Abstract
BACKGROUND
As outlined in the 2004 American Heart Association guidelines, the diagnosis of Kawasaki disease (KD) is supported by results of clinical laboratory studies. However, detailed information regarding the evolution of these results during illness has not been previously reported. The goals of this project were to characterize the evolution of clinical laboratory values in KD before and after treatment with intravenous immunoglobulin (IVIG).
METHODS
Laboratory values from 380 unselected, consecutive KD patients were analyzed at 3 times: acute (illness day 2-10, illness day 1= first day of fever and before IVIG), subacute (illness day 11-21) and convalescent (illness day 22-60). Results were stratified by IVIG response and coronary artery outcome.
RESULTS
While white blood cell count, percentage bands, erythrocyte sedimentation rate (ESR), and CRP values were highest and age-adjusted hemoglobin was lowest in the acute phase before IVIG, platelet count was highest in the subacute phase and percentage lymphocytes and eosinophils were highest in the convalescent phase after IVIG. KD patients with coronary artery aneurysms had a higher WBC count in the subacute phase and higher ESR in the subacute and convalescent phases compared with those with dilated or normal coronary arteries.
CONCLUSIONS
A consistent evolution of laboratory values is associated with KD before and after treatment. Understanding the dynamic changes in laboratory values can assist physicians in using laboratory criteria to diagnose KD following the American Heart Association guidelines.
Keywords: Kawasaki disease, laboratory values
Introduction
Kawasaki disease (KD), the leading cause of pediatric acquired heart disease, is diagnosed according to clinical criteria supported by laboratory studies indicating marked systemic inflammation. The American Heart Association (AHA) 2004 guidelines for incomplete KD incorporate laboratory values to support the diagnosis 1. However, the evolution of these laboratory values before and after treatment with IVIG has not been previously reported.
In his landmark paper reporting the first 50 cases of KD, Dr. Kawasaki noted that the illness was characterized by an elevated white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) value, as well as anemia for age 2. Subsequent studies comparing laboratory values between KD patients and febrile controls presenting in the first 14 days after onset of fever found a higher ESR, higher values of CRP, γ glutamyl transferase (GGT), and alanine amionotransferase (ALT), higher percentage eosinophils, and a lower age-adjusted hemoglobin (zHgb), in acute KD patients 3-7. Conversely, a WBC count less than 10 × 103/mm3 and a platelet count below 200 × 103/mm3 were significantly more common in the febrile controls3. The importance of laboratory testing in establishing the diagnosis of KD was highlighted in the 2004 AHA guidelines, which recommend measuring the ESR, WBC count in blood and urine, platelet count, and values of CRP, albumin, Hgb, and ALT in the evaluation of a child with suspected KD 1, 8.
Several studies have used multivariate logistic regression to identify laboratory values that predict resistance to therapy with intravenous immunoglobulin (IVIG) and an increased risk for coronary artery aneurysms 9-15. Elevated counts of immature neutrophils (bands) and platelets, and elevated values of GGT, CRP, ALT, and aspartate aminotransferase (AST), and bilirubin, as well as low zHgb and albumin, have been combined with age and illness day and incorporated into scoring systems to predict IVIG-resistance. In addition, low serum sodium has been shown to be a predictor of giant coronary artery aneurysms 14, 16. Most recently, low albumin and an elevated ESR and WBC count have been associated with noncoronary cardiac abnormalities, including left ventricular systolic dysfunction and mitral regurgitation, in children with acute KD 17.
Despite several studies on the laboratory value abnormalities differentiating acute KD from other febrile illnesses and predicting IVIG-resistance and coronary artery abnormalities, no study has described the change in laboratory indices during the course of illness. This study characterizes the evolution of clinical laboratory values in children with KD prior to treatment with IVIG and over time in children treated for KD within the first 10 days of illness, stratified by IVIG response and coronary artery outcome.
Materials and Methods
Subjects and Samples
We performed a retrospective chart review of 380 unselected, consecutive patients treated for KD between January 1, 2002 and June 30, 2009 at Rady Children’s Hospital, San Diego, the only pediatric acute care hospital serving the general population (approximately 3 million inhabitants) of San Diego County. All patients were managed according to a standardized protocol with laboratory testing and outpatient follow-up visits dictated by the protocol. The following data were prospectively collected and entered into an electronic database: demographic and clinical data, including illness day at diagnosis (illness day 1= first day of fever), response to IVIG therapy, coronary artery status (Z scores), and laboratory values (WBC, hemoglobin, platelet count, percentage bands, percentage lymphocytes, percentage eosinophils, absolute cell count (neutrophils, bands, lymphocytes, and eosinophils), ESR and plasma values of CRP, ALT, and GGT). We calculated zHgb according to the following formula: ([Observed hemoglobin] -[Mean hemoglobin for age])/Standard deviation for age 18. The standard deviations for zHgb were estimated as one quarter of the reported range of normal hemoglobin concentrations for each age interval. Laboratory values were plotted for patients presenting within the first 20 days of fever onset and before IVIG treatment and also analyzed in 3 intervals: pre-IVIG acute (illness day 2-10, illness day 1= first day of fever), subacute (illness day 11-21) and convalescent (illness day 22-60). For subjects with multiple laboratory values within a time interval, only the first laboratory value within that interval was analyzed. The subacute and convalescent zHgb values from subjects who were transfused for low zHgb were excluded from the analysis.
The response to IVIG therapy was classified as resistant or responsive for patients treated within the first 10 days after fever onset. For patients who received late treatment (≥ 11 days after fever onset), were not treated (initial diagnosis beyond the acute febrile phase), or received infliximab in addition to IVIG as initial therapy for coronary artery abnormality, only the pre-treatment laboratory studies were analyzed. IVIG-resistance was defined as persistent or recrudescent fever (T ≥38.0°C rectally or orally) at least 36 hours but not longer than 7 days after completion of the first IVIG infusion (2 g/kg) 9. Subjects were classified as having normal (<2.5 standard deviation units [Z-score] from the mean, normalized for body surface area), dilated (Z-score ≥ 2.5), or aneurysmal (focal dilation of an arterial segment at least 1.5 times the diameter of the adjacent segment) coronary arteries on the basis of the maximal internal diameters of the right coronary artery (RCA) and left anterior descending artery (LAD) measured by echocardiography at the time of diagnosis and at 2 to 6 weeks after onset of fever 19. The study received Institutional Review Board approval at the University of California, San Diego.
Statistics
Descriptive analyses were performed to 1) study the relationship between the pre-treatment lab values and illness days, and 2) to describe the changes of the laboratory values over time for individuals treated within the first 10 days of illness. Laboratory values were compared between IVIG-resistant and -responsive patients by Wilcoxon rank sum test and across groups based on coronary artery status by the Kruskal-Wallis test at different time points. For laboratory values that were outside the dynamic range of the test (ie. ESR of >140 mm/hour and CRP <0.3 mg/dL), the maximal or minimal value detected by the assay was used, as appropriate. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed in R version 2.10.0 (http://www.R-project.org).
Results
The demographic and clinical data for the 380 subjects are shown in Table 1. Evaluation of the first set of pretreatment laboratory values in the 360 KD subjects who presented within the first 20 days of illness showed that the percentage bands and CRP value peak within the first 10 days of illness while the platelet count, percentage and absolute lymphocyte count, and percentage and absolute eosinophil counts peak between illness days 11 and 20 (see Figure, Supplemental Digital Content 1).
