Abstract
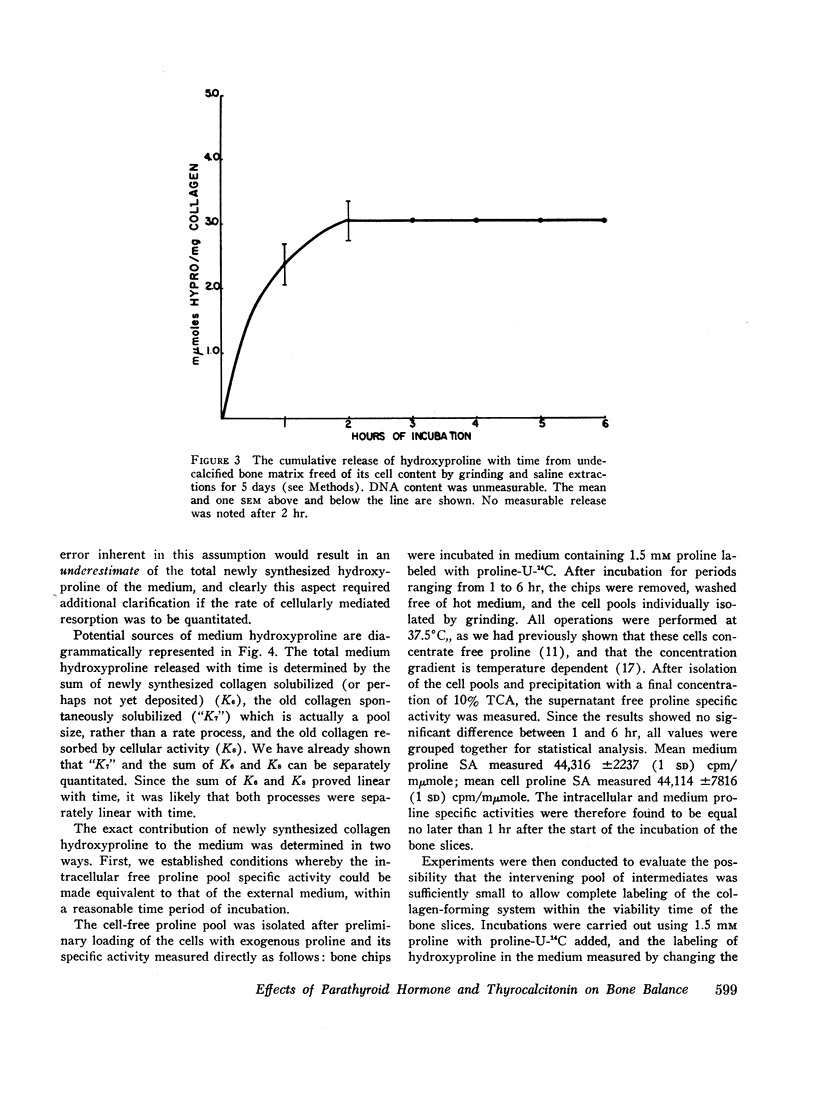
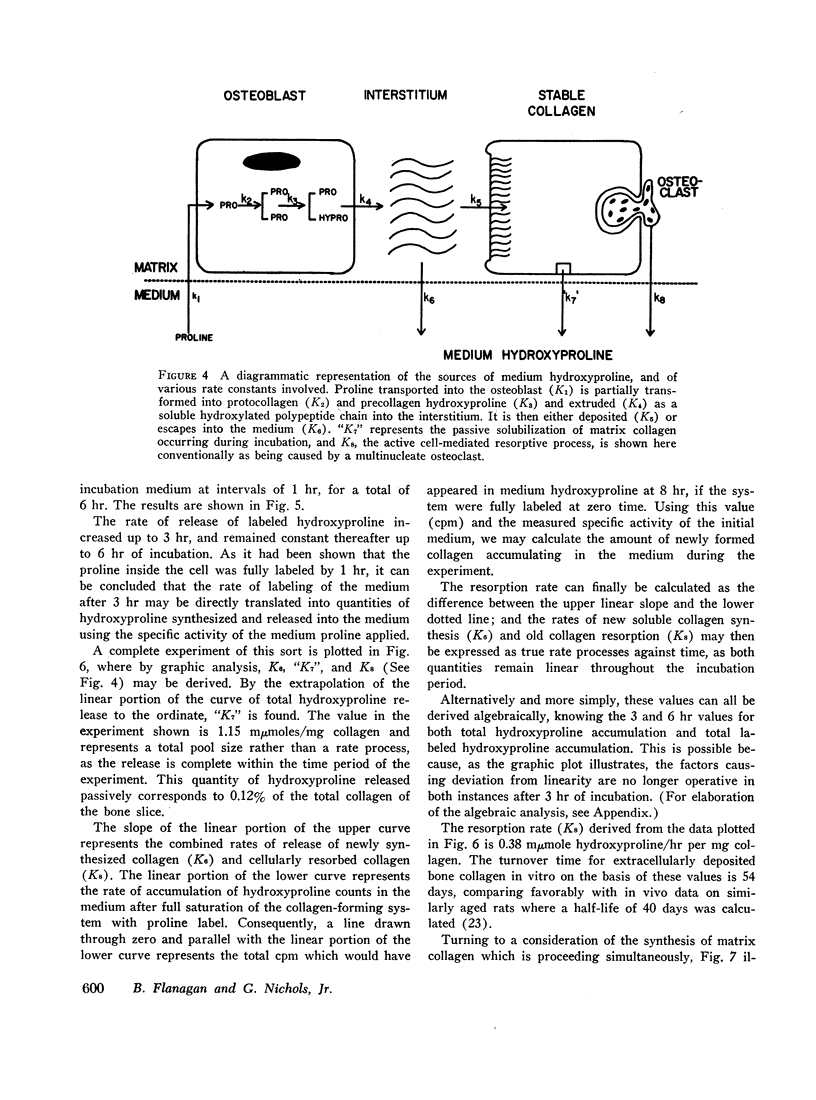
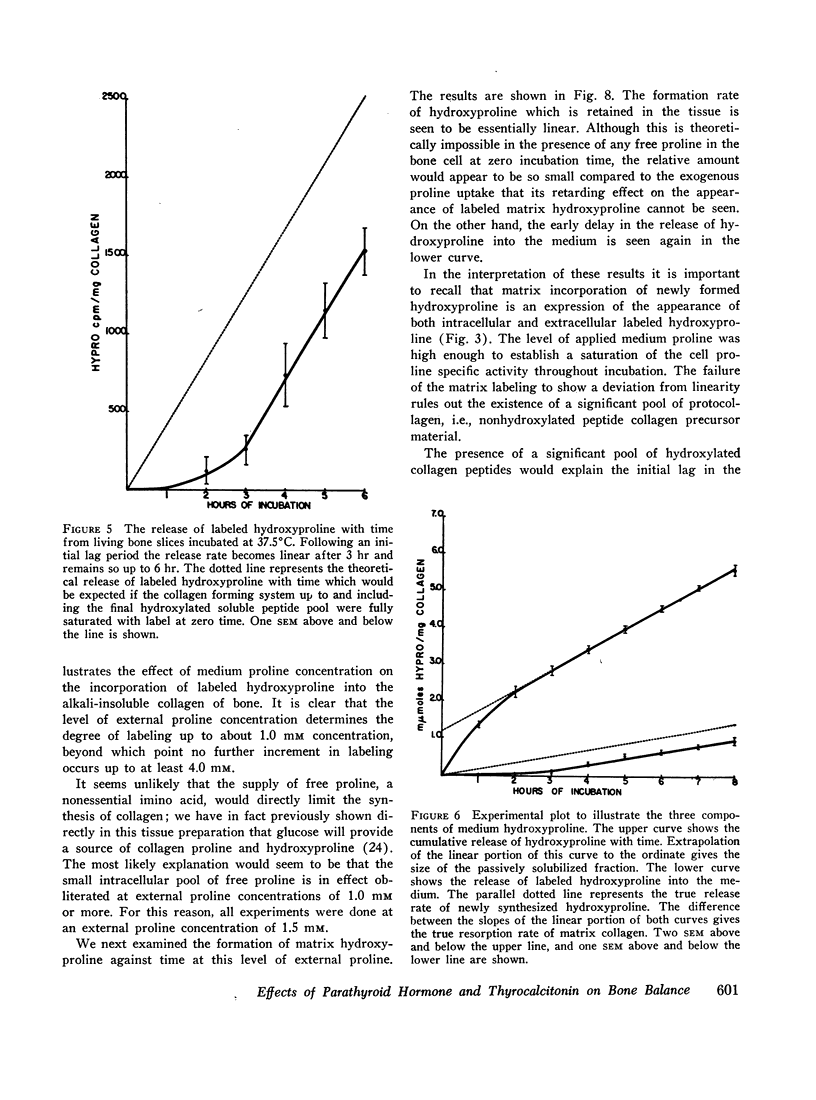
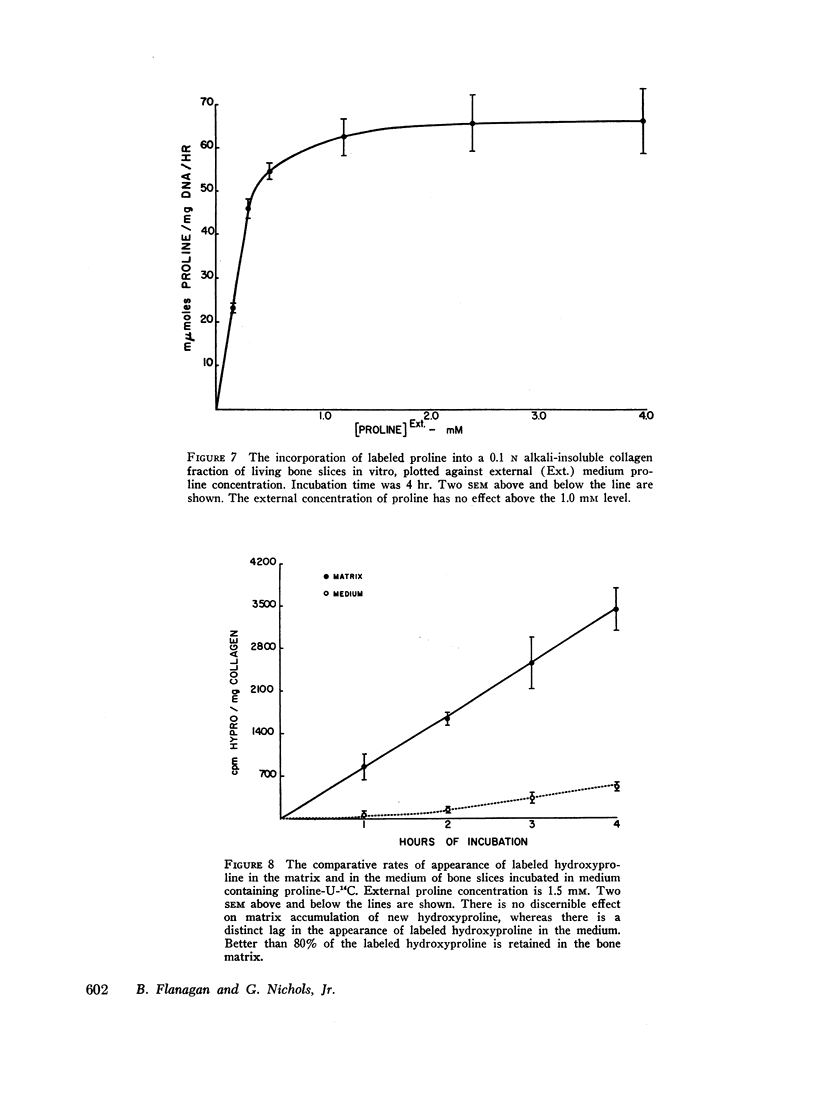
Labeled proline from incubation media has been shown to be incorporated into living bone matrix collagen in vitro. Hydroxyproline is released from fresh bone slices in similar systems in a characteristic curve against time. This hydroxyproline is derived from three distinct sources, each of which may be separately quantitated. Part of the total represents passive solubilization of matrix collagen, part is derived from new synthesis of soluble collagen occurring in vitro, and the remainder is released by cell-mediated resorptive action.
