Abstract
Context
Our previous case-control study identified human neutrophil peptide (HNP) as a potential biomarker for bronchiolitis obliterans syndrome (BOS) in lung transplant recipients.
Objective
To prospectively validate HNP as a biomarker for BOS.
Materials and Methods
HNP was measured by ELISA in bronchoalveolar lavage (BAL) fluid in lung transplant recipients.
Results
The first HNP measurement after reaching baseline pulmonary function was predictive of developing BOS ≥2 (p=0.0419). HNP remained elevated in those that developed BOS. The effect of potential confounders did not significantly impact BOS-free survival time.
Conclusion
HNP levels are elevated early and persistently in those that develop BOS.
Keywords: Biomarkers, Rejection, Lung transplant
Introduction
Lung transplantation is an effective treatment for end-stage lung disease, with two-year survival rates approaching 70% (Anyanwu et al., 1999). However, long-term survival remains low compared to most other solid organ transplants, mainly due to the development of chronic allograft rejection. Chronic rejection is manifested histologically by obliterative bronchiolitis (OB) with bronchiolitis obliterans syndrome (BOS) the clinical surrogate. BOS is diagnosed by a progressive decline in pulmonary function, specifically forced expiratory volume in one second (FEV1) and/or mid-expiratory flow rates (FEF25–75) (Estenne et al., 2002). However, the pathobiology of BOS appears to be heterogeneous, as obliterative bronchiolitis is only one of many different factors contributing to loss of lung function after transplantation. Regardless of the etiology, development of chronic allograft dysfunction confers significant morbidity and subsequent mortality.
Certain markers of inflammation and fibroproliferation have been associated with BOS development; however, none of these putative biomarkers have been validated prospectively (Belperio et al., 2002a, Belperio et al., 2001, Belperio et al., 2002c, Charpin et al., 2000, DiGiovine et al., 1996, Elssner and Vogelmeier, 2001, Kelly et al., 1998, Meyer et al., 2001). We have previously identified the antimicrobial peptide, human neutrophil α-defensin, also called human neutrophil peptide (HNP), as a potential biomarker of BOS in a small case control study using BAL fluid samples collected between 1993-96 (Nelsestuen et al., 2005, Zhang et al., 2005). HNP is an essential member of the innate immune system and belongs to a family of anti-microbial peptides referred to as defensins (Cole and Ganz, 2000, Ganz, 2002b). Four of the six α-defensins are present in neutrophils and some lymphocytes, and can be found in lungs. The release of HNP is stimulated by microbial products and certain cytokines and they act directly against many bacteria, fungi, and certain viruses (Borregaard et al., 2000). In addition to their role in innate immunity defensins have concentration-dependent roles in both cellular repair and injury, and cell proliferation (Austin et al., 2000, Ganz, 2002a). Both HNP and BAL neutrophilia have been independently reported to be elevated in lung transplant recipients at risk for and those that have already developed BOS (Nelsestuen et al., 2005, Riise et al., 1999, Riise et al., 1998, Elssner and Vogelmeier, 2001, DiGiovine et al., 1996, Zheng et al., 2000, Anderson et al., 2008). This present study was designed to validate BAL fluid HNP as a biomarker of BOS. HNP was measured prospectively in BAL fluid from lung transplant recipients undergoing either surveillance or clinically indicated bronchoscopies over a six-year period.
Methods
Subjects
Between January 2002 and December 2009, 244 patients underwent lung transplantation at the University of Minnesota. From these patients, 149 individuals had 577 BAL fluid samples collected after reaching baseline post-transplant pulmonary function tests (FEV1 or FEF25/75) (Estenne et al., 2002) and these patients and samples are the basis of this study (Table 1). The median age of these subjects was 61 and the most common diseases were COPD and idiopathic pulmonary fibrosis. Our primary outcome was the development of BOS grade 2 or higher (BOS ≥ 2) (Estenne and Hertz, 2002). BOS grades and baseline pulmonary function date were assigned based on International Society for Heart and Lung Transplantation (ISHLT) guidelines (Estenne et al., 2002). Baseline pulmonary function is defined as the average of the 2 highest measurements, not necessarily sequential, obtained at least 3 weeks apart (Estenne et al., 2002). All patients with a BOS diagnosis had the assigned BOS grade for a minimum of six months at the time of study closure. Acute rejection was defined using the 2007 consensus criteria (Stewart et al., 2007). The study was approved by the University of Minnesota Institutional Review Board (Protocol 0107M04822).
Table 1. Subject Characteristics.
| Category | Subjects with HNP levels after baseline pulmonary function (N=149)(%) |
|---|---|
| Age | 27-74 (median 61) |
| Female | 68 (46%) |
| Underlying Disease | |
|
| |
| COPD/Emphysema | 57 (39%) |
| Idiopathic Pulmonary Fibrosis | 30 (20%) |
| Alpha-1-Antitrypsin Deficiency | 22 (15%) |
| Cystic Fibrosis | 15 (10%) |
| Pulmonary Hypertension | 7 (5%) |
| Sarcoidosis | 5 (3%) |
| Bronchiectasis | 2 (1%) |
| Miscellaneous | 11 (7%) |
| BOS Grade | |
| 0 | 73 (49%) |
| 1 | 33 (22%) |
| 2 | 25 (17%) |
| 3 | 18 (12%) |
Bronchoalveolar lavage
An average of 3.87 BAL fluid samples/subjects were collected either as part of standard surveillance for acute rejection or when clinically indicated (Gimino et al., 2003). Standard surveillance bronchoscopies started at one month post-operatively and then continued for every two months until the recipient went one year without any episodes of acute rejection. BAL fluid was sent for routine clinical evaluations including cytology, viral rapid antigen detection (CMV and seasonal RSV and influenza), culture (bacterial, fungal, viral) and differential cell counts. The remainder of the BAL fluid was placed on ice and transported to the laboratory where it was centrifuged; supernatant was stored in aliquoted samples at −80°C until use. HNP ELISA was performed on all samples in batches of 40. Samples obtained after baseline pulmonary function was established were used for our primary outcomes.
HNP Measurements
A commercial ELISA kit (HyCult Biotechnology, Uden, Netherlands) was used to measure cumulative HNP1-3 in all samples as previously reported (Nelsestuen et al., 2005).
Statistical Methods
While this cohort study was powered for detecting differences in BOS-free survival rates, we employed several different statistical approaches to fully understand the nature of the relationship between this potential biomarker and development of BOS. Our primary analysis was based on examining differences in BOS-free survival between those with HNP levels exceeding and those less than the median after reaching baseline pulmonary function. In addition to this primary analysis, we tested for a more immediate prognostic effect of HNP using a time varying Cox proportional hazards model to predict time to BOS ≥ 2 given the most recent HNP level. The value of this approach is that it is similar to how HNP would be used as a clinical tool however it entails assumptions such as proportional hazards and the functional form of the relationship between HNP and the hazard for developing BOS ≥ 2. A third mixed model approach adds considerable insight into the nature of the relationship between HNP levels and the development of BOS ≥ 2. This approach utilizes a linear model to assess the effect of time since transplantation and if a patient develops BOS 2 on the level of HNP. We constructed a receiver operating characteristics (ROC) curve using hold one out cross validation in the context of our time varying Cox model. Specific statistical methods are available in the online supplemental material.
Results
Initial HNP level after reaching baseline pulmonary function
In our previous study we observed transiently high HNP levels within the first several months after transplantation, possibly due to post-operative changes and/or infection (Nelsestuen et al., 2005, Zhang et al., 2005). Therefore to determine if HNP is predictive of developing BOS we chose the first HNP level after the baseline pulmonary function date was established in order to avoid early post-operative changes. In this study we had 149 patients with a BAL sample obtained after reaching baseline pulmonary function. The time to reach baseline pulmonary function varied from 0.16 to 0.65 years (Table 2) and all of the subjects had reached their baseline pulmonary function by one year after transplant. Using a log rank test to compare those subjects with an HNP level below the median to those above the median we found that the initial HNP value after reaching baseline pulmonary function is significantly predictive of developing BOS ≥ 2 (p=0.0419); whereas the first HNP level after transplantation is not statistically significant (p=0.086). In the subjects that developed BOS ≥ 2, the time from their first HNP measurement after reaching baseline pulmonary function until developing BOS ranged from 0.07 to 4.32 years (median 0.88 with quartiles 0.61 and 2.12). Figure 1 displays the associated Kaplan-Meier curves of individuals that remain free from BOS ≥ 2 using values above the median (bottom line) versus those below the median (top line). A time varying Cox proportional hazards model also confirmed a statistically significant impact of the most recent HNP measurement after baseline pulmonary function on the time to development of BOS ≥ 2 (p=0.0490). A 95% confidence interval for the risk associated with each unit increase in the logarithm of the HNP level is (1.0, 1.2) indicates that higher levels of HNP are associated with an increased risk of developing BOS ≥ 2. The Grambsch Therneau test for proportional hazards gave a p-value of 0.663 indicating that the data are not at odds with the proportional hazards assumption. Although HNP was predictive of developing BOS ≥ 2 we did not find HNP to be predictive of developing BOS grade 1. BOS grade 1 was documented in 33/43 individuals (77%) that developed BOS ≥ 2. Therefore the smaller sample size was likely underpowered to detect a correlation.
Table 2.
Time from lung transplantation to baseline pulmonary function tests
| BOS Grade | Time to baseline PFT (years) |
|---|---|
| 0 | 0.65 (0.28, 1.6) |
| 1 | 0.36 (0.12, 0.83) |
| 2 | 0.16 (0.12, 0.41) |
| 3 | 0.65 (0.28, 1.4) |
Figure 1.
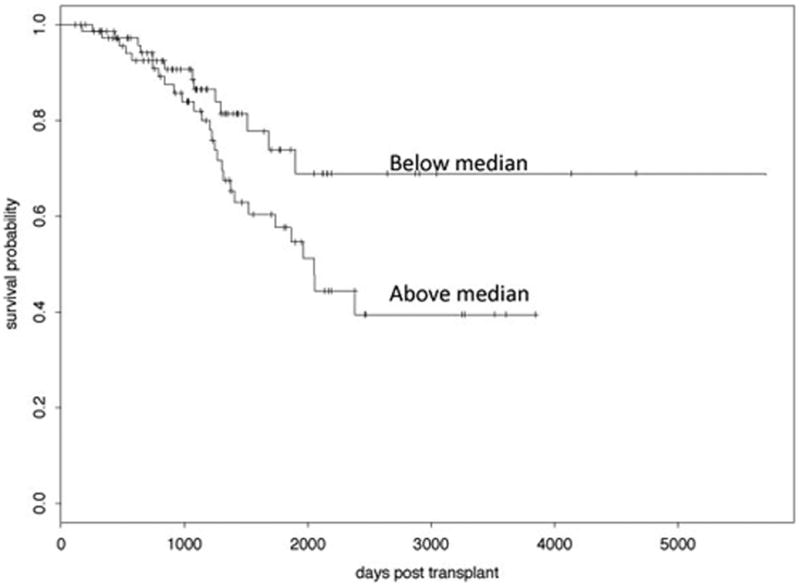
Kaplan Meier curve for HNP value after baseline pulmonary function date comparing BOS ≥ 2 free-survival for those with values below versus above the median HNP value.
HNP Trends
Using a mixed-model approach we found the log HNP values to be significantly higher in patients that developed BOS ≥ 2 compared to those that did not (p=0.0013 95% CI: 0.563, 2.274) throughout all time points; and the effect of days since transplantation not significant (p=0.113). The p-value for the interaction between time since transplantation and BOS ≥ 2 status was 0.899 indicating that there is not a significant difference in the HNP trajectories over time among patients who developed BOS ≥ 2 versus those who did not. This model found that the log HNP values were elevated in the first bronchoscopy after baseline pulmonary function was reached and remained persistently (17%) higher in those that developed BOS ≥ 2 compared to those that did not develop BOS ≥ 2 (C.I. of 6% to 26%).
HNP Sensitivity and specificity
From these data we generated a ROC curve using the time varying Cox model approach. The resulting ROC curve demonstrates an area under the curve (AUC) of 0.73, which is in close agreement with our previously reported value of 0.79 (Figure 2) (Nelsestuen et al., 2005). Although a higher AUC is desirable, this lower AUC likely reflects the heterogeneous etiology of BOS and that no single biomarker will reflect the diversity of causes for organ dysfunction following lung transplantation. Using this information, we can use the most recent HNP level to estimate sensitivities and specificities (Table 3).
Figure 2.
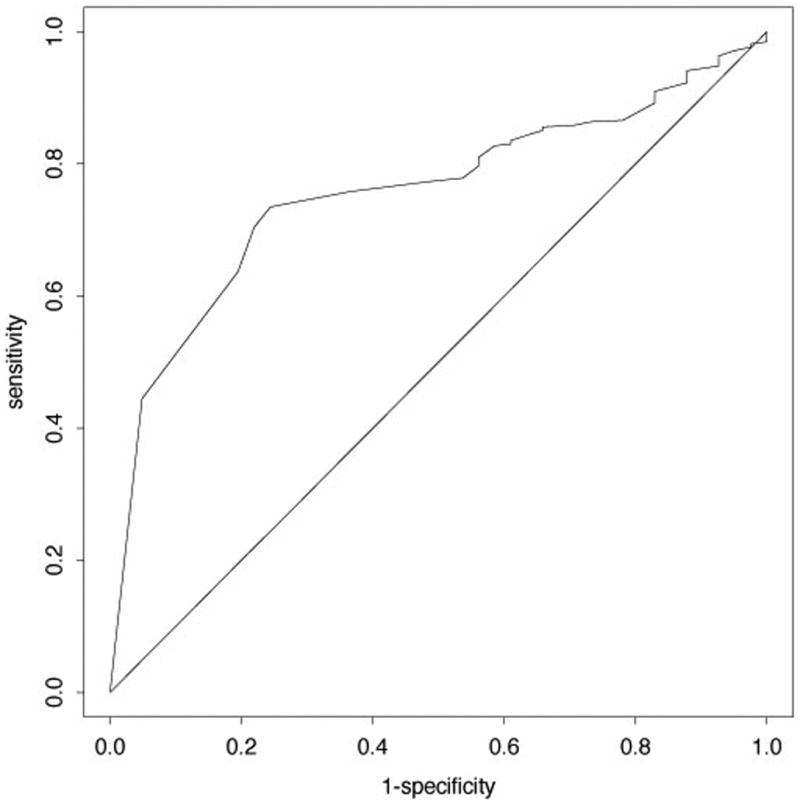
Receiver operator curve for HNP levels and BOS ≥ 2 based on a time varying Cox proportional hazards model. The ROC area under the curve is 0.73.
Table 3.
The sensitivity and specificity with specific HNP cut-off levels on the first bronchoscopy after the baseline pulmonary function date to predict development of BOS ≥2
| Raw HNP level | Logarithmic Transformed HNP level | Sensitivity | Specificity |
|---|---|---|---|
| 4881.6 | 8.49 | 0.71 | 0.44 |
| 12215.9 | 9.41 | 0.66 | 0.56 |
| 27824.0 | 10.2 | 0.54 | 0.65 |
HNP and neutrophil counts
To test for an association between HNP levels and neutrophil counts we used a mixed effect model using the log of HNP and neutrophil counts. From this model we found that HNP levels were associated with elevated neutrophil counts (p < 0.0001) (Figure 3). This suggests that elevated HNP levels in BOS are secondary to an elevation in neutrophils along with neutrophil activation; however we did not find neutrophil counts to be predictive of time to BOS ≥ 2 in a statistically significant fashion (p=0.513). This discrepancy may reflect that this study was not powered to look at effects of neutrophilia and BOS and thus the power for detecting a difference is lacking.
Figure 3.
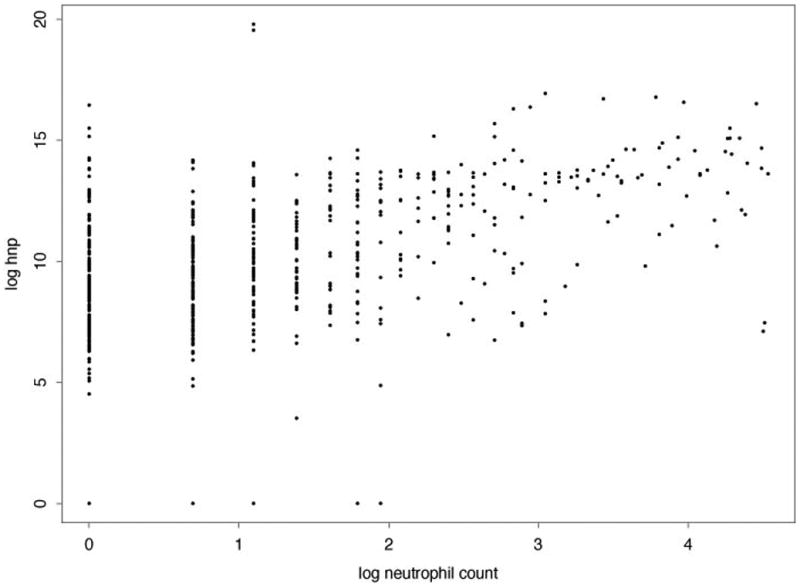
Scatterplot of HNP versus neutrophils. The estimated variance of the random effects was near 0 and not statistically different from 0, hence we summarized the association with Pearson's correlation coefficient. The value of the correlation coefficient was 0.52 and the usual p-value for testing if the correlation is 0 is less than 0.0001.
