Abstract
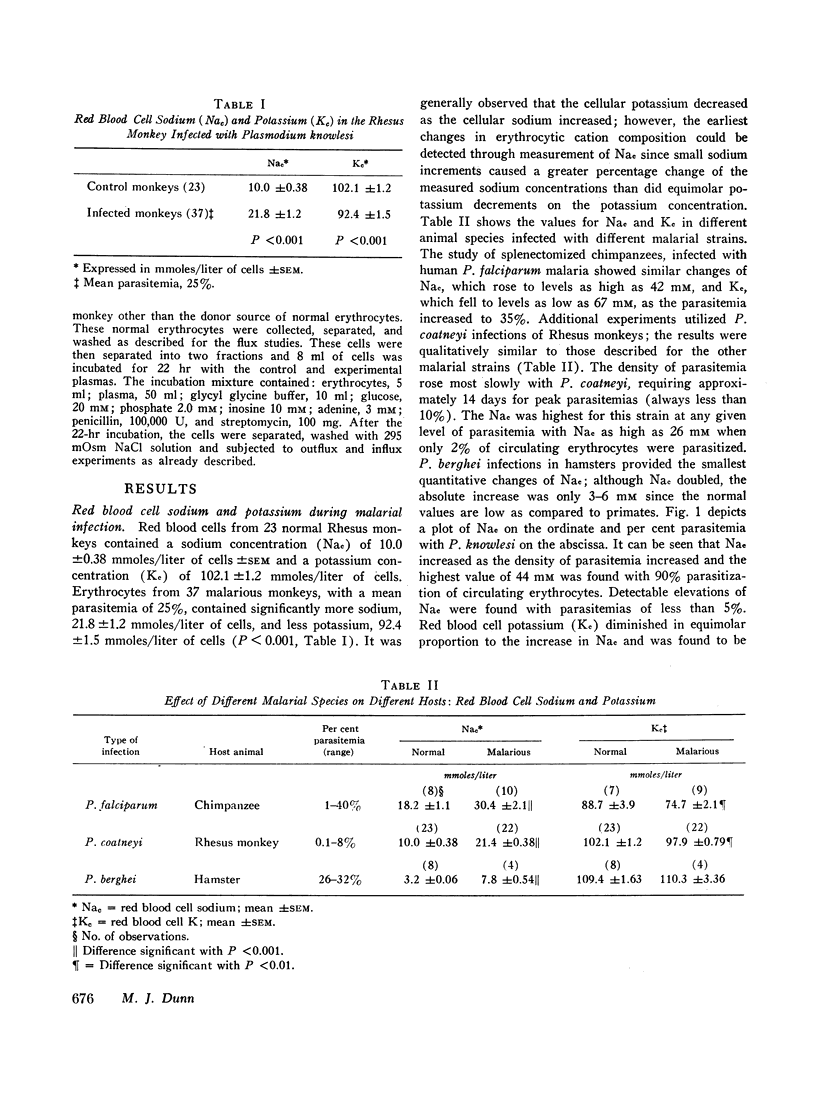
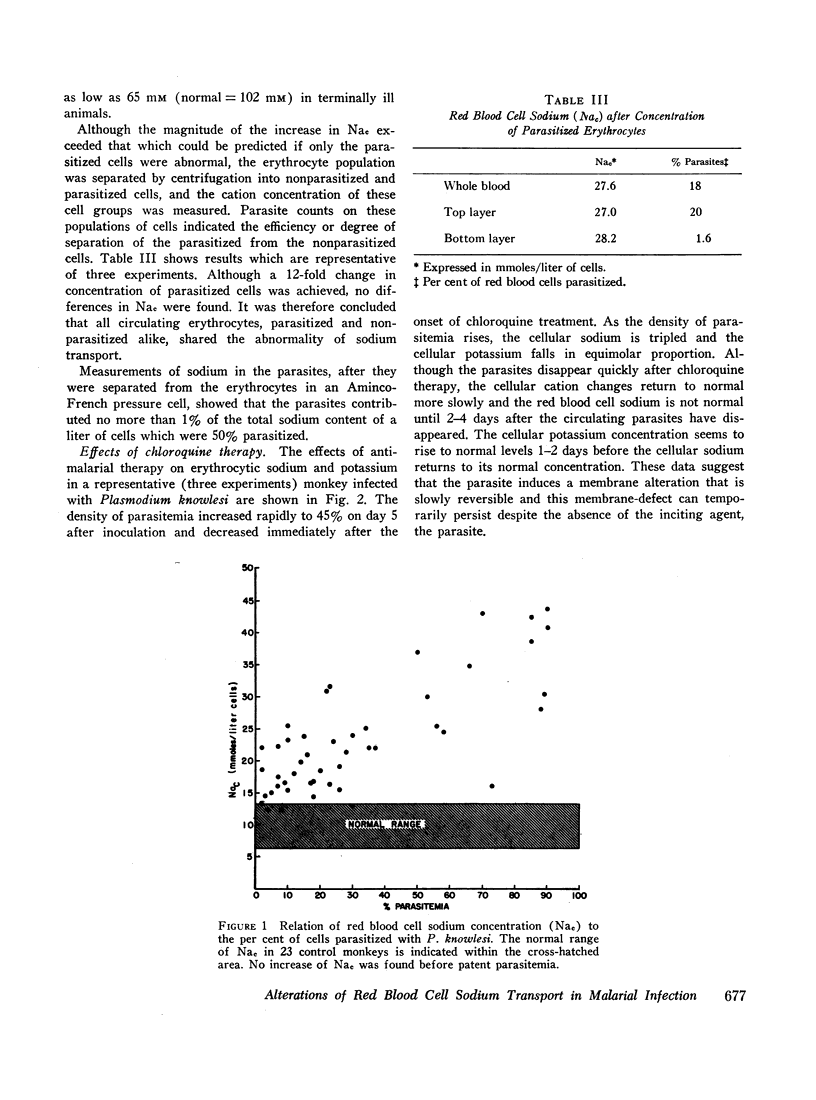
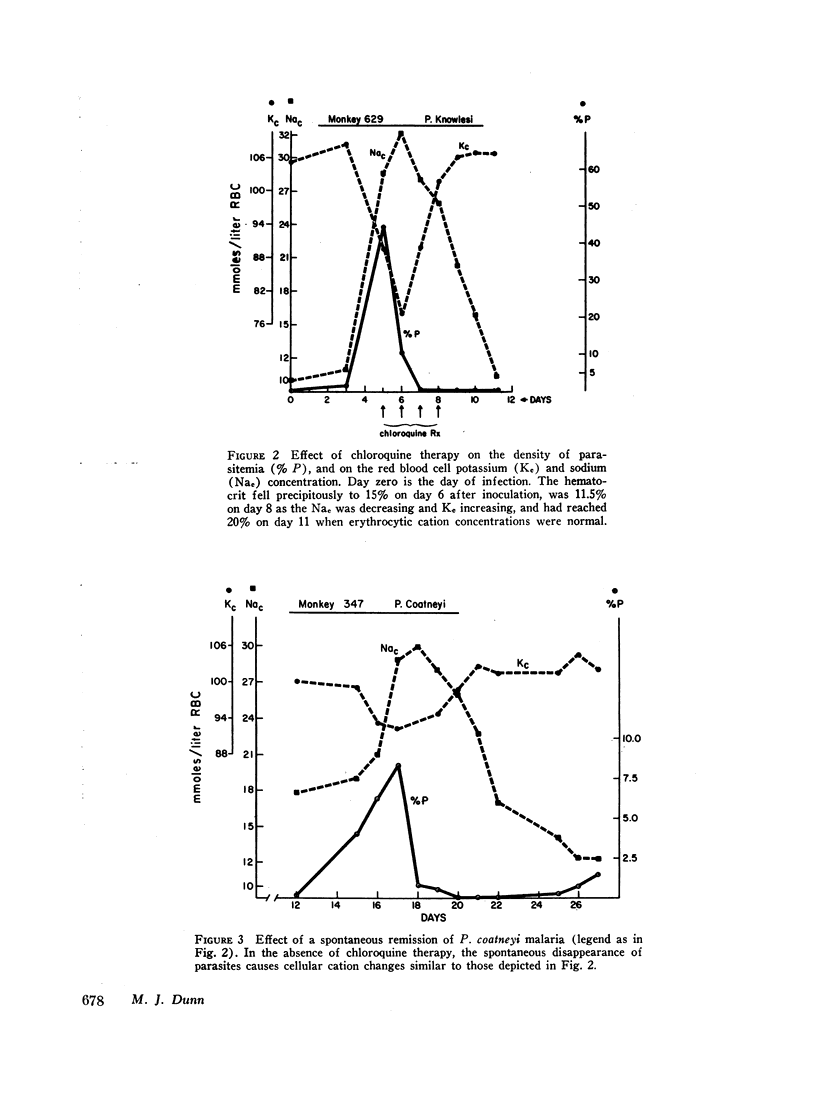
Previous studies have suggested that malaria induces changes in erythrocytic membrane permeability and susceptibility to osmotic lysis. The present study investigated erythrocytic transport of sodium with cells