Abstract
Robust expansion and genetic manipulation of human embryonic stem cells (hESCs) and induced-pluripotent stem (iPS) cells are limited by poor cell survival after enzymatic dissociation into single cells. Although inhibition of apoptosis is implicated for the single-cell survival of hESCs, the protective role of attenuation of apoptosis in hESC survival has not been elucidated. Bcl-xL is one of several anti-apoptotic proteins, which are members of the Bcl-2 family of proteins. Using an inducible system, we ectopically expressed Bcl-xL gene in hESCs, and found a significant increase of hESC colonies in the single-cell suspension cultures. Overexpression of Bcl-xL in hESCs decreased apoptotic caspase-3+ cells, suggesting attenuation of apoptosis in hESCs. Without altering the kinetics of pluripotent gene expression, the efficiency to generate embryoid bodies (EBs) in vitro and the formation of teratoma in vivo were significantly increased in Bcl-xL-overexpressing hESCs after single-cell dissociation. Interestingly, the number and size of hESC colonies from cluster cultures was not affected by Bcl-xL overexpression. Several genes of extracellular matrix and adhesion molecules were upregulated by Bcl-xL in hESCs without single-cell dissociation, suggesting that Bcl-xL regulates adhesion molecular expression independent of cell dissociation. In addition, the gene expression of FAS and several TNF signaling mediators were downregulated by Bcl-xL.
These data support a model in which Bcl-xL promotes cell survival and increases cloning efficiency of dissociated hESCs without altering hESC self-renewal by i) attenuation of apoptosis, and ii) upregulation of adhesion molecules to facilitate cell-cell or cell-matrix interactions.
Keywords: human embryonic stem cells, Bcl-xL, apoptosis, caspase-3, adhesion molecules
Pluripotent stem cells, including human embryonic stem cells (hESCs) and induced-pluripotent stem (iPS) cells, are capable of self-renewal and multilineage differentiation. Pluripotent stem cells not only have enormous potential as a source of therapeutic tissues, but also provide a unique system for studying lineage commitment and early human development [1, 2]. Due to low survival rate as single cells, hESCs are commonly grown as small clusters after collagenase treatment following mechanical scraping, resulting in limited expansion of hESCs [3]. Enhancement of hESC survival is a critical step for rapid hESC expansion and lineage differentiation. Recent studies demonstