Abstract
Dentin matrix protein-1 (DMP1) is a key regulator of biomineralization. Here, we examine changes in structural, geometric, and material properties of cortical bone in a transgenic mouse model overexpressing DMP1. Micro-computed tomography and three-point bending were performed on 90 femora of wild type and transgenic mice at 1, 2, 4, and 6 months. Fourier transform infrared imaging was performed at 2 months. We found that the transgenic femurs were longer (p<0.01), more robust in cross-section (p<0.05), stronger (p<0.05), but had less post-yield strain and displacement (p<0.01), and higher tissue mineral density (p<0.01) than the wild type femurs at 1 and 2 months. At 2 months, the transgenic femurs also had a higher mineral-to-matrix ratio (p<0.05) and lower carbonate substitution (p<0.05) compared to wild type femurs. These findings indicate that increased mineralization caused by overexpressing DMP1 led to increased structural cortical bone properties associated with decreased ductility during the early post-natal period.
Introduction
Biomineralization of bone and dentin is a highly regulated process that is still not completely understood. During this process, it is known that both osteoblasts and odontoblasts secrete type I collagen and non-collagenous proteins (NCPs). In bone, the NCPs help mineralize the osteoid and can be divided into four general groups: proteoglycans, glycosylated proteins, glycosylated proteins with potential cell-attachment activities, and γ-carboxylated proteins (Robey and Boskey2006). Each group contains a number of proteins, all of which are being studied to understand their roles in mineralization. Dentin matrix protein-1 (DMP1) is a member of the small integrin-binding ligand, n-glycosylated protein and other glycoproteins with cell attachment activity (SIBLING) family, along with osteopontin (OPN), bone sialoprotein (BSP), matrix extracellular phosphoglycoprotein (MEPE), and dentin sialophosphoprotein (DSPP) (Fisher et al. 2001, Fisher and Fedarko2003). DMP1 was first discovered in dentin (George et al. 1993), but it was later found in bone and other tissues such as the kidney, pancreas, brain, and salivary and eccrine sweat glands (D'Souza et al. 1997, Hirst et al. 1997, MacDougall et al. 1998, Ogbureke and Fisher2004, Ogbureke and Fisher2005, Ogbureke and Fisher2007, Terasawa et al. 2004). While this protein has been found in various tissues, DMP1’s most influential function is its role as a key regulator in the complex process of biomineralization.
In vitro, DMP1 was first shown to be involved in mineralization when cells overexpressing DMP1 showed accelerated differentiation and mineralization (Narayanan et al. 2001). Later, DMP1 was shown to nucleate hydroxyapatite (He et al. 2003). In vivo, DMP1 knockout mice have delayed secondary ossification centers, enlarged growth plates with dramatic expansion of hypertrophic chondrocyte zones, and short limbs (Feng et al. 2006, Ling et al. 2005, Ye et al. 2005). Additionally, deficiencies in mineralization in these mice contribute to osteomalacia (Feng et al. 2006, Ling et al. 2005, Ye et al. 2005).
Overexpressing DMP1 in vivo through a gain-of-function approach confirmed the earlier in vitro studies and showed accelerated mineralization, chondrocyte hypertrophy in osseous tissues, and a five-fold increase of DMP1 expression (RNA and protein) in both teeth and bone when compared to liver in the transgenic mice (Albazzaz et al. 2009). In this study, we use this transgenic model to test if overexpressing DMP1 leads to altered structural, geometric or material properties during post-natal maturation of the appendicular skeleton. Micro-computed tomography (μCT), three-point bending tests, and Fourier transform infrared imaging (FTIRI) demonstrated accelerated mineralization and less ductile mechanical behavior during rapid phases of longitudinal growth in the mouse femur.
Materials and Methods
Animals
This study used 90 mice that were divided among wild type and transgenic animals at 1, 2, 4, and 6 months (Table 1). CD-1 mice from Charles River Laboratories overexpressed DMP1 through preparation of a cytomegalovirus (CMV) -DMP1 construct that induces ubiquitous expression of DMP1 (Albazzaz et al. 2009). The mice were housed in a controlled temperature environment with daylight photoperiod-controlled lighting. They were allowed free access to food and water. The experiments were conducted according to University of Illinois at Chicago animal care-approved protocols, and animals were maintained in accordance with the Institute of Laboratory Animal Research’s Guide for the Care and Use of Laboratory Animals.
Table 1.
Sample size distribution.
| Months | Wild type | Transgenic | Totals |
|---|---|---|---|
| 1 | 13 | 17 | 30 |
| 2 | 9 | 15 | 24 |
| 4 | 8 | 8 | 16 |
| 6 | 10 | 10 | 20 |
| Totals | 40 | 50 | 90 |
Following necropsy at respective ages, right femurs were dissected, freed of adherent soft tissue, and refrigerated in saline at 4°C for subsequent analysis including μCT, biomechanical testing, and FTIRI. The femurs were measured along the proximal-distal direction using a digital caliper (Control Company, Texas). Genders were combined and represented consistently at each group and age. Of 94 femurs initially included, four were eliminated due to errors in mechanical testing, resulting in a sample size of 90 for all analyses. Representative contralateral femurs at 2 months were analyzed by FTIRI (n=4 per group).
μCT
Desktop micro-computed tomography (μCT 40, Scanco Medical AG, Basserdorf, Switzerland) was used to nondestructively evaluate bone cross-sectional geometric properties. Femurs were scanned submerged in saline solution and oriented orthogonally to the axis of the X-ray beam, using 55 kVp, 145 µA, and an integration time of 300 ms to collect data from a 1.4 mm-long segment of the mid-diaphysis. Scans were performed at a nominal resolution of 12 µm. Sigma gauss was 0.8 and support gauss was 1. All samples were thresholded at 260 and the manufacturer’s software was used to determine average cortical thickness (Ct.Th; mm), cortical bone area (Ct.Ar; mm2), total cross-sectional area inside the periosteal envelope (Tt.Ar; mm2), medullary area (Ma.Ar; mm2), the moment of inertia about the medial-lateral axis (Iml; mm4), maximum and minimum moments of inertia (Imax; mm4, Imin; mm4), section modulus (Imax/Cmax, Imin/Cmin), and polar moment of inertia (J; mm4). In addition, the tissue mineral density of the cortical bone was determined using the manufacturer’s calibration phantom and software set at 1200 mgHA/ccm. We use the standard nomenclature and reporting guidelines for assessment of bone microstructure in rodents with μCT (Bouxsein et al. 2010).
Biomechanical Testing
Three-point bending was performed in position control using a custom-made materials testing machine, which consists of a linear actuator with optical encoder (Digit series, UltraMotion), a 250 N load cell (Entran, EL series, Measurement Specialties, Hampton, VA), a custom-made specimen holder, and a water chamber. The test was performed in 9 ml of physiological saline. Since femur length changed with each age, the span lengths (L) were varied per bone and were normalized to approximately 67% of the total bone length. Therefore, a set of 8 custom-made specimen holders were constructed with span lengths of 7, 8, 9, 10, 10.5, 11, 11.5, and 12 mm. The cross-head speed of 0.2 mm/s was held constant throughout and force-displacement data were recorded at a sampling rate of 100 Hz. Load was applied in the anterior-posterior direction, midway between the two support points in the exact region that underwent μCT. From the force-displacement curve, bending stiffness (S; N/mm), yield moment (FY; N), maximum moment (FU; N), energy to failure (mJ), energy to yield (mJ), post-yield energy to failure, ultimate displacement (D), pre-yield displacement, and post-yield displacement (DPY) were determined. Bending stiffness was defined as the slope of the initial linear portion of the load displacement curve. Maximum force was defined as the peak of the curve on the y-axis. Yield moment was defined using a 0.015 mm offset parallel to the stiffness (Schriefer et al. 2005). Energy to yield was defined as the area under the curve until the yield point. Energy to failure was defined as the area under the entire curve. Post-yield energy to failure was defined as the area under the curve between the yield and failure points. Ultimate displacement was defined as the total distance traveled by the load starting from 0.4 N until failure. Pre-yield and post-yield displacements were also calculated.
Since the bones varied in size, the actual span between loading points were of various sizes. Fortunately, this can be normalized in terms of bending moment. For three-point bending, the maximum bending moment is registered at mid-span on the test specimen, allowing normalization of data in terms of bending strain, which is a more appropriate way of describing data regardless of sizes. A similar analysis was performed by Draper and Goodship for four-point bending tests (Draper and Goodship2003). Derivation of equations from first principles (Euler-Bernoulli beam theory) is included in the Appendix.
From these structural properties and the geometric properties from μCT, material properties were calculated as follows:
where σU is the ultimate stress (MPa), ε is strain, E is Young’s modulus (GPa), u is modulus of toughness (MPa), L is the length between the two spans, c is one-half of the bone diameter measured perpendicular to the neutral axis (mm), d is the bone diameter at the mid-shaft (mm), U is the energy to failure, and the other terms are as defined (Schriefer et al. 2005), above.
Fourier Transform Infrared Imaging
Femurs were embedded in methylmethacrylate, 2 mm thick transverse sections were cut at the mid-diaphysis with a diamond-tipped blade (Isomet 5000, Buehler, Lake Bluff, IL), and then ground and polished with a final finish using 9 micron red diamond suspension paste (Phoenix 4000, Buehler, Lake Bluff, IL) to prepare a flat smooth surface. Fourier Transform Infrared Imaging (FTIRI) was performed using a Bruker Vertex 80V Step-Scan FTIR spectrometer and Hyperion 3000 infrared microscope equipped with a 64×64 pixel focal plane array detector. Images were collected in reflection mode from the surface of the polished bone with 4 cm−1 resolution, 256 scans/image and an effective pixel resolution of 20 × 20 µm2. Spectra were converted to absorbance using a custom, Matlab-based Kramers-Kronig transformation routine.
Spectra were analyzed for three characteristic peaks which have been associated with specific chemical components of bone tissue. Protein (1600 – 1700 cm−1, baseline: 1300 – 1800 cm−1), carbonate (1414 – 1424 cm−1, baseline: 1300 – 1800 cm−1) and phosphate (900 – 1200 cm−1, baseline: 900 – 1200 cm−1) contents were determined by area integration (Miller et al. 2007). Mineral-to-matrix ratio was calculated as the phosphate / protein ratio and carbonate substitution was calculated as the carbonate / phosphate ratio. The ratio of nonreducible/reducible collagen cross-linking was determined from a peak height ratio of 1660 / 1690 cm−1 (baseline: 1300 – 1800 cm−1) (Paschalis et al. 2001) and crystallinity was determined from a peak height ratio of 1020 / 1030 cm−1 (baseline: 900 – 1200 cm−1) (Paschalis et al. 1996).
Statistical Analysis
Gender differences were examined and found to be minimal, so this factor was ignored in the statistical analyses. For μCT and biomechanical testing, two-way analyses of variance (ANOVA) were used to evaluate differences between the femurs from the four ages (1, 2, 4 and 6 months) and the two groups (wild type and transgenic). Where group was significant or there was a significant group-by-age interaction term, independent Student t-tests were used to assess differences between transgenic and wild type animals at each age. For the FTIRI analysis at 2 months, we used the Mann-Whitney U test to assess statistical difference due to the smaller sample size. Significance was noted at p < 0.05. All statistical analyses were conducted using SPSS (SPSS 15.0 for Windows; SPSS, Chicago, IL) statistical software. All data are represented as mean ± standard deviation.
Results
Geometric properties (Figure 1)
Figure 1.
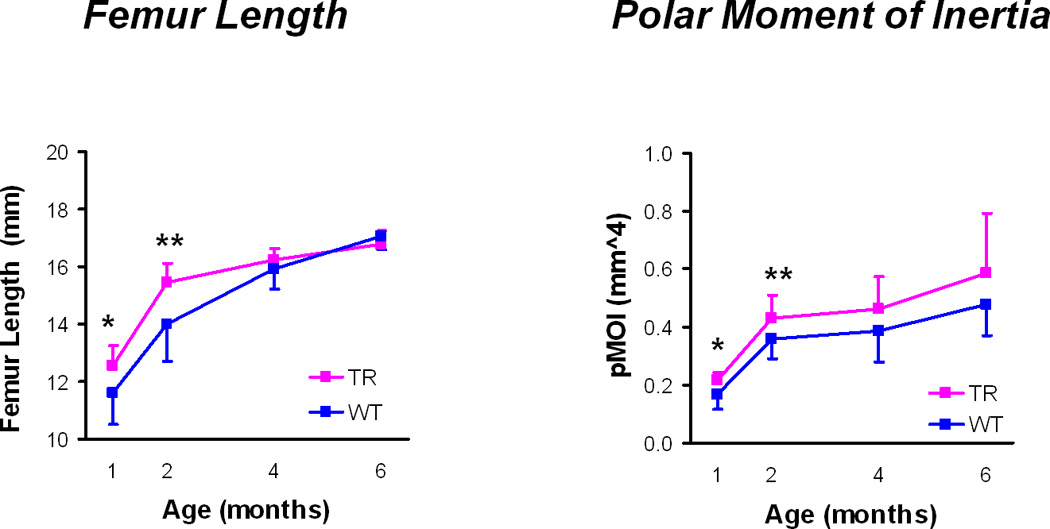
Geometrical properties: Femur length and polar moment of inertia (mean ± standard deviations). TR = transgenic mice, WT = wild type mice, * p < 0.05, ** p < 0.01 for TR v WT mice at a given age.
Transgenic mouse femurs were significantly longer compared to wild-type mouse femurs at 1 and 2 months (Table 2). However, by 4 and 6 months, there were no differences in bone length. The transgenic mouse femurs had significantly greater cross-sectional geometric properties than the wild-type mouse femurs at one month, including Ct.Ar, Tt.Ar, Ma.Ar, Imax, Imin, Imax/Cmax, Imin/Cmin, and J (Table 2). At 2 months, Tt.Ar, Imin, Imin/Cmin, and J were greater in the transgenic femurs. At 4 months, Imax was the only geometric property that was significantly greater in the transgenic femurs compared to the wild type femurs. There were no differences in any of these geometric properties between transgenic and wild type femurs at 6 months. There were no differences in cortical thickness at any time point.
Table 2.
Geometric (length and mid-shaft femoral cross-sectional) properties (mean ± standard deviations).
| Age (months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | ANOVA | ||||||
| Femur length (mm) | TR | 12.56* | (.69) | 15.44** | (.67) | 16.22 | (.43) | 16.80 | (.46) | a,b,c |
| WT | 11.62 | (1.11) | 14.01 | (1.32) | 15.92 | (.71) | 17.06 | (.46) | a,b,c | |
| Ct.Ar (mm2) | TR | 0.64* | (.06) | 0.99 | (.10) | 1.02 | (.13) | 1.14 | (.14) | a,b |
| WT | 0.55 | (.12) | 0.91 | (.09) | 0.92 | (.12) | 1.06 | (.10) | ||
| Ct.Th (mm) | TR | 0.18 | (.01) | 0.24 | (.02) | 0.26 | (.02) | 0.26 | (.01) | a |
| WT | 0.16 | (.03) | 0.23 | (.02) | 0.24 | (.02) | 0.26 | (.02) | ||
| Tt.Ar (mm2) | TR | 1.32** | (.07) | 1.81* | (.16) | 1.83 | (.21) | 2.10 | (.40) | a,b |
| WT | 1.19 | (.17) | 1.65 | (.14) | 1.69 | (.22) | 1.90 | (.25) | ||
| Ma.Ar (mm2) | TR | .70** | (.04) | .82 | (.10) | .78 | (.13) | .96 | (.27) | a,b |
| WT | .65 | (.07) | .74 | (.06) | .70 | (.10) | .84 | (.21) | ||
| Imax (mm4) | TR | 0.13* | (.02) | 0.27 | (.06) | 0.28* | (.10) | 0.35 | (.13) | a,b |
| WT | 0.10 | (.04) | 0.23 | (.04) | 0.21 | (.05) | 0.28 | (.07) | ||
| Imin (mm4) | TR | 0.08** | (.01) | 0.16** | (.02) | 0.15 | (.03) | 0.23 | (.08) | a,b |
| WT | 0.06 | (.02) | 0.13 | (.02) | 0.14 | (.03) | 0.19 | (.04) | ||
| Imax/Cmax (mm3) | TR | 0.18* | (.02) | 0.29 | (.05) | 0.30 | (.07) | 0.36 | (.09) | a,b |
| WT | 0.14 | (.04) | 0.26 | (.04) | 0.25 | (.05) | 0.32 | (.05) | ||
| Imin/Cmin (mm3) | TR | 0.14** | (.01) | 0.24** | (.03) | 0.23 | (.03) | 0.30 | (.06) | a,b |
| WT | 0.11 | (.03) | 0.20 | (.03) | 0.21 | (.03) | 0.27 | (.04) | ||
| J (mm4) | TR | 0.21* | (.03) | 0.43* | (.08) | 0.43 | (.13) | 0.59 | (.21) | a,b |
| WT | 0.17 | (.05) | 0.36 | (.07) | 0.34 | (.08) | 0.48 | (.11) | ||
p < 0.05,
p < 0.01,
p < 0.001, vs. WT at same age
Two-way ANOVA significant for age (p<0.05)
Two-way ANOVA significant for group (p<0.05)
Two-way ANOVA significant for the age-by-group interaction (p<0.05)
TR = Transgenic, WT = Wild type
Ct.Ar = cortical bone area, Ct.Th = average cortical thickness, Tt.Ar = total cross-sectional area inside the periosteal envelope, Ma.Ar = medullary area, Imax = maximum moment of inertia, Imin = minimum moment of inertia, Imax/Cmax, Imin/Cmin = section modulus, J = polar moment of inertia
Structural mechanical properties (Figure 2)
Figure 2.
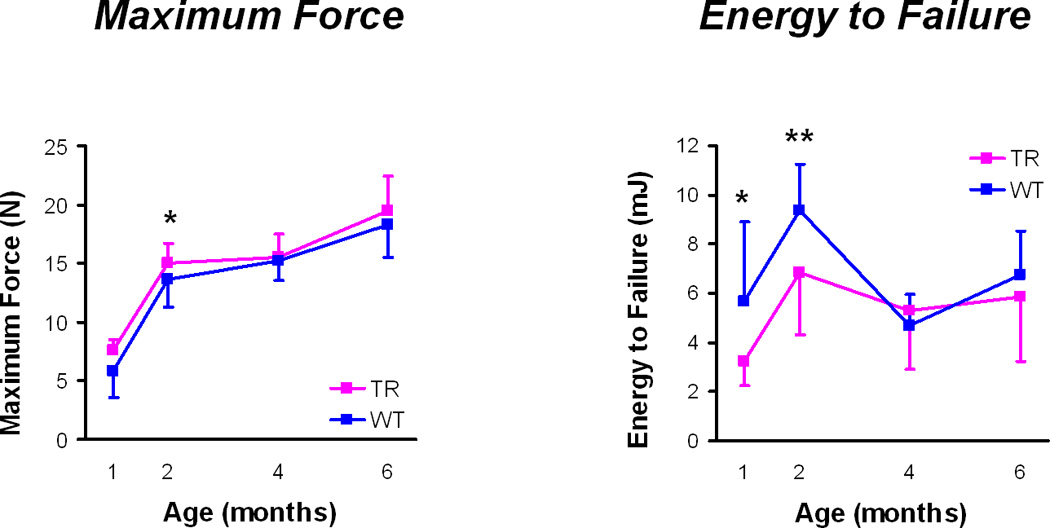
Whole bone mechanics: Maximum force and energy to failure (mean ± standard deviations). TR = transgenic mice, WT = wild type mice, * p < 0.05, ** p < 0.01 for TR v WT mice at a given age.
At 1 month, transgenic mouse femurs had higher yield and maximum moments compared to the wild type mouse femurs (Table 3). At 1 and 2 months, it took significantly less energy to fracture the transgenic femurs and it took less energy to break the transgenic femurs in the post-yield phase, although the energy to yield was significantly higher in the transgenic femurs at 2 months. In addition, the transgenic femurs had less ultimate displacement and less post-yield displacement at 1 and 2 months than the wild type femurs. There were no differences in bending stiffness or pre-yield displacement at any time point and there were no differences in any structural mechanical properties between transgenic and wild type femurs at either 4 or 6 months.
Table 3.
Structural mechanical properties (mean ± standard deviations)
| Age (months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | ANOVA | ||||||
| Bending Stiffness (N/mm) | TR | 40.30 | (6.68) | 56.49 | (11.01) | 64.04 | (15.12) | 75.37 | (19.01) | a |
| WT | 28.79 | (13.79) | 56.94 | (12.85) | 61.74 | (10.90) | 68.16 | (18.51) | ||
| Maximum moment (N) | TR | 15.91* | (2.84) | 40.15 | (5.50) | 44.19 | (6.57) | 55.87 | (8.08) | a,b |
| WT | 12.6 | (6.07) | 34.36 | (11.53) | 41.64 | (6.22) | 54.04 | (8.78) | ||
| Yield moment (N) | TR | 12.06* | (3.01) | 31.07* | (3.92) | 36.04 | (5.89) | 50.42 | (6.86) | a,b |
| WT | 9.08 | (4.94) | 24.33 | (9.86) | 34.27 | (4.62) | 43.26 | (10.99) | ||
| Total Energy (mJ) | TR | 3.26* | (1.00) | 6.83* | (2.51) | 5.29 | (2.40) | 5.85 | (2.63) | a,b |
| WT | 5.69 | (3.24) | 9.39 | (1.88) | 4.69 | (1.25) | 6.74 | (1.80) | ||
| Energy to Yield (mJ) | TR | 0.49 | (.14) | 1.52* | (.55) | 1.56 | (.64) | 2.47 | (.64) | a,b |
| WT | 0.39 | (.27) | 0.97 | (.39) | 1.63 | (.53) | 1.88 | (.56) | ||
| Post-yield Energy (mJ) | TR | 2.77* | (.95) | 5.31** | (2.15) | 3.73 | (2.15) | 3.38 | (2.83) | a,b,c |
| WT | 5.29 | (3.13) | 8.42 | (1.91) | 3.05 | (1.56) | 4.86 | (1.69) | ||
| Ultimate Displacement (mm) | TR | 0.55*** | (.16) | 0.64*** | (.21) | 0.48 | (.15) | 0.47 | (.12) | a,b,c |
| WT | 1.34 | (.52) | 0.94 | (.20) | 0.46 | (.11) | 0.55 | (.16) | ||
| Pre-yield Displacement (mm) | TR | 0.14 | (.02) | 0.26 | (.09) | 0.22 | (.05) | 0.28 | (.06) | a,b,c |
| WT | 0.14 | (.05) | 0.18 | (.03) | 0.23 | (.06) | 0.24 | (.05) | ||
| Post-yield Displacement (mm) | TR | 0.41*** | (.16) | 0.38*** | (.15) | 0.26 | (.13) | 0.19 | (.15) | a,b,c |
| WT | 1.20 | (.53) | 0.76 | (.19) | 0.23 | (.14) | 0.31 | (.14) | ||
p < 0.05,
p < 0.01,
p < 0.001, vs. WT at same age
Two-way ANOVA significant for age (p<0.05)
Two-way ANOVA significant for group (p<0.05)
Two-way ANOVA significant for the age-by-group interaction (p<0.05)
TR = Transgenic, WT = Wild type
Material properties (Figure 3)
Figure 3.
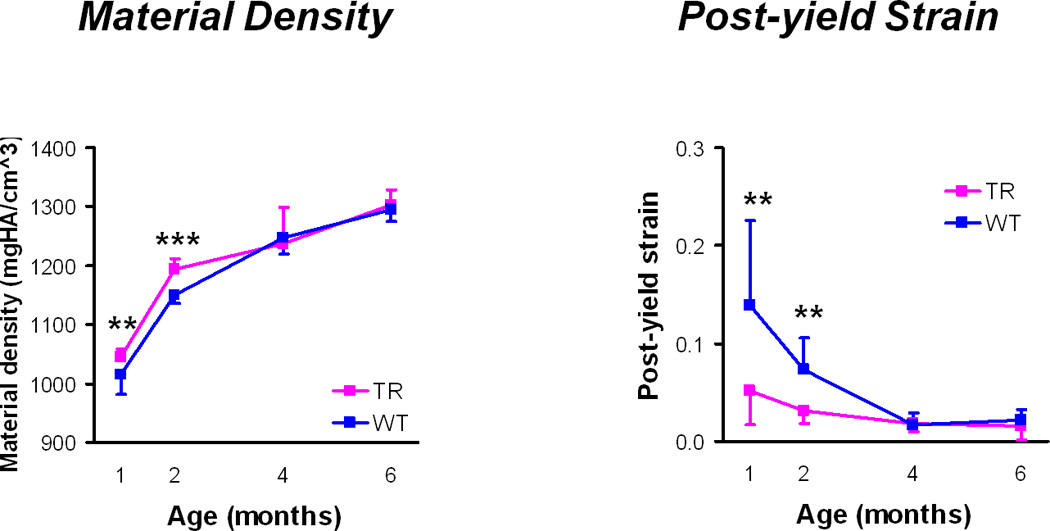
Material properties: Material density and post-yield strain (mean ± standard deviations). TR = transgenic mice, WT = wild type mice, ** p < 0.01, *** p < 0.001 for TR v WT mice at a given age.
Transgenic femurs had significantly higher tissue mineral density and lower post-yield strain and modulus of toughness at 1 and 2 months than the wild type femurs (Table 4). Differences in Young’s modulus, ultimate stress, and pre-yield strain were not significant between the transgenic and wild type femurs at 1 and 2 months. At 4 and 6 months, there were no significant differences in any material property. At 2 months, transgenic femurs had significantly higher mineral-to-matrix ratios and lower carbonate substitution as compared to wild type femurs (Fig. 4, Table 5). There were no differences in mineral crystallinity or collagen cross-linking as assessed by FTIRI.
Table 4.
Material Properties (mean ± standard deviations)
| Age (months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | ANOVA | ||||||
| Tissue Mineral Density (mgHA/cm3) | TR | 1045.32** | (13.99) | 1194.43*** | (17.99) | 1270.07 | (38.56) | 1302.30 | (25.90) | a,b,c |
| WT | 1014.25 | (33.18) | 1149.95 | (13.87) | 1262.49 | (13.84) | 1295.24 | (20.11) | ||
| Ultimate Stress (MPa) | TR | 104.23 | (13.67) | 157.02 | (14.41) | 187.79 | (28.17) | 185.80 | (36.11) | a |
| WT | 98.83 | (30.62) | 148.97 | (22.06) | 182.59 | (22.17) | 177.57 | (22.78) | ||
| Pre-yield Strain | TR | 0.030 | (0.005) | 0.040 | (0.012) | 0.030 | (0.007) | 0.042 | (0.008) | a |
| WT | 0.031 | (0.005) | 0.036 | (0.009) | 0.032 | (0.006) | 0.035 | (0.010) | ||
| Post-yield Strain | TR | 0.05** | (.04) | 0.03** | (.01) | 0.02 | (.01) | 0.02 | (.01) | a,b,c |
| WT | 0.14 | (.09) | 0.07 | (.03) | 0.02 | (.01) | 0.02 | (.01) | ||
| Young's Modulus (GPa) | TR | 5.20 | (1.55) | 7.62 | (1.98) | 11.20 | (2.41) | 9.49 | (2.49) | a |
| WT | 4.37 | (2.00) | 6.47 | (1.98) | 10.37 | (2.11) | 9.63 | (3.37) | ||
| Modulus of toughness (MPa) | TR | 2.25* | (4.87) | 1.31** | (1.74) | 0.57 | (.59) | 0.34 | (.22) | a,b,c |
| WT | 16.68 | (19.18) | 4.15 | (1.76) | 0.49 | (.41) | 0.61 | (.49) | ||
p < 0.05,
p < 0.01,
p < 0.001, vs. WT at same age
Two-way ANOVA significant for age (p<0.05)
Two-way ANOVA significant for group (p<0.05)
Two-way ANOVA significant for the age-by-group interaction (p<0.05)
TR = Transgenic, WT = Wild type
Figure 4.
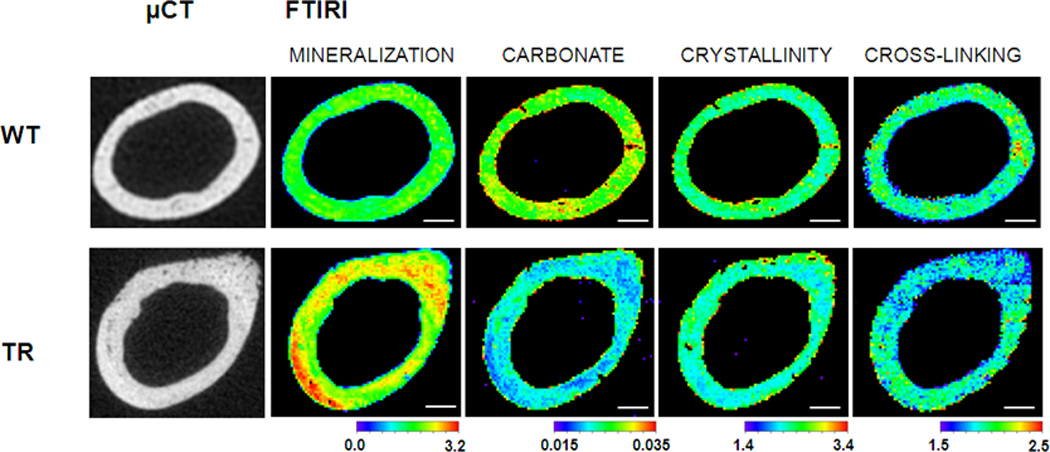
μCT and FTIRI images. Examples of μCT cross-sections of wild type (WT) and transgenic (TR) femurs with respective FTIRI images, including mineralization, carbonate substitution, mineral crystallinity, and collagen cross-linking.
Scale bar is 200 microns.
Mineralization range: 0 – 3.2
Carbonate range: 0.015 – 0.035
Crystallinity range: 1.4 – 3.4
Cross-linking range: 1.5 – 2.5
Table 5.
FTIR (mean ± standard deviations)
| 2 months | ||||
|---|---|---|---|---|
| TR | WT | |||
| Mineral:Matrix | 2.40* | (0.17) | 1.82 | (0.05) |
| Crystallinity | 1.94 | (0.24) | 2.01 | (0.29) |
| Collagen Cross-Linking | 2.01 | (0.11) | 2.17 | (0.23) |
| Carbonate Substitution | 0.023* | (0.002) | 0.031 | (0.003) |
p < 0.05,
p < 0.01,
p < 0.001, vs. WT at 2 months
TR = Transgenic, WT = Wild type
Discussion
Our objective was to determine if the overexpression of DMP1 affected bone geometry, structural (whole bone) mechanical or material properties. Overall, we found effects at 1 and 2 months that were no longer present at 4 and 6 months. Specifically, overexpression of DMP1 was associated with more rapid longitudinal and cross-sectional bone growth, increased strength combined with decreased post-yield energy dissipation as a structure, and depressed toughness and increased density as a material. Thus, even though the transgenic mouse femurs were stronger, they also required less energy to fracture. The transgenic femurs demonstrated less post-yield ability to withstand deformation or strain during mechanical testing; implying that overexpression of DMP1 caused the cortical bone to behave in a less ductile manner.