from Rhesus monkeys infected with Plasmodium knowlesi. Red blood cell sodium concentration was significantly elevated in 37 parasitized animals (21.8±1.2 mM; mean ±SEM), as compared to 23 control animals (10.0±0.38 mM). The cellular sodium increased with the density of parasitemia and the cellular potassium decreased in proportion to the elevation of sodium. Nonparasitized as well as parasitized erythrocytes possessed this abnormality of cation metabolism. Effective chloroquine therapy reversed the changes over a period of 4 days.
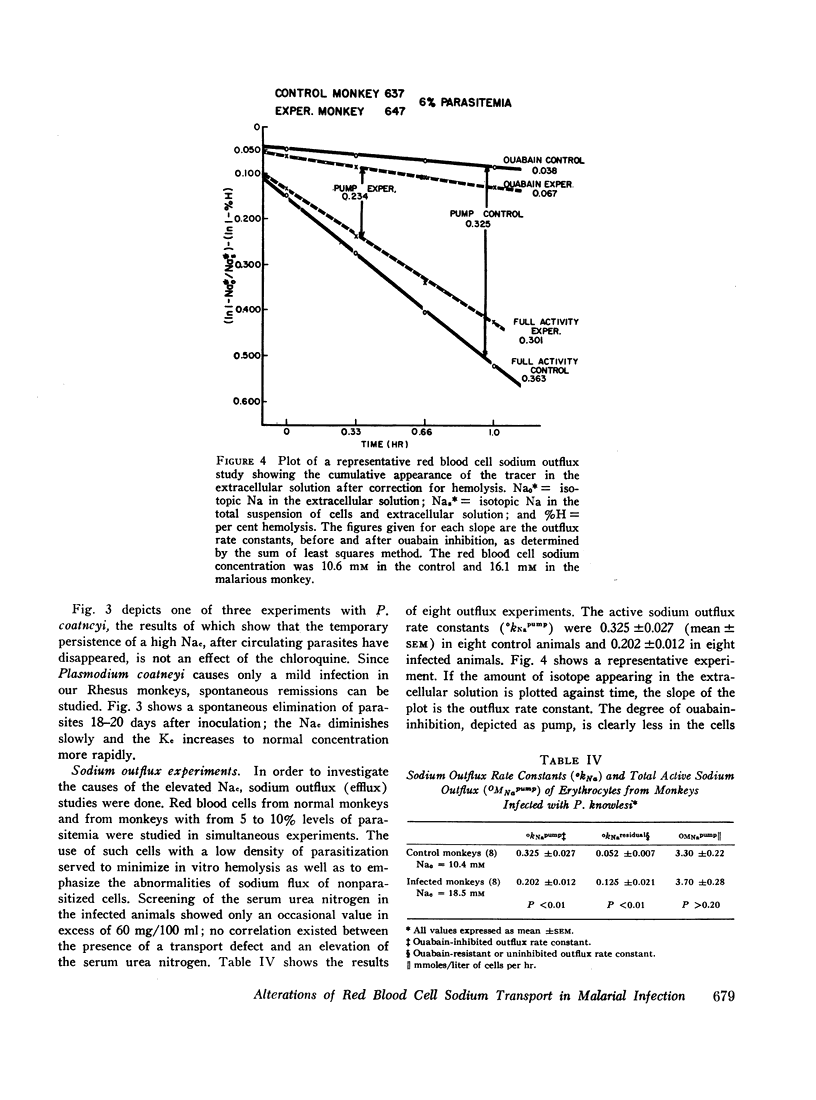
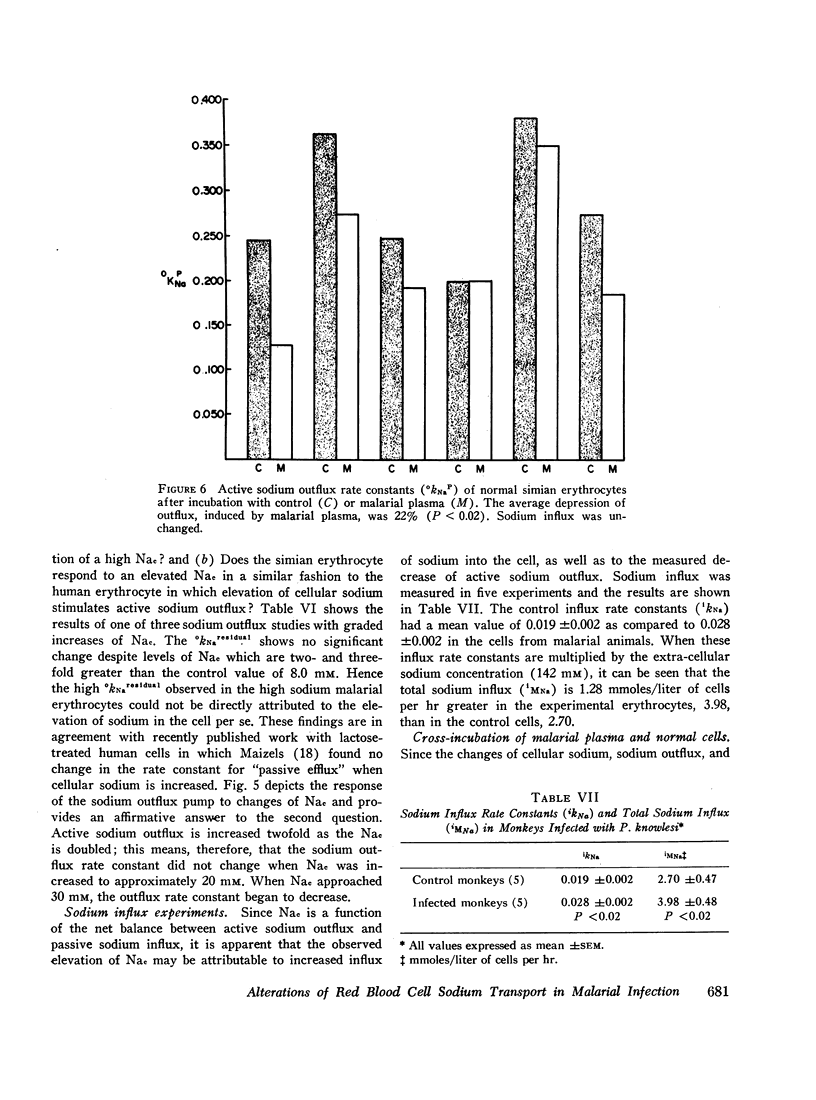
Active sodium outflux rate constants were depressed in animals with malaria (0.202±0.012), as compared to controls (0.325±0.027). Passive sodium influx rate constants were higher in infected monkeys (0.028±0.002) than in control animals (0.019±0.002). The cross incubation of malarial plasma with normal red blood cells induced a 22% diminution in active sodium outflux but no changes were observed in sodium influx.
