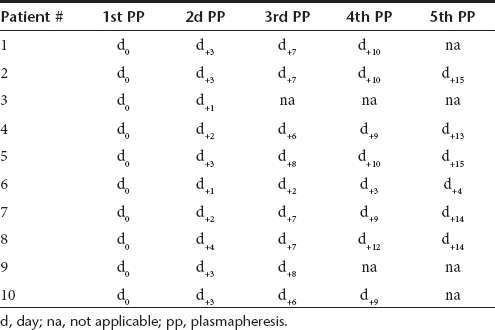
Table 3. Effect of multiple successive PP on the AAV2-specific NAF titers.
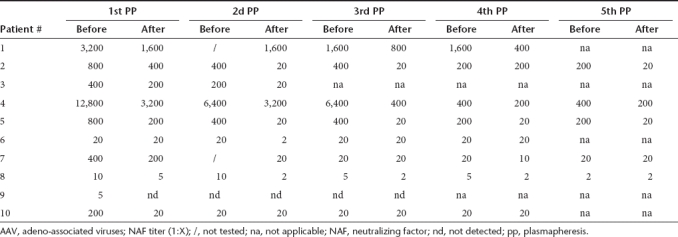
Table 4. Effect of multiple successive PP on the AAV1-specific NAF titers.
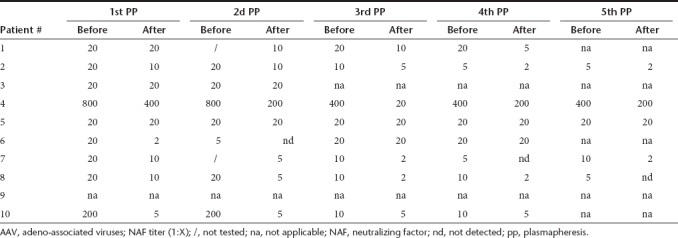
Table 5. Effect of multiple successive PP on the AAV6-specific NAF titers.
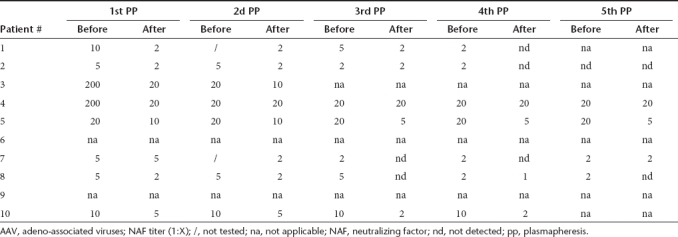
Initial titers of NAF against AAV2 prior PPs were quite heterogeneous and patients could be attributed to three groups: group 1 with titers >1:800, group 2 with titers >1:200 <1:800, and group 3 with titers <1:200 (Table 3). When the “rebound” phenomenon was observed it appeared rapidly after the second or third PP. The NAF titer fold decreases were between 1 to 10 after the first, fourth, and fifth PP, between 1 to 20 after the second and third PP (Table 3). Ultimately, despite the “rebound” effects observed, the total NAF titer fold decreases between the first and the fifth PP were between 1 (patient #6) to 64 (patient #4). Nevertheless, none of the final titer ended up <1:20, except for patients #8 and #9 who had low initial titers (1:10 and 1:5, respectively) (Table 3).
In contrast to AAV2, initial NAF titers against AAV1 were more homogeneous. Indeed, all exhibited titers of 1:20, except patients #4 and #10 who had titers ≥ 1:200 (Table 4). As observed for AAV2, the “rebound” event appeared as early as the second or third PP and its intensity decreased with further PP courses (Table 4). The NAF titer fold decreases were between 1 and 40 after the first and the second PP, between 1 and 20 after the third PP, and between 1 and 10 after the fourth and fifth PP. Finally, the total NAF titer fold decreases between the first and the fifth PP were between 1 (patients #3; #5, and #6) to 40 (patient #10) and the final titer was ≤ 1:5 for four out of nine patients (patients #1, #2, #7, and #10) and undetectable for one out of nine patients (patient #8). The multiple regimens of PP were inefficient on patients #5 and #6, as observed for one of these patients in AAV2 (patient #6).