Impact of potential confounders
We examined the role of infection and other potential confounders on the association between HNP levels and time to the development of BOS ≥ 2 by including these variables as covariates in our time varying covariate Cox proportional hazards model in conjunction with HNP levels. In addition to infection we also included acute rejection and cystic fibrosis as an underlying disease as variables since elevated HNP levels have been reported in these populations. CMV was defined as a positive rapid antigen test or culture. A positive bacterial culture was defined as a positive culture other than normal oral flora. In models that included each of these variables and HNP no statistically significant effect was detected for any of these variables (Table 4). We did not investigate if these variables by themselves were predictive of time to BOS ≥ 2 since the goal was to determine HNP role and this study was not powered to investigate these factors.
Table 4.
Summary of time varying covariate models that include HNP and potential confounding variables. All models included just one of these covariates in addition to HNP levels
| CMV | Bacterial infection | Fungal infection | Acute rejection | CF | |
|---|---|---|---|---|---|
| p-value | 0.45 | 0.84 | 0.54 | 0.58 | 0.18 |
| HR (95% CI) | 0.71 (0.30, 1.71) |
0.92 (0.40, 2.09) |
1.24 (0.63, 2.44) |
1.51 (0.35, 6.50) |
2.06 (0.72, 5.86) |
Discussion
BOS is a leading cause of morbidity and mortality in lung transplant recipients and occurs in 60-70% of long-term survivors. We previously identified HNP in BAL fluid as a potential biomarker of BOS using mass spectrometry. In this current study, we sought to prospectively validate these findings in patients undergoing surveillance bronchoscopy using a standard ELISA. In our previous study we observed that many individuals had transiently elevated HNP levels within the first year following lung transplantation. We felt this was likely due to early post-operative changes influencing HNP levels such as wound healing and infection. To avoid this confounder we used the first HNP level obtained after reaching baseline pulmonary function in this study and all samples were obtained within the first post-transplant year We found that HNP levels were elevated in the first BAL fluid obtained after reaching baseline spirometry and remained consistently higher throughout the entire observation period in those patients that eventually developed BOS 2 or higher. These elevated levels were evident up to 4 years prior to the clinical diagnosis of BOS. This suggests that events early after transplantation are setting the stage for the subsequent development of BOS and these conditions persist throughout the post-transplantation period until BOS manifests itself clinically.
HNP is a small neutrophil peptide that is key in the host innate immune response that exhibits anti-microbial activity and is seen in lung diseases associated with infection (Aarbiou et al., 2002b, Ashitani et al., 2002, Bals and Hiemstra, 2004, Boyton and Openshaw, 2002, Ganz, 2002a, Hiemstra et al., 1998, Hiratsuka et al., 2003, Ihi et al., 1997, Mukae et al., 2002, Borregaard et al., 2000). Elevated HNP levels have also been associated with non-infectious lung diseases including Idiopathic Pulmonary Fibrosis, sarcoid, and Chronic Obstructive Pulmonary Disease (Mukae et al., 2002, Hiemstra et al., 1998). Although infections, particularly CMV infection, have been identified as risk factors for developing BOS, we did not find that viral, fungal and bacterial infection were predictive of the time to the development of BOS ≥ 2 given the effect of HNP. Elevated HNP levels have also been measured in individuals with cystic fibrosis (Aarbiou et al., 2002b), presumably secondary to chronic inflammation and infection. In this study, we did not find that cystic fibrosis as an underlying disease was predictive of time to BOS ≥ 2 given the effect of HNP. Previous studies have identified BAL neutrophilia as a risk factor for BOS. Although we found an association between HNP levels and neutrophilia, neutrophilia itself did not reach statistical significance in this study to predict BOS. Elevated HNP levels may reflect neutrophil activation and therefore be a more sensitive biomarker.