Several factors may contribute to this bone phenotype. Overexpressing DMP1 has been shown to accelerate mineralization in vitro (Narayanan et al. 2001). The present study showed that the cortical bone had a significantly higher tissue mineral density and mineral-to-matrix ratio and that these changes in material properties were associated with less ductile mechanical behavior at a material and, to some extent, structural level. This association between increased mineralization and less ductile bone behavior is consistent with many previous studies of the role of mineralization in determining how bone behaves mechanically (Currey1962). While we have focused largely on the inorganic phase (tissue mineral density), other aspects of mineral quality or differences in the organic phase may also contribute to DMP1’s effects on the bone’s mechanical behavior (Ling et al. 2005).
The bone phenotype observed in the present study, accelerated mineralization and less ductile mechanical behavior in mice overexpressing DMP1, is consistent with earlier studies in the same mouse model in which overexpression of DMP1 was associated with accelerated mineralization, as assessed by whole mount staining, up to one month post natally (Albazzaz et al. 2009). Interestingly, opposite effects were observed by previous investigators in mice with DMP1 expression knocked out [14,15,16]. Together, these studies suggest that lack of DMP1 inhibits mineralization and depresses bone growth while increased DMP1 accelerates mineralization and stimulates bone growth. While the skeleton does not compensate for the absence of DMP1 post-natally [15], the phenotypic effects of DMP1 overexpression observed in the present study were evident only during rapid bone growth but did not persist as the animals approach skeletal maturity. While femur length was increased early on, it is unlikely that the stimulation in bone growth at 1 and 2 months is related to size of the mice because earlier work in this model showed no statistical difference of weight vs age or group (unpublished).
As the role of DMP1 in mineralization is further understood, various mechanisms may explain these changes in bone phenotype that occur only during rapid phases of growth. One possible explanation revolves around the role of DMP1 expression in preosteoblasts, late osteoblasts and osteocytes (Eapen et al. 2010, Narayanan et al. 2003). In the preosteoblasts one of the co-author’s laboratory has demonstrated that DMP1 is in the nucleus and acts as a transcription factor (Narayanan et al. 2003). Further, the George laboratory has recently demonstrated that DMP1 can trigger Runx 2 expression (Eapen et al. 2010). At later stages during osteoblast differentiation, DMP1 is exported out to the matrix and initiates hydroxyapatite nucleation (Narayanan et al. 2003). Based on these observations in vitro, we suggest that during early stages of development, DMP1 can trigger the osteoblast differentiation cascade. At later stages there might be other factors that inhibit this process.
A limitation in this study is the ability to estimate tissue level mechanical properties using beam theory. The value of Young’s modulus was relatively low (4 – 11 GPa) in our study, and upon reviewing the literature, we have found a high variation of reported values of E. The reported values for E are dependent on the method employed. Raum et al, reported values in the range of mid teens (16.3 +/− 1.9 GPa) when using ultrasound and acoustic microscopy (Raum et al. 2007). Somerville et al reported even higher numbers at 22.3 GPa for males (Somerville et al. 2004). Van Lenthe et al used a micro finite element model and arrived at values of approximately 12.0 GPa for B6 WT mice (van Lenthe et al. 2008) and emphasized the underestimation using beam theory as a motivation for using micro finite element analysis. Chattah et al explored the influence of freezer storage time and obtained values between 8.6 and 10.9 GPa for BL6 mice femurs using Electronic Speckle Interferometry (Chattah et al. 2009). Brodt et al reported E values in the neighborhood of 10 GPa for 12 and 16 week old mice (Brodt et al. 1999). One of the co-authors found an even lower E value for Balb/C WT mice femurs: 6.57 GPa (Espinoza Orias et al. 2008). There seems to be sensitivity to the testing method, but our results still are in general concordance with values found in the literature. The experiments were carefully carried out and our analysis method is outlined in the Appendix. Clearly, newer technology is finding some variation, enabling researchers to fine tune the analysis methods. This would not necessarily mean that the results using beam theory, especially for comparative purposes, are wrong, however if exact values are needed, then micro modeling with the finite element method may be preferable.
Various pharmacological and mechanical perturbations are known to alter the response of bone cells at the endocortical and periosteal surfaces differently. With DMP1 overexpression, both the endocortical and periosteal areas increased at 1 and 2 months, implying increased net bone resorption at the endocortical surface and increased net bone formation at the periosteal surface. Further histological evaluation is needed to understand the relative importance of bone formation and resorption to these changes at the endocortical and periosteal surfaces.
Since the main goal of this study was to assess the effect of DMP1 overexpression on cortical bone, we combined males and females to increase our sample size. However, we did conduct initial three-way analyses of variance with age, group, and gender, and did not find any gender effects. Since males and females were equally divided at each age and group, it is unlikely that gender played a significant role in this study. Therefore, we decided to ignore gender and focus on age and genotype effects. Nevertheless, without having a much larger sample it is not possible to conclude that gender has no influence on the skeletal response to overexpression of DMP1.