Initial NAF titers against AAV6 were ≤1:200 (Table 5). For the AAV6 serotype the “rebound” event appeared to have moderate impact (Table 5). The NAF titer fold decreases were between 1 and 10 after the first PP, between 1 and 2.5 after the second and between 1 and 5 after the third and fourth PP, and between 1 and 4 after the fifth PP. Finally, the total NAF titer fold decreases between the first and the fifth PP were between 2.5 (patient #7) and 20 (patient #3). Interestingly, the final titer was of 1:5 for 1 patient (patients #5); 1:2 for 2 patients (patients #7 and #10); and undetectable for three out of eight patients (patients #1, #2, and #8).
Initial NAF titers against AAV8 were also rather homogeneous. Except for patient #4 who had a titer >1:400, all patients presented titers ≤1: 20 (Table 6). As for AAV6, the “rebound” phenomenon had a moderate impact since the initial titers were low (Table 6). The NAF titer fold decreases were between 1 and 20 after the first PP and between 1 and 10 after the second, third, and fourth PP and between to 2 and 10 after the fifth PP (Table 6). Ultimately, the total NAF titer fold decreases between the first and the fifth PP were between 1 (patients #3 and #6) and 20 (patients #2; #4, and #8), and the final titer was ≤ 1:5 for 2 out of 10 patients (patients #1 and #10) and undetectable for 4 out of 10 patients (patients #2, #7, #8, and #9). The multiple regimens of PP were almost inefficient in patients #5 and #6, as observed for other AAV serotypes.
Table 6. Effect of multiple successive PP on the AAV8-specific NAF titers.
Fate of AAV serum Ig after 3–5 successive plasmaphereses
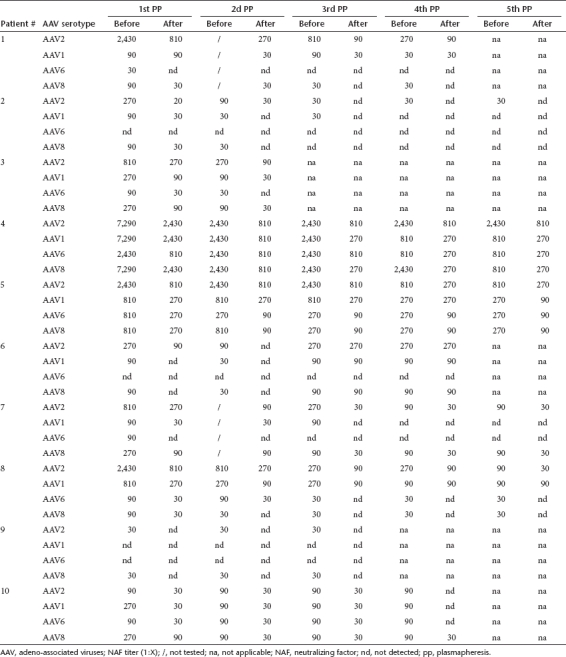
To look for sensible correlation between NAF and Ig antibodies against AAV, we determined the seroprevalences of total Ig antibodies to AAV of serotypes 1, 2, 6, or 8 using AAV-specific enzyme-linked immunosorbent assay (ELISA) on serum samples from adult patients. This prevalence of Ig is a relevant indicator of the frequency of individuals who are not naive for one of the AAV serotypes. Whatever the AAV serotype analyzed, the Ig titer fold decrease was usually three after each PP, but reached 90 in some cases (Table 7). The total Ig titer fold decreases between the first and the fifth PP were between 1 (patient #6) and 270 (patients #4 for AAV1, 6, and 8; patient #5 and #6 for AAV2; patient #10 for AAV1). Interestingly, the Ig-specific final titer was <1:30 for three patients for AAV2 (patients #2, #9, and #10) and AAV1 (patients #2, #7, and #10). It was also < 1:30 for five out of seven patients for AAV6 (patients #1, #3, #7, #8, and #10) and for 4 out of 10 patients for AAV8 (patients #1, #2, #8, and #9). Of note, the sensitivity of the ELISA which is lower (serum dilution factor 1:30) than that of neutralizing assay (serum dilution factor 1:2) may explain some discrepancies between these results. Altogether, the ELISA results correlate to the NAF findings.
Table 7. Effect of multiple successive PP on the AAV2, 1, 6 and 8-specific Ig titers.
Discussion
rAAV vectors are potential and powerful therapeutic candidates in several clinical indications. They allow permanent gene expression in many different animal species and long-term data are accumulating in patients. Despite the well-established safety and efficacy of AAV vectors in preclinical and clinical studies, challenges remain to be addressed. A major obstacle that can attenuate the efficacy of AAV vectors in vivo is the prevalence of preexisting NAF, including antibodies, in humans previously exposed to AAV.21,22,23
In this study, we evaluated the effect of one to five PP, at 1–5 day intervals on NAF titers specific for AAV types 1, 2, 6, and 8 in AAV seropositive patients with diverse pathologies and immunosuppressor treatments. Indeed, PP, a process in which blood is withdrawn, the plasma removed, and the red cells reinfused after resuspension in a replacement solution, has been used for a variety of applications and pathologies such as organ transplant, neurological disease (e.g., Guillain–Barré), and hematological disorders (e.g., thrombotic thrombocytopenic purpura). Plasma exchange and immunoadsorption columns are two powerful techniques for therapeutic PP in the management of such diseases by physically eliminating free antibodies and circulating immune complexes from the plasma. Applications of therapeutic PP can be broadly subdivided into two general categories: (i) acute toxicity for which PP is used to lowers the circulating pathogenic agent/molecules and (ii) chronic diseases, in which there is constant production of pathogenic antibodies. Because PP does not address the underlying pathology, and, due to the phenomenon of rebound antibody production, its use in chronic diseases has been more controversial than in acute toxicity indications.
To overcome this rebound effect, novel techniques of serological immunomodulation, which act directly on pathogenic antibodies and involve IVIg26 are used. In gene therapy mediated by AAV the use of IVIg as a serological immunomodulator is excluded due to the presence of AAV NAF at high titer in such preparations (data not shown). For this reason, patients who received IVIg or heterologous plasma were excluded from this study. Nevertheless, the use of PP and classical pharmacological immunosuppressive treatments (such as corticosteroids, cyclophosphamid, and rituximab) may help to design optimal clinical trial.
It has been previously reported that even low levels of neutralizing antibodies (1:5–1:10) specific for AAV can completely abrogate transduction with high-titer vectors.11,12,13 Hurlbut et al. recently reported the effect of PP on neutralizing antibodies, but it was only for the serotype 8 and after only one PP.27 In our study, we showed that after 1–5 PPs performed in a short period of time, NAF specific for AAV2, 1, 6, and 8 became undetectable for some patients when initial titers at the screening steps were ≤1:20. Furthermore, it was also possible to reduce the NAF titer to 1:5–1:2 but also for patients with low initial titers (≤1:20). Overcoming these residual NAF can be achieved by increasing vector dose, as previously described.27 For patients with initial intermediate NAF titers (>1:20 ≤1:800) or high titers (>1:800), significant titer reductions were also observed, but only to respectively titers 1:20 and 1:200, even after five PP sessions. Therefore, we can assume that, for these patients, the use of PP in combination with B cell targeted immunosuppression, use of alternate serotypes, or additional PP rounds might be necessary to drastically reduce NAF titers. Of note, the NAF titer decrease is mainly correlated with an Ig titer reduction. Nevertheless, it cannot be excluded, as reported for other viruses,28,29,30,31 that in certain cases some other unidentified factors present in the serum can at least in part be responsible for the virus neutralization. Interestingly, therapeutic PP remove components that may include antibodies, immune complexes, mediators of inflammation or complement activation, toxins, lipids, and other potentially harmful molecules. T-cell response against the capsid was also described as a limitation to vector transduction even when baseline levels of anti-AAV antibodies were not very high.7,8,9,10 Nevertheless, for these patients the use of PP in combination with T-cell immunosuppressive treatments should also be necessary to overcome these capsid-T cell-specific responses
The reasons for the clinical effectiveness of PP are not well understood, but they do not appear to be limited to the reduction of antibody and immune complex levels.32 In addition to antibodies and immune complexes, the removal of proinflammatory agents and soluble adhesion molecules seems to enhance the beneficial effect.