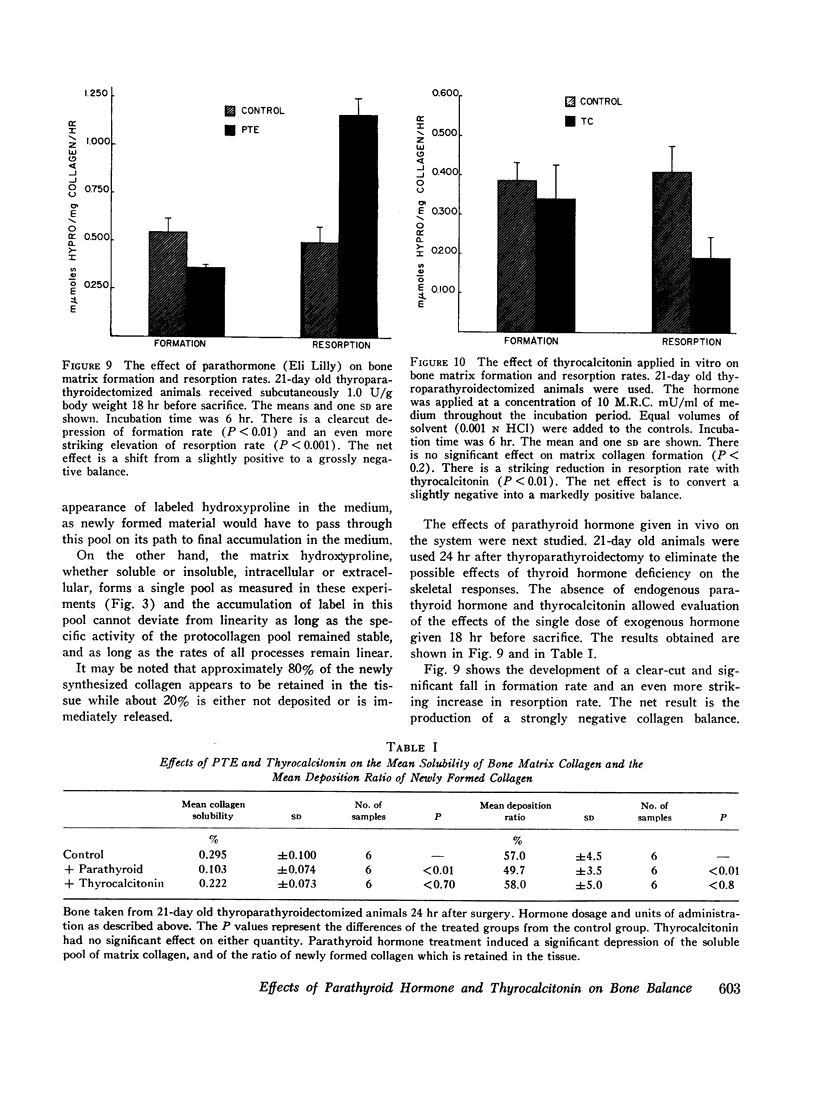
The latter two processes are linear with time up to 8 hr; the former decays to zero at about 2 hr. Consequently, rates of collagen synthesis and of new collagen deposition and resorption can be quantitated simultaneously in the same system. The ability to measure these parameters of bone collagen metabolism provides methods both for the accurate evaluation of organic matrix resorption in vitro and for the accurate measurement of rates of collagen synthesis and collagen deposition. The application of the method is illustrated using parathyroid hormone and thyrocalcitonin. Parathyroid hormone diminishes collagen synthesis and stimulates collagen resorption. It reduces slightly the deposition of newly formed collagen in stable matrix. The net effect of these changes is to produce a marked negative balance. It does not significantly affect the solubility of matrix collagen.