We found a number of age-by-group interactions. These usually occurred when there were significant differences between wild type and transgenic animals at 1 and 2 months, but not at 4 and 6 months. Although the differences between wild type and transgenic animals at 4 and 6 months were smaller then at 1 and 2 months, it is not possible to conclude that they were not actually present as the study was not adequately powered to detect such small differences. In any case, however, it is safe to conclude that the effect of DMP1 overexpression was at least much diminished at 4 and 6 months and for some variables was completely eliminated. Thus, the value of examining different ages when trying to assess a skeletal phenotype in transgenic mouse models is clear.
The data of the present study indicate that DMP1 overexpression engendered differences in bone geometry, structural mechanical properties and material properties during phases of rapid growth and that these effects diminished as the animals approached skeletal maturity. Thus, DMP1 overexpression is associated with more highly mineralized cortical bone which behaves in a less ductile manner and with increases in cross-sectional properties. The net effect of these geometric and material effects is increased strength coupled with depressed post-yield energy absorption at the structural (whole bone) level during phases of rapid longitudinal bone growth.
Supplementary Material
Acknowledgments
Funding sources: NIH Grants R01DE11657, T32AR052272, and the Grainger Foundation. Funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No author has a conflict of interest.
Contributor Information
Ankush Bhatia, Email: ankush_bhatia@rush.edu.
Michael Albazzaz, Email: anneg@uic.edu.
Alejandro A. Espinoza Orías, Email: alejandro_espinoza@rush.edu.
Nozomu Inoue, Email: nozomu_inoue@rush.edu.
Lisa M. Miller, Email: lmiller@bnl.gov.
Alvin Acerbo, Email: acerbo@bnl.gov.
Anne George, Email: anneg@uic.edu.
Dale R. Sumner, Email: rick_sumner@rush.edu.
Reference List
- Albazzaz M, Narayanan K, Hao J, Andheri R, Ramachandran A, Ravindran S, George A. Impaired skeletal formation in DMP1 overexpressing mice. Orthopaedics Research and Reviews. 2009;1:1–10. [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner.Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Brodt MD, Ellis CB, Silva MJ. Growing C57B1/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–2166. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- Chattah NL, Sharir A, Weiner S, Shahar R. Determining the elastic modulus of mouse cortical bone using electronic speckle pattern interferometry (ESPI) and micro computed tomography: a new approach for characterizing small-bone material properties. Bone. 2009;45:84–90. doi: 10.1016/j.bone.2009.03.664. [DOI] [PubMed] [Google Scholar]
- Currey JD. Strength of bone. Nature. 1962;195:513–514. [Google Scholar]
- D'Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J.Bone Miner.Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- Draper ER, Goodship AE. A novel technique for four-point bending of small bone samples with semi-automatic analysis. J.Biomech. 2003;36:1497–1502. doi: 10.1016/s0021-9290(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Eapen A, Sundivakkam P, Song Y, Ravindran S, Ramachandran A, Tiruppathi C, George A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J Biol Chem. 2010;285:36339–36351. doi: 10.1074/jbc.M110.145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza Orias AA, Sen S, Lassova L, Cohen AJ, Yerramalli CS, Adams SL, Elliott DM. Col3A deficiency alters bone geometry and bending mechanics. Trans ORS. 2008;33:913. (Abstract) [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat.Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect.Tissue Res. 2003;44 Suppl 1:33–40. [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem.Biophys.Res.Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J.Biol.Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by selfassembled dentin matrix protein 1. Nat.Mater. 2003;2:552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- Hirst KL, Ibaraki-O'Connor K, Young MF, Dixon MJ. Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J.Dent.Res. 1997;76:754–760. doi: 10.1177/00220345970760030701. [DOI] [PubMed] [Google Scholar]
- Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J.Bone Miner.Res. 2005;20:2169–2177. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J.Bone Miner.Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- Miller LM, Little W, Schirmer A, Sheik F, Busa B, Judex S. Accretion of bone quantity and quality in the developing mouse skeleton. J.Bone Miner.Res. 2007;22:1037–1045. doi: 10.1359/jbmr.070402. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J.Biol.Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc.Natl.Acad.Sci.U.S.A. 2001;98:4516–4521. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J.Dent.Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J.Histochem.Cytochem. 2007;55:403–409. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Dicarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif.Tissue Int. 1996;59:480–487. doi: 10.1007/BF00369214. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner.Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- Raum K, Hofmann T, Leguerney I, Saied A, Peyrin F, Vico L, Laugier P. Variations of microstructure, mineral density and tissue elasticity in B6/C3H mice. Bone. 2007;41:1017–1024. doi: 10.1016/j.bone.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Robey PG, Boskey AL. Extracellular Matrix and Biomineralization of Bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Chicago: American Society for Bone and Mineral Research; 2006. pp. 12–19. [Google Scholar]
- Schriefer JL, Robling AG, Warden SJ, Fournier AJ, Mason JJ, Turner CH. A comparison of mechanical properties derived from multiple skeletal sites in mice. J.Biomech. 2005;38:467–475. doi: 10.1016/j.jbiomech.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int. 2004;74:469–475. doi: 10.1007/s00223-003-0101-x. [DOI] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J.Bone Miner.Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- van Lenthe GH, Voide R, Boyd SK, Muller R. Tissue modulus calculated from beam theory is biased by bone size and geometry: Implications for the use of three-point bending tests to determine bone tissue modulus. Bone. 2008;43:717–723. doi: 10.1016/j.bone.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J.Biol.Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