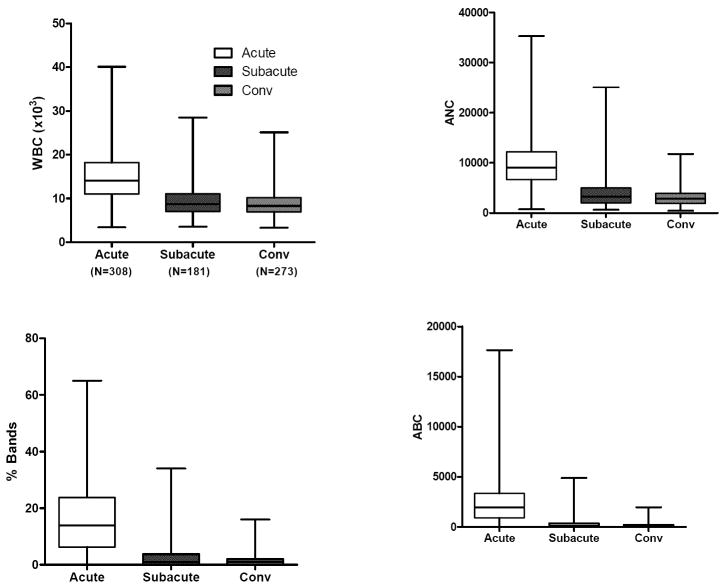
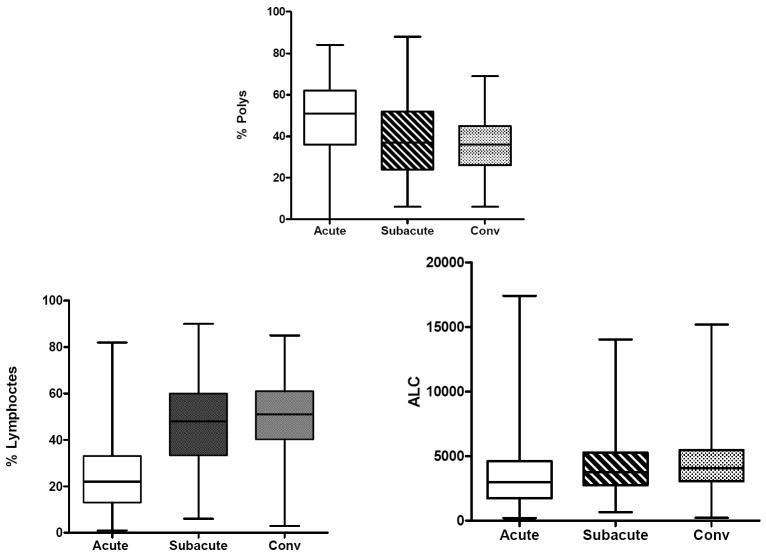
Evaluation of the laboratory values by stage of illness (acute, subacute, and convalescent) for KD subjects treated within the first 10 days of illness (N=324) showed that the WBC count, percentage and absolute band count, percentage and absolute polymorphonuclear leukocyte count, ESR and CRP value were highest in the acute phase. zHgb was low in the acute and subacute phases and normalized by the convalescent phase without specific treatment or intervention. In 36 of 45 subjects (80%) with a zHgb less than -3 in the acute or subacute phase, both the mean corpuscular volume and the mean corpuscular hemoglobin concentration were normal, confirming a normocytic, normochromic anemia. The other 9 subjects had a microcytic, normochromic anemia. Platelet count was highest in the subacute phase and percentage and absolute lymphocyte and percentage and absolute eosinophil counts were highest in the convalescent phase after treatment with IVIG, However, the absolute lymphocyte and eosinophil counts did not increase as rapidly as their percentage of the total white blood cell count. (Figure 1).
Figure 1.
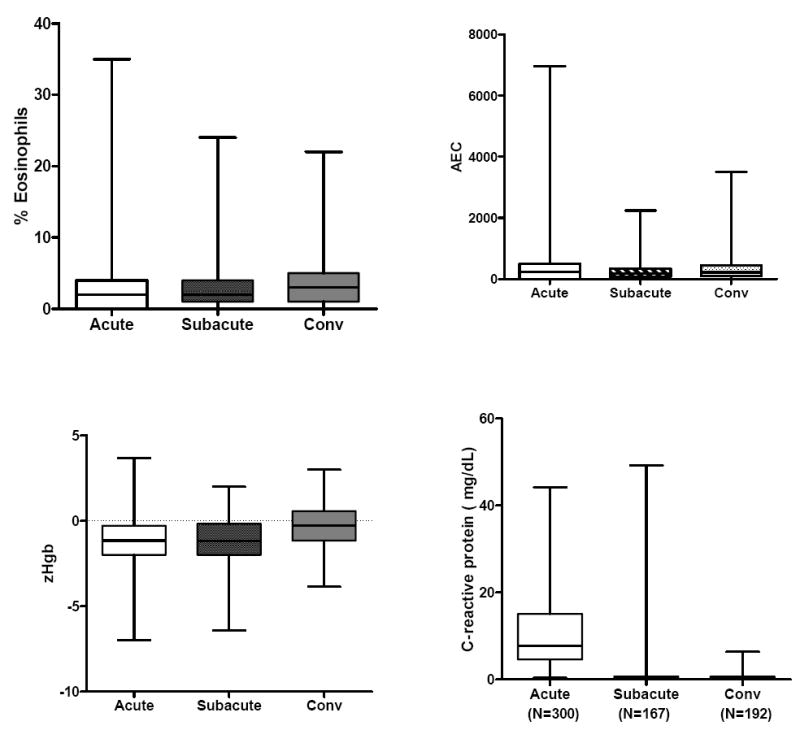
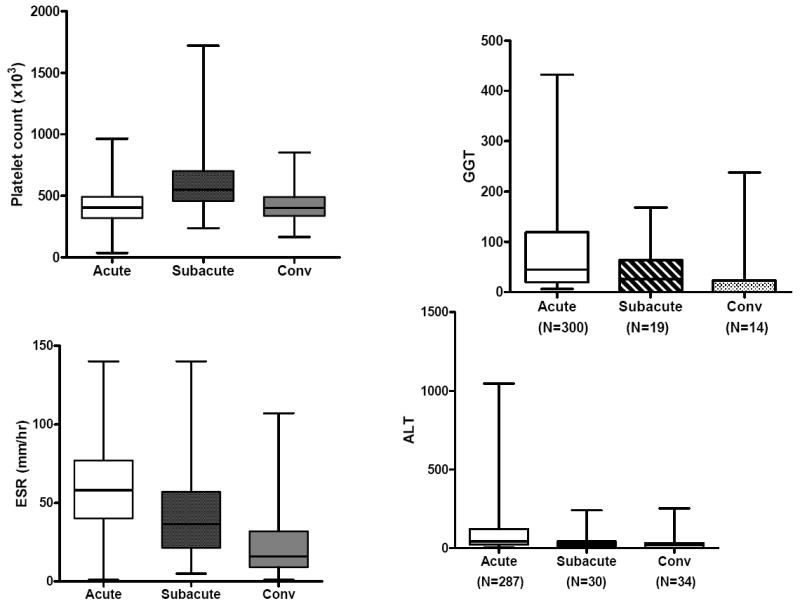
Laboratory values by stage of illness for KD subjects treated within the first 10 days of illness. The three stages are pre-IVIG acute (illness day 2-10, illness day 1= first day of fever), subacute (illness day 11-21) and convalescent (illness day 22-60). Box plots show median, 25%, 75%, minimum and maximum. The number of subjects for each interval is shown for WBC (representing all cell counts), CRP, GGT, and ALT.
GGT and ALT values were only measured during the acute period in KD subjects treated within the first 10 days of illness. Of the 306 subjects for whom GGT was measured, 192 (62.7%) of these KD subjects had an elevated GGT value based on age-adjusted reference values. In the 300 subjects for whom ALT was measured, 121 (40.3%) had an elevated ALT value based on age-adjusted reference values.
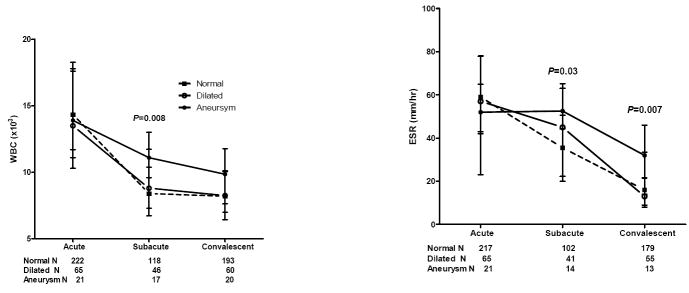
Of the 324 subjects who presented within the first 10 days of illness, 12 received IVIG followed immediately by infliximab (5 mg/kg) for coronary artery abnormalities, thus confounding a determination of treatment response specifically to IVIG. Therefore, 312 of the subjects treated within the first 10 days of illness could be classified as either IVIG-resistant or -responsive. Of the laboratory values measured in these 312 KD subjects, WBC, zHgb, platelet count, ESR, and ALT were statistically different between IVIG-resistant and –responsive subjects either in the subacute or convalescent phase of KD (see Figure, Supplemental Digital Content 2). IVIG-resistant subjects also had a higher band count, CRP value, ALT, and GGT in the acute phase than IVIG-responsive subjects (P<0.0001, 0.001, <0.001, and 0.006, respectively). In this same cohort of KD subjects treated within the first 10 days of illness, subjects with coronary artery aneurysms had a higher WBC count in the subacute phase and higher ESR in the subacute and convalescent phases compared to those with dilated or normal coronary arteries (Figure 2). Because administration of IVIG can artifactually accelerate the ESR, we analyzed the ESR in aneurysm patients stratified by number of IVIG doses received to determine if the higher ESR in aneurysm patients was a sign of greater inflammation or was confounded by additional IVIG treatment. For the 13 aneurysm subjects for whom an ESR was determined in the convalescent phase, 6 were IVIG-resistant and received an additional dose (2g/kg) of IVIG and 7 were IVIG-responsive. There was no statistical difference in the median ESR between these two groups. KD subjects with coronary artery aneurysms had a higher CRP in the acute phase than those with either normal or dilated coronary arteries (P=0.005).
Figure 2.
Laboratory values by coronary artery status for KD subjects treated in the first 10 days of illness. The three stages are pre-IVIG acute (illness day 2-10, illness day 1= first day of fever), subacute (illness day 11-21) and convalescent (illness day 22-60). Plots show median and 25-75%.
Of the 25 subjects with aneurysms treated within the first 10 days of illness, the IVIG response could be determined in 21 subjects as 4 were treated with both IVIG and infliximab at initial presentation. Of these 21 subjects, 10 (48%) were also IVIG-resistant compared to 39 of 222 subjects (18%) with normal coronaries and 13 of 65 subjects (20%) with dilated coronary arteries.
Discussion
The clinical diagnosis of KD can be challenging and laboratory testing can help the clinician evaluate the degree of inflammation and can aid in the diagnosis of incomplete KD as outlined by the 2004 AHA guidelines 1. However, a clear understanding of the dynamic nature of these laboratory values is critical to this process. Here, we have comprehensively defined the evolution of laboratory values over time in KD subjects before and after IVIG treatment.
In his original paper describing the first 50 cases of KD, Dr. Kawasaki noted that both the ESR and CRP were elevated during the initial presentation and became normal “within 3 to 4 weeks” although “the timing for resolution was not the same” with the CRP returning to normal earlier in the course of illness.20, 21 There was also a “tendency toward” a high WBC and “various degrees of left shift observed in 41 of the 50 subjects”, a “mild, transient elevation …of serum ALT”, and Coombs negative anemia for 34 of 35 cases during the initial presentation. Our data confirm these clinical observations. Dr. Kawasaki did not note eosinophilia at the initial presentation. By contrast, our data and that of others show that the percentage eosinophils and absolute eosinophil count are elevated in acute KD and that the percentage of eosinophils continues to rise, peaking in the convalescent phase.6 Dr. Kawasaki also described that “generally the lymphocyte count [%] was low at the time of hospitalization but recovered in convalescence”. While we also noted this change, the absolute lymphocyte count was stable over time.