It is concluded that malaria alters erythrocytic sodium transport in all erythrocytes. The elevated intracellular sodium concentration is the net result of decreased sodium outflux and increased sodium influx. The plasmodium organism or the affected host may produce a circulating substance that is deleterious to erythrocytic membrane cation transport.
Full text
PDF

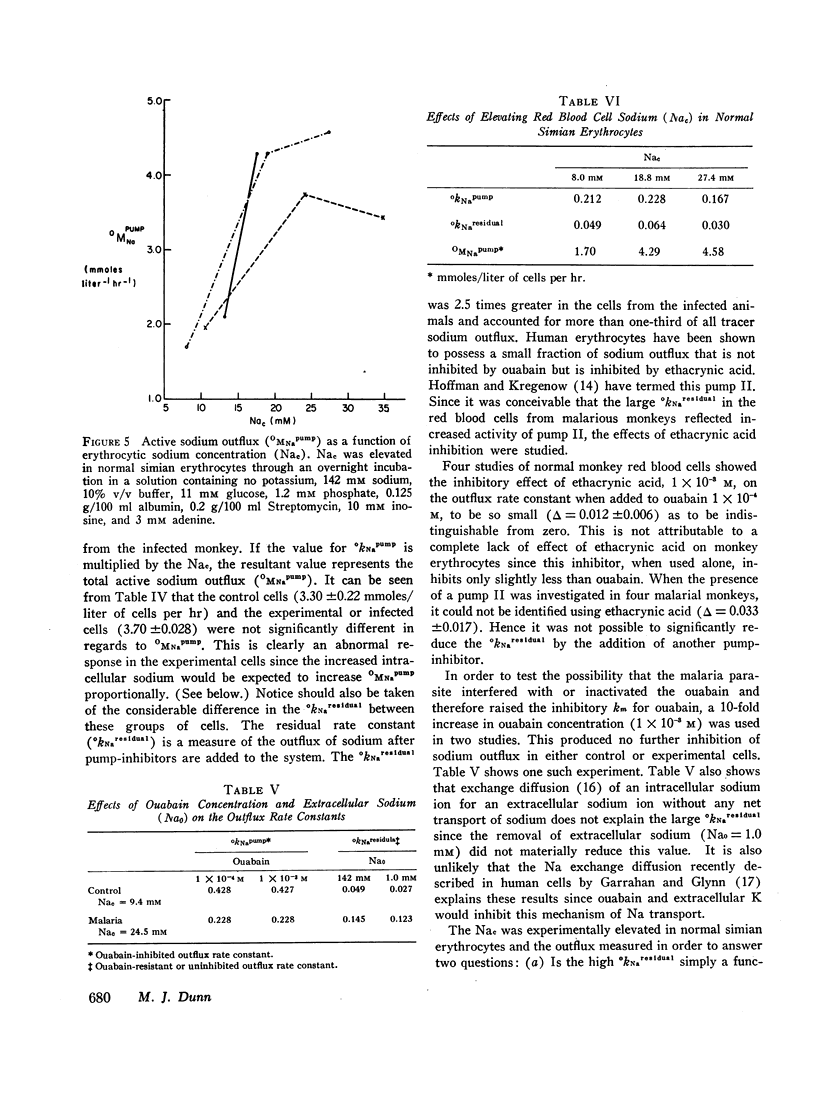



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTLES J. F. Sodium transport across the surface membrane of red blood cells in hereditary spherocytosis. J Clin Invest. 1957 Jun;36(6 Pt 1):816–824. doi: 10.1172/JCI103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antonio L. E., Von Doenhoff A. E., Jr, Fife E. H., Jr A new method for isolation and fractionation of complement fixing antigens from Plasmodium knowlesi. Proc Soc Exp Biol Med. 1966 Oct;123(1):30–34. doi: 10.3181/00379727-123-31394. [DOI] [PubMed] [Google Scholar]
- DANON D., GUNDERS A. E. On the relationship between parasitaemia and increased osmotic fragility of erythrocytes in rodent malaria. Bull Res Counc Isr Sect E Exp Med. 1962 Jul;10E:59–64. [PubMed] [Google Scholar]
- DEVAKUL K., MAEGRAITH B. G. Lysis and other circulatory phenomena in malaria (Plasmodium knowlesi). Ann Trop Med Parasitol. 1959 Dec;53:430–450. doi: 10.1080/00034983.1959.11685943. [DOI] [PubMed] [Google Scholar]
- Fogel B. J., Shields C. E., Von Doenhoff A. E., Jr The osmotic fragility of erythrocytes in experimental malaria. Am J Trop Med Hyg. 1966 May;15(3):269–275. doi: 10.4269/ajtmh.1966.15.269. [DOI] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. The action of antibody and complement on mammalian cells. Ann N Y Acad Sci. 1960 May 31;87:352–362. doi: 10.1111/j.1749-6632.1960.tb23205.x. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Stokes E. F., Wicker D. J., Conrad M. E. Studies of the mechanism of hemolysis in experimental malaria. Mil Med. 1966 Sep;131(9 Suppl):1217–1224. [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H. LEAKY RED CELLS. Blood. 1965 Sep;26:367–382. [PubMed] [Google Scholar]