Acute rejection is also a known risk factor for developing BOS and is characterized histologically by perivascular and peribronchial lymphocytic inflammation. These lymphocytes are primarily CD8 T-cell lymphocytes that are cytotoxic (Riise et al., 1997). Although localized neutrophilia has been identified in BOS, it has not been associated with acute rejection. In this study we did not find a statistically significant association between HNP levels and incidence of acute rejection, likely a reflection of absence of neutrophilia and different pathological processes.
The role HNP plays in the pathogenesis of BOS is unknown. HNP has also been implicated in adaptive immunity (Biragyn et al., 2002, Chaly et al., 2000, van Wetering et al., 2002, van Wetering et al., 2000). In lung epithelial cells HNP induces expression of co-stimulatory ligands and enhances interaction with CD4+ lymphocytes (van Wetering et al., 2000). HNP can also induce cellular proliferation of lung epithelial cells and fibroblasts, along with increased collagen production (Aarbiou et al., 2002a, Aarbiou et al., 2002b, Aarbiou et al., 2004, Chaly et al., 2000). However, at very high concentrations HNP has been shown to be cytotoxic in vitro (Aarbiou et al., 2002b). These high HNP levels were similar to the elevated concentrations measured in this study suggesting that HNP could be cytotoxic in the setting of developing BOS. Interestingly, we found HNP levels to be elevated in the first BAL fluid obtained after reaching baseline lung function. In addition, HNP remained elevated in those that subsequently develop BOS, suggesting early pathological changes occur in those that develop BOS and that HNP plays a role in the pathogenesis and/or acceleration of airway injury and fibrosis that is seen in chronic lung allograft dysfunction.
The pathogenesis of BOS remains poorly understood and is likely multifactorial. Therefore it is not a surprise that no individual or set of biomarkers adequately predicts those at risk for developing BOS and likely explains the relatively modest sensitivity and specificity of HNP as a biomarker. It would be advantageous to identify individuals at risk of developing BOS prior to irreversible loss of lung function to allow for interventions in immuno- or non-immunotherapy to prevent the final common pathway of airway dysfunction. A number of biomarkers in BALF have been described for BOS including neutrophilia (Riise et al., 1999, Riise et al., 1998, Whitford et al., 2001, Zheng et al., 2000, DiGiovine et al., 1996, Elssner and Vogelmeier, 2001, Neurohr et al., 2009) and certain molecular markers (Belperio et al., 2002a, Belperio et al., 2002b, Belperio et al., 2001, Belperio et al., 2002c, Charpin et al., 2000, Meyer et al., 2001). However, to date, none of these biomarkers have been introduced into standard medical practice. Therefore until precise phenotypes are defined it is likely that multiple biomarkers may be necessary to determine the risk of developing BOS. HNP may be one such biomarker that, when persistently elevated after the recipient has reached baseline pulmonary function, portends an increased risk for developing BOS.
Conclusions
In those that develop BOS, HNP levels are elevated as early as the first bronchoscopy after reaching stable pulmonary functions and are predictive of those that will subsequently develop BOS. In addition these levels remain elevated until the development of BOS, even up to four years. This suggests pathological changes occur early after lung transplantation and that HNP may be a key player in the development of BOS. The sensitivity and specificity of HNP as a biomarker for BOS is relatively low and this may reflect the heterogeneity of BOS as a clinical surrogate for chronic rejection.
Supplementary Material
Acknowledgments
Dr. C. Reilly conducted all the statistical analyses and wrote the manuscript with Dr. C. Wendt. Dr. Cervenka edited the manuscript, was involved in the initial recruitment of subjects, sample collection, establishing the database and measurements. Dr. Hertz edited the manuscript and provided expertise in clinical assessments. Ms. Becker assisted with sample and data collection, including quality assurance of the samples. Dr. Wendt supervised the sample and data collection, wrote the manuscript with Dr. Reilly and assisted with the interpretation of results. The authors would like to thank Dr. Gary Nelsestuen for technical advice and the use of laboratory equipment.