The changes in laboratory values in IVIG-treated and untreated subjects followed a similar pattern during the first 20 days of illness. The abnormalities in the ESR, platelet count and zHgb resolved over a period of 6 weeks. Because the anemia is normochromic and normocytic, it most likely reflects decreased RBC production in the setting of acute inflammation and resolved spontaneously without the need for nutritional supplementation with folate or iron.
Steroids, commonly used by many centers for IVIG-resistant KD, can increase the WBC count. In our study, only 2 of the 63 IVIG-resistant subjects received steroids and neither had an increase in the WBC after steroids were initiated. It has also been described that high-dose IVIG increases the ESR 22. This is thought to occur as a consequence of the net positive charge on the IgG molecule that neutralizes the net negative charge on the surface of the RBC (zeta potential) and leads to enhanced rouleaux formation and accelerated RBC sedimentation. In our study, many subjects who developed coronary artery abnormalities were also IVIG-resistant and received 2 doses of IVIG, a fact which could explain the increased ESR in the subacute and convalescent phases. However, a comparison of the convalescent ESR of IVIG-resistant vs. –responsive KD subjects with aneurysms did not show a difference between the groups, suggesting that the higher ESR in subjects with aneurysms was due to more inflammation rather than an effect of a second dose of IVIG. Failure to normalize markers of inflammation in the convalescent phase suggests that a subset of patients with aneurysms have persistent inflammation that is not well controlled by standard therapy.
Limitations of our study include a diminishing sample size for increasing illness day after illness day 10 and measurement of GGT and ALT values only in the acute phase, which precluded an analysis of change in values over time. We were also unable to do pairwise comparisons of the laboratory values across stages of illness as some laboratory values were not available during the subacute and convalescent phases.
Conclusions
Understanding the evolution of standard clinical laboratory test results in KD patients both before and after IVIG therapy should help physicians calibrate their expectations of changes in laboratory values over time. These data may also be useful in applying laboratory criteria to diagnose incomplete KD following the AHA guidelines.
Supplementary Material
Evolution of laboratory values prior to therapy during the first 20 days of illness (illness day 1= first day of fever). Scatterplots show medians. The number of subjects for each illness day is shown for the WBCs and is representative of the approximate number of samples for each day for the other laboratory values.
Laboratory values by IVIG treatment response and stage of illness for KD subjects treated within the first 10 days of illness. The three stages are pre-IVIG acute (illness day 2-10, illness day 1= first day of fever), subacute (illness day 11-21) and convalescent (illness day 22-60). Plots show median and 25-75%.
Acknowledgments
We are grateful to Dee Anna Scherrer for technical assistance, Joan Pancheri for data and sample collection, and the families for participation in this study.
This work is supported in part by grants from the National Heart, Lung, and Blood Institute (HL69413 and K24 HL074864) awarded to JCB, and National Institute of Child Health and Human Development (K23 HD56939) and the Harold Amos Faculty Development Award to AHT.
Footnotes
The authors do not have any conflicts of interest to report.
These data were presented in part at the 2011 Pediatric Academic Societies annual meeting in Denver, Colorado.