- Maizels M. Effect of sodium content on sodium efflux from human red cells suspended in sodium-free media containing potassium, rubidium, caesium or lithium chloride. J Physiol. 1968 Apr;195(3):657–679. doi: 10.1113/jphysiol.1968.sp008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARON N., JEANLOZ R. W. The diaminohexose component of a polysaccharide isolated from Bacillus subtilis. J Biol Chem. 1960 Jan;235:1–5. [PubMed] [Google Scholar]
- Sachs J. R., Conrad M. E. Effect of tetraethylammonium on the active cation transport system of the red blood cell. Am J Physiol. 1968 Oct;215(4):795–798. doi: 10.1152/ajplegacy.1968.215.4.795. [DOI] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C. The effects of sickling on ion transport. II. The effect of sickling on sodium and cesium transport. J Gen Physiol. 1955 Sep 20;39(1):55–67. doi: 10.1085/jgp.39.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELT L. G., SACHS J. R., MCMANUS T. J. AN ION TRANSPORT DEFECT IN ERYTHROCYTES FROM UREMIC PATIENTS. Trans Assoc Am Physicians. 1964;77:169–181. [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCKERMAN A. AUTOIMMUNIZATION AND OTHER TYPES OF INDIRECT DAMAGE TO HOST CELLS AS FACTORS IN CERTAIN PROTOZOAN DISEASES. Exp Parasitol. 1964 Apr;15:138–183. doi: 10.1016/0014-4894(64)90014-1. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN A. Blood loss and replacement in plasmodial infections. III. Plasmodium cynomolgi, Plasmodium gonderi and Plasmodium knowlesi in Macaca mulatta mulatta, the rhesus monkey. J Infect Dis. 1960 Mar-Apr;106:123–140. doi: 10.1093/infdis/106.2.123. [DOI] [PubMed] [Google Scholar]
- Zuckerman A. Recent studies on factors involved in malarial anemia. Mil Med. 1966 Sep;131(9 Suppl):1201–1216. [PubMed] [Google Scholar]