Altogether, our results show that the applicability of this PP approach to eliminate preexisting immunity to AAV in the human population may be efficient for serotypes 6 and 8 and possibly 1, for which initial titers are moderate (≤1:20) compared to AAV2, and despite the fact that we have a limited number of eligible and uncontrolled patients. Our results also indicate that for patients with intermediate or high NAF titers, PPs allow to significantly reduce these titers, but not <1:20. Indeed, a rigorous PP regimen involving frequent sessions and association with pharmacological immunosuppressive treatment may expend AAV gene therapy indication in cases of primo-administration to AAV seropositive patients or for patient readministration.
Materials and Methods
Patients. Ten patients with pathologies such as autoimmune disease or waiting for deceased-donor transplants received PP, combined or not with immunomodulatory treatments (Table 1). Serum samples from these patients were harvested in the connecting tubes. This study has been approved by the Ethical Review Committee of the Cochin Hospital, Paris, France. Patients were analyzed for the AAV-specific NAF titer before and immediately after each PP that they received. The treatment interval was every 1–5 days. PP was done on a cell separator COBE spectra (COBE BCT, Lakewood, CO). The standard PE procedure consisted of removal of one and half plasma volume during each session, without specificity for an Ig subclass, with replacement of 100% of the volume removed. The red and white blood cells are returned, while the plasma is discarded and replaced with other fluids. The replacement fluid was albumin (5% solution). Donors who received fresh frozen plasma or high-dose IVIg treatment in the 4 weeks before the first PP were excluded.
Production of AAV vectors and contaminant proteins. Pseudotyped AAV vectors were generated by packaging AAV2-based recombinant genomes in AAV1, 2, 6, and 8 capsids, as previously described.23 Briefly, all the vectors used in the study were produced using the three-plasmid transfection protocol as described elsewhere. Briefly, HEK293 cells were tri-transfected with the adenovirus helper plasmid pXX6,33 a pAAV packaging plasmid expressing the rep and cap genes pACG2.1 for AAV2,34 pLT-RC02 for AAV1,35 pLTRCO6 for AAV6,35 and p5e18-VD2-8 for AAV836 and the relevant pAAV2 vector plasmid. Single-stranded AAV vectors were produced with conventional pGG2 AAV2 vector plasmid-expressing luciferase37 under the transcriptional control of the cytomegalovirus immediate early (CMV IE) promoter associated with the SV40 polyA signal. The contaminant proteins were obtained using the same transfection protocol on HEK293 cells but lacking the packaging plasmid. Recombinant vectors and contaminant proteins were purified by double-cesium chloride ultracentrifugation followed by dialysis against sterile phosphate-buffered saline. Viral genomes (vg) were quantified by real-time PCR and vector titers are expressed as vg/ml and contaminant proteins were quantified by the Bradford protein assay.