References
- 1.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T. Acute febrile mucocutaneous syndrome with lympyhoid involvement with specific desquamation of the fingers and toes in children [in Japanese] Arerugi = [Allergy] 1967;16:178. [PubMed] [Google Scholar]
- 3.Burns JC, Mason WH, Glode MP, et al. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. United States Multicenter Kawasaki Disease Study Group. The Journal of pediatrics. 1991;118:680–6. doi: 10.1016/s0022-3476(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 4.Xiu-Yu S, Jia-Yu H, Qiang H, Shu-Hui D. Platelet count and erythrocyte sedimentation rate are good predictors of Kawasaki disease: ROC analysis. Journal of clinical laboratory analysis. 24:385–8. doi: 10.1002/jcla.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang MY, Gupta-Malhotra M, Huang JJ, Syu FK, Huang TY. Acute-phase reactants and a supplemental diagnostic aid for Kawasaki disease. Pediatric cardiology. 31:1209–13. doi: 10.1007/s00246-010-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terai M, Yasukawa K, Honda T, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. The Pediatric infectious disease journal. 2002;21:777–81. doi: 10.1097/00006454-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Ting EC, Capparelli EV, Billman GF, Lavine JE, Matsubara T, Burns JC. Elevated gamma-glutamyltransferase concentrations in patients with acute Kawasaki disease. The Pediatric infectious disease journal. 1998;17:431–2. doi: 10.1097/00006454-199805000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Yellen ES, Gauvreau K, Takahashi M, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 125:e234–41. doi: 10.1542/peds.2009-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremoulet AH, Best BM, Song S, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. The Journal of pediatrics. 2008;153:117–21. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano T, Kurotobi S, Matsuzaki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. European journal of pediatrics. 2007;166:131–7. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 12.Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. The Journal of pediatrics. 2006;149:237–40. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. The Journal of pediatrics. 2008;153:365–8. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Yashiro M, Uehara R, et al. Use of laboratory data to identify risk factors of giant coronary aneurysms due to Kawasaki disease. Pediatr Int. 2004;46:33–8. doi: 10.1111/j.1442-200X.2004.01840.x. [DOI] [PubMed] [Google Scholar]
- 15.Eladawy M, Dominguez SR, Anderson MS, Glode MP. Abnormal liver panel in acute Kawasaki disease. The Pediatric infectious disease journal. 30:141–4. doi: 10.1097/INF.0b013e3181f6fe2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song D, Yeo Y, Ha K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. European journal of pediatrics. 2009;168:1315–21. doi: 10.1007/s00431-009-0925-0. [DOI] [PubMed] [Google Scholar]
- 17.Printz BF, Sleeper LA, Newburger JW, et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute kawasaki disease. Journal of the American College of Cardiology. 57:86–92. doi: 10.1016/j.jacc.2010.08.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunn L, Nechyba C. The Harriet Lane Handbook. 16. Philadelphia: Mosby; 2002. [Google Scholar]
- 19.Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–9. doi: 10.1007/s00246-009-9599-7. [DOI] [PubMed] [Google Scholar]
- 20.Abe J. Immunological aspects of Kawasaki disease. Nippon rinsho. 2008;66:267–71. [PubMed] [Google Scholar]
- 21.Kawasaki T. Pediatric acute febrile mucocutaneous lymph node syndrome with characteristic desquamation of fingers and toes: my clinical observation of fifty cases (translated from original 1967 publication) The Pediatric infectious disease journal. 2002;21:1–38. [Google Scholar]
- 22.Lee KY, Lee HS, Hong JH, Han JW, Lee JS, Whang KT. High-dose intravenous immunoglobulin downregulates the activated levels of inflammatory indices except erythrocyte sedimentation rate in acute stage of Kawasaki Disease. Journal of tropical pediatrics. 2005;51:98–101. doi: 10.1093/tropej/fmh087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evolution of laboratory values prior to therapy during the first 20 days of illness (illness day 1= first day of fever). Scatterplots show medians. The number of subjects for each illness day is shown for the WBCs and is representative of the approximate number of samples for each day for the other laboratory values.
Laboratory values by IVIG treatment response and stage of illness for KD subjects treated within the first 10 days of illness. The three stages are pre-IVIG acute (illness day 2-10, illness day 1= first day of fever), subacute (illness day 11-21) and convalescent (illness day 22-60). Plots show median and 25-75%.