Funding sources: This work was supported by the National Institutes of Health [Grants HL080041, HL07741]
Work performed at the University of Minnesota
Funding: NIH [R01HL080041], [2 T32 HL07741]
Footnotes
Declaration of Interest: Cavan Reilly has no conflicts of interest to disclose
Tereza Cervenka has no conflicts of interest to disclose
Marshall Hertz has no conflicts of interest to disclose
Trisha Becker has no conflicts of interest to disclose
Chris Wendt has no conflicts of interest to disclose
References
- Aarbiou J, Ertmann M, Van Wetering S, Van Noort P, Rook D, Rabe KF, Litvinov SV, Van Krieken JH, de Boer WI, Hiemstra PS. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002a;72:167–74. [PubMed] [Google Scholar]
- Aarbiou J, Rabe KF, Hiemstra PS. Role of defensins in inflammatory lung disease. Ann Med. 2002b;34:96–101. doi: 10.1080/07853890252953482. [DOI] [PubMed] [Google Scholar]
- Aarbiou J, Verhoosel RM, Van Wetering S, de Boer WI, Van Krieken JH, Litvinov SV, Rabe KF, Hiemstra PS. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30:193–201. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- Anderson RL, Hiemstra PS, Ward C, Forrest IA, Murphy D, Proud D, Lordan J, Corris PA, Fisher AJ. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. European Respiratory Journal. 2008;32:670–677. doi: 10.1183/09031936.00110807. [DOI] [PubMed] [Google Scholar]
- Anyanwu AC, Rogers CA, Murday AJ. Review of the current state of thoracic transplantation: a national prospective cohort study. UK Cardiothoracic Transplant Audit Steering Group. Transplantation Proceedings. 1999;31:165. doi: 10.1016/s0041-1345(98)01485-7. [DOI] [PubMed] [Google Scholar]
- Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, Matsukura S. Elevated levels of alpha-defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest. 2002;121:519–26. doi: 10.1378/chest.121.2.519. [DOI] [PubMed] [Google Scholar]
- Austin JH, Gougoutas CA, Schulman LL. Short air bubble in the gastric fundus during fasting: radiographic sign of gastroparesis after lung transplantation. J Thorac Imaging. 2000;15:65–70. doi: 10.1097/00005382-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–33. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Digiovine B, Keane MP, Burdick MD, Ying Xue Y, Ross DJ, Lynch JP, 3rd, Kunkel SL, Strieter RM. Interleukin-1 receptor antagonist as a biomarker for bronchiolitis obliterans syndrome in lung transplant recipients. Transplantation. 2002a;73:591–9. doi: 10.1097/00007890-200202270-00020. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002b;110:1703–16. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–56. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002c;169:1037–49. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Elsbach P, Ganz T, Garred P, Svejgaard A. Innate immunity: from plants to humans. Immunol Today. 2000;21:68–70. doi: 10.1016/s0167-5699(99)01570-4. [DOI] [PubMed] [Google Scholar]
- Boyton RJ, Openshaw PJ. Pulmonary defences to acute respiratory infection. Br Med Bull. 2002;61:1–12. doi: 10.1093/bmb/61.1.1. [DOI] [PubMed] [Google Scholar]
- Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–66. [PubMed] [Google Scholar]
- Charpin JM, Stern M, Grenet D, Israel-Biet D. Insulinlike growth factor-1 in lung transplants with obliterative bronchiolitis. Am J Respir Crit Care Med. 2000;161:1991–8. doi: 10.1164/ajrccm.161.6.9905049. [DOI] [PubMed] [Google Scholar]
- Cole AM, Ganz T. Human antimicrobial peptides: analysis and application. Biotechniques. 2000;29:822–6. 830–1. doi: 10.2144/00294rv01. [DOI] [PubMed] [Google Scholar]
- Digiovine B, Lynch JP, 3rd, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, Morris SB, Kunkel SL, Strieter RM. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157:4194–202. [PubMed] [Google Scholar]
- Elssner A, Vogelmeier C. The role of neutrophils in the pathogenesis of obliterative bronchiolitis after lung transplantation. Transpl Infect Dis. 2001;3:168–76. doi: 10.1034/j.1399-3062.2001.003003168.x. [DOI] [PubMed] [Google Scholar]
- Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–4. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002a;109:693–7. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Immunology. Versatile defensins. Science. 2002b;298:977–9. doi: 10.1126/science.1078708. [DOI] [PubMed] [Google Scholar]