ELISA. ELISA was performed as previously described.23 Briefly, rAAV particles were diluted in coating buffer (0.1 mol/l carbonate buffer at pH 9.5) to a final concentration of 2 × 1010 vg/ml. Fifty microliters were added to each well in a 96-well Nunc Maxisorp Immunoplate (Thermo Fisher Scientific, Roskilde, Denmark). At the same time, proteins corresponding to contaminants purified during the steps of rAAV production, but in the absence of viral particle formation, were diluted in the same buffer and seeded, in parallel, in different wells in the same immunoplate, at a concentration of 3.4 µg/ml. This amount of protein corresponds to an amount of contaminant protein equivalent to that seeded in the AAV wells and the signal obtained at the end corresponds to the nonspecific signal that was removed from the signal obtained with the same serum in the corresponding AAV wells. Plates were then incubated overnight at 4 °C. The next day, plates were washed three times with blocking buffer (6% fat milk buffer in phosphate-buffered saline) then blocked with blocking buffer for 2 hours at room temperature. Plates were washed again three times with wash buffer (0.05% Tween-20 in phosphate-buffered saline) then incubated with heat-inactivated serum (at 56 °C for 30 minutes) diluted at 1/30 to 1/65610, for 1 hour at 37 °C. After three washes, horse-radish peroxidase-conjugated antibodies specific for Ig purchased from Southern Biotech (Birmingham, UK) were added and incubated 1 hour at 37 °C. Finally, plates were washed three times with wash buffer and revealed with TMB substrate solution (Becton Dickinson Biosciences, Mountain View, CA), 30 minutes in the dark. The reaction was stopped with H2SO4 solution and measurements were made at 450 nm. The results are expressed in arbitrary units: optic density (OD) using a colorimetric amplification system based on peroxidase.
The AAV-specific signal was reported as the OD from AAV-coated ELISA after removal of the OD obtained on contaminant protein ELISA, which is a nonspecific signal (OD AAV-OD NSS). Sera were considered positive for AAV-specific Ig when OD signal were ≥0.5 (cutoff based on mean OD of 58 negative healthy donors + 3 SD) at dilution ≥1:30.
Neutralizing assay. The neutralizing assay was performed as previously described.23 Briefly, on day 1, 48-well plates were seeded either with 5 × 104 Hela cells/well or with 5 × 104 human hepatoma cell line Huh7 cells/well for 24 hours. On day 2, rAAV-CMV-Luciferase was diluted in Dulbecco's modified Eagle's medium (Invitrogen Life Technology, Auckland, CA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT) and incubated with a two- to tenfold serial dilutions (1:2–1:12,800) of heat-inactivated (at 56 °C for 30 minutes) serum samples for 1 hour at 37 °C. Subsequently, the serum-vector mixtures corresponding to 5 × 103 to 2 × 104 vg/cell, depending on the serotype, were added to cells plated on day 1 and incubated in Dulbecco's modified Eagle's medium + 10% fetal calf serum for 48 hours at 37 °C and 5% CO2. Each mix was performed in duplicate. Cells were then washed in phosphate-buffered saline and lysed for 10 minutes in 0.2% Triton lysis buffer at 4 °C. The lysate was transferred to 96-well plates and the luciferase activity was read on a luminometer (VICTOR2; Perkin Elmer/Life Sciences, Waltham, MA). Transduction efficiency was measured as relative light units, per second per well and normalized per amount of protein per well expressed as optical density (relative light units/second/w/OD).
The neutralizing titer was reported as the highest serum dilution that inhibited the rAAV transduction by ≥50% compared with the control without serum and correlated with the amount of protein quantified in each well after cell lysis by the Bradford assay.
Acknowledgments
This work was supported by the Association Française contre les Myopathies (AFM) and Advanced Diagnostics for New Therapeutic Approaches (ADNA), a program dedicated to personalized medicine, coordinated by Institut Mérieux and supported by research and innovation aid from the French public agency, OSEO. We thank Fréderic Barnay-Toutain for providing viral vectors. We thank Susan Cure for critical reading of the manuscript. The authors declared no conflict of interest.