Thyrocalcitonin does not affect collagen synthesis or its deposition. It causes a marked fall in resorption rate. It has no effect on matrix collagen solubility. The net effect is to produce a marked positive balance of matrix collagen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliapoulios M. A., Goldhaber P., Munson P. L. Thyrocalcitonin inhibition of bone resorption induced by parathyroid hormone in tissue culture. Science. 1966 Jan 21;151(3708):330–331. doi: 10.1126/science.151.3708.330. [DOI] [PubMed] [Google Scholar]
- BORLE A. B., NICHOLS N., NICHOLS G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960 Apr;235:1206–1210. [PubMed] [Google Scholar]
- BORLE A. B., NICHOLS N., NICHOLS G., Jr Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960 Apr;235:1211–1214. [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- FLANAGAN B., NICHOL S. G., Jr PARATHYROID INHIBITION OF BONE COLLAGEN SYNTHESIS. Endocrinology. 1964 Feb;74:180–186. doi: 10.1210/endo-74-2-180. [DOI] [PubMed] [Google Scholar]
- FLANAGAN B., NICHOLS G., Jr METABOLIC STUDIES OF BONE IN VITRO. V. GLUCOSE METABOLISM AND COLLAGEN BIOSYNTHESIS. J Biol Chem. 1964 Apr;239:1261–1265. [PubMed] [Google Scholar]
- FLANAGAN B., NICHOLS G., Jr Metabolic studies of bone in vitro. IV. Collagen biosynthesis by surviving bone fragments in vitro. J Biol Chem. 1962 Dec;237:3686–3692. [PubMed] [Google Scholar]
- Flanagan B., Nichols G., Jr Bone matrix turnover and balance in vitro. II. The effects of aging. J Clin Invest. 1969 Apr;48(4):607–612. doi: 10.1172/JCI106019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan B., Nichols G., Jr Metabolic studies of human bone in vitro. I. Normal bone. J Clin Invest. 1965 Nov;44(11):1788–1794. doi: 10.1172/JCI105286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan B., Nichols G., Jr Metabolic studies of human bone in vitro. II. Changes in hyperparathyroidism. J Clin Invest. 1965 Nov;44(11):1795–1804. doi: 10.1172/JCI105287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Raisz L. G. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science. 1965 Dec 10;150(3702):1465–1467. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- GERBER G., GERBER G., ALTMAN K. I. Studies on the metabolism of tissue proteins. I. Turnover of collagen labeled with proline-U-C14 in young rats. J Biol Chem. 1960 Sep;235:2653–2656. [PubMed] [Google Scholar]
- HANCOX N. M., BOOTHROYD B. ELECTRON MICROSCOPY OF THE EARLY STAGES OF OSTEOGENESIS. Clin Orthop Relat Res. 1965 May-Jun;40:153–161. [PubMed] [Google Scholar]
- HARKNESS R. D., MARKO A. M., MUIR H. M., NEUBERGER A. The metabolism of collagen and other proteins of the skin of rabbits. Biochem J. 1954 Apr;56(4):558–569. doi: 10.1042/bj0560558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- LEACH A. A. The determination of gelatin in blood and urine. Anal Biochem. 1961 Dec;2:529–534. doi: 10.1016/0003-2697(61)90020-3. [DOI] [PubMed] [Google Scholar]
- MARTIN C. J., AXELROD A. E. A modified method for determination of hydroxyproline. Proc Soc Exp Biol Med. 1953 Jul;83(3):461–462. doi: 10.3181/00379727-83-20386. [DOI] [PubMed] [Google Scholar]
- NEUMAN R. E., LOGAN M. A. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. [PubMed] [Google Scholar]
- Nichols G., Jr, Flanagan B. Osteoporosis--a disorder of bone cell metabolism. Fed Proc. 1966 May-Jun;25(3):922–927. [PubMed] [Google Scholar]
- PETERKOFSKY B., PROCKOP D. J. A method for the simultaneous measurement of the radioactivity of proline-C14 and hydroxyproline-C14 in biological materials. Anal Biochem. 1962 Nov;4:400–406. doi: 10.1016/0003-2697(62)90141-0. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P., Flanagan B., Nichols G., Jr Active transport of amino acids into bone cells. Biochim Biophys Acta. 1967 Sep 9;135(4):732–740. doi: 10.1016/0005-2736(67)90104-6. [DOI] [PubMed] [Google Scholar]
- SCHARTUM S., NICHOLS G., Jr Concerning pH gradients between the extracellular compartment and fluids bathing the bone mineral surface and their relation to calcium ion distribution. J Clin Invest. 1962 May;41:1163–1168. doi: 10.1172/JCI104569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAES G. M., NICHOLS G., Jr Effects of a dose of parathyroid extract on bone metabolic pathways. Endocrinology. 1962 Apr;70:546–555. doi: 10.1210/endo-70-4-546. [DOI] [PubMed] [Google Scholar]
- WOODS J. F., NICHOL S. G., Jr COLLAGENOLYTIC ACTIVITY IN MAMMALIAN BONE. Science. 1963 Oct 18;142(3590):386–387. doi: 10.1126/science.142.3590.386. [DOI] [PubMed] [Google Scholar]