- Gimino VG, Lande JD, Berryman TR, King RA, Hertz MI. Gene Expression Profiling of Bronchoalveolar Lavage Cells in Acute Lung Rejection. Am J Respir Cell Mol Biol. 2003 doi: 10.1164/rccm.200305-644OC. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS, Van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J. 1998;12:1200–8. doi: 10.1183/09031936.98.12051200. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T, Mukae H, Iiboshi H, Ashitani J, Nabeshima K, Minematsu T, Chino N, Ihi T, Kohno S, Nakazato M. Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax. 2003;58:425–30. doi: 10.1136/thorax.58.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihi T, Nakazato M, Mukae H, Matsukura S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin Infect Dis. 1997;25:1134–40. doi: 10.1086/516075. [DOI] [PubMed] [Google Scholar]
- Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation. 1998;66:764–71. doi: 10.1097/00007890-199809270-00011. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Cardoni AL, Xiang Z, Cornwell RD, Love RB. Vascular endothelial growth factor in human lung transplantation. Chest. 2001;119:137–43. doi: 10.1378/chest.119.1.137. [DOI] [PubMed] [Google Scholar]
- Mukae H, Iiboshi H, Nakazato M, Hiratsuka T, Tokojima M, Abe K, Ashitani J, Kadota J, Matsukura S, Kohno S. Raised plasma concentrations of alpha-defensins in patients with idiopathic pulmonary fibrosis. Thorax. 2002;57:623–8. doi: 10.1136/thorax.57.7.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsestuen GL, Martinez MB, Hertz MI, Savik K, Wendt CH. Proteomic identification of human neutrophil alpha-defensins in chronic lung allograft rejection. Proteomics. 2005;5:1705–13. doi: 10.1002/pmic.200401036. [DOI] [PubMed] [Google Scholar]
- Neurohr C, Huppmann P, Samweber B, Leuschner S, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, Frey L, Ueberfuhr P, Bittmann I, Behr J. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28:468–74. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, Schersten H. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J. 1999;14:1123–30. doi: 10.1183/09031936.99.14511239. [DOI] [PubMed] [Google Scholar]
- Riise GC, Kjellstrom C, Ryd W, Schersten H, Nilsson F, Martensson G, Andersson BA. Inflammatory cells and activation markers in BAL during acute rejection and infection in lung transplant recipients: a prospective, longitudinal study. European Respiratory Journal. 1997;10:1742–6. doi: 10.1183/09031936.97.10081742. [DOI] [PubMed] [Google Scholar]
- Riise GC, Williams A, Kjellstrom C, Schersten H, Andersson BA, Kelly FJ. Bronchiolitis obliterans syndrome in lung transplant recipients is associated with increased neutrophil and decreased antioxidant status in the lung. Eur Respir J. 1998;12:82–88. doi: 10.1183/09031936.98.12010082. [DOI] [PubMed] [Google Scholar]
- Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, McNeil KD, Reed EF, Reinsmoen NL, Scott JP, Studer SM, Tazelaar HD, Wallwork JL, Westall G, Zamora MR, Zeevi A, Yousem SA. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Hiemstra PS. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm Res. 2002;51:8–15. doi: 10.1007/pl00000282. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Van Der Linden AC, Van Sterkenburg MA, de Boer WI, Kuijpers AL, Schalkwijk J, Hiemstra PS. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. Am J Physiol Lung Cell Mol Physiol. 2000;278:L51–8. doi: 10.1152/ajplung.2000.278.1.L51. [DOI] [PubMed] [Google Scholar]
- Whitford HM, Orsida B, Pais M, Levvey B, Ward C, Reid S, Reid D, Williams TJ, Kotsimbos T, Walters EH, Snell GI. Bronchoalveolar lavage(BAL) neutrophilia in lung transplant recipients(LTR): infection and bronchiolitis obliterans syndrome(BOS), the chicken or the egg? J Heart Lung Transplant. 2001;20:260. doi: 10.1016/s1053-2498(00)00599-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomis. 2005 doi: 10.1002/pmic.200500105. in press. [DOI] [PubMed] [Google Scholar]
- Zheng L, Walters EH, Ward C, Wang N, Orsida B, Whitford H, Williams TJ, Kotsimbos T, Snell GI. Airway neutrophilia in stable and bronchiolitis obliterans syndrome patients following lung transplantation. Thorax. 2000;55:53–9. doi: 10.1136/thorax.55.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