REFERENCES
- Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Chérel Y, Chenuaud P.et al. (2008Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle J Virol 827875–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A.et al. (2000Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector Nat Genet 24257–261. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR.et al. (2003AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B Blood 1012963–2972. [DOI] [PubMed] [Google Scholar]
- Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D.et al. (2004Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial Chest 125509–521. [DOI] [PubMed] [Google Scholar]
- Nierman, M, Twisk J, Hermens WT, Baker D, Van Den Deventer S, Rekke B.et al. (2007Safety and efficacy of AMT-010, an adeno-associated virus based gene therapy vector administered to lipoprotein lipase-deficient subjects Hum Gene TherA8: Inv3.
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP.et al. (2008Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients Arterioscler Thromb Vasc Biol 282303–2304. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg J, Hui DJ, Basner-Tschakarajan E, de Jong A, Pos P.et al. (2007Capsid-specific T cell responses in humans upon intramuscular administration of an AAV-1 vector expressing LPLs447X transgene Hum Gene Ther 1894 [Google Scholar]
- Mingozzi F, Meulenberg J, Hui DJ, Basner-Tschakarajan E, de Jong A, Pos P.et al. (2007Intramuscular administration of an AAV-1 vector in humans results in capsid-specific T cell-responses Mol Ther 15S403 [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA.et al. (2009AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Blood 1142077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S.et al. (2006Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice Blood 1071810–1817. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA.et al. (2000Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system Proc Natl Acad Sci USA 973428–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ.et al. (2003Adeno-associated viruses undergo substantial evolution in primates during natural infections Proc Natl Acad Sci USA 1006081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos T, Graham IR, Foster H., and, Dickson G. Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD) Gene Ther. 2004;11 Suppl 1:S109–S121. doi: 10.1038/sj.gt.3302379. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L.et al. (2006rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice Nat Med 12787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, Fu FH., and, Xiao X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009;27:421–426. doi: 10.1002/jor.20781. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J.et al. (2005Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart Nat Biotechnol 23321–328. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA.et al. (2006Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8 Mol Ther 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SL, Li H, Mingozzi F, Sabatino DE, Hui DJ, Edmonson SA.et al. (2009Diverse IgG subclass responses to adeno-associated virus infection and vector administration J Med Virol 8165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF.et al. (2010Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors Hum Gene Ther 21704–712. [DOI] [PubMed] [Google Scholar]
- Poullin P, Pietri PA., and, Lefèvre P. Heparin-induced thrombocytopenia with thrombosis: successful treatment with plasma exchange. Br J Haematol. 1998;102:630–631. doi: 10.1046/j.1365-2141.1998.0847b.x. [DOI] [PubMed] [Google Scholar]
- Kolins J., and, Jones JE. American Association of Blood Banks, Arlington; 1983. Therapeutic Apheresis. [Google Scholar]
- Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E.et al. (2010Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy Mol Ther 181983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Ihle JN, Oleszko O., and, Barnes RD. Virus-specific neutralization by a soluble non-immunoglobulin factor found naturally in normal mouse sera. Proc Natl Acad Sci USA. 1975;72:5071–5075. doi: 10.1073/pnas.72.12.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Jensen FC., and, Cooper NR. Neutralization of Epstein-Barr virus by nonimmune human serum. Role of cross-reacting antibody to herpes simplex virus and complement. J Clin Invest. 1982;70:1081–1091. doi: 10.1172/JCI110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SL, Frank I, Yee A, Cohen GH, Eisenberg RJ., and, Friedman HM. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990;162:331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Ebenbichler CF, Thielens NM, Vornhagen R, Marschang P, Arlaud GJ., and, Dierich MP. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KM. Plasmapheresis and immunoadsorption: different techniques and their current role in medical therapy. Kidney Int Suppl. 1998;64:S61–S65. [PubMed] [Google Scholar]
- Xiao X, Li J., and, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Samulski RJ., and, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière C, Danos O., and, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B.et al. (1997Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice Hum Gene Ther 81891–1900. [DOI] [PubMed] [Google Scholar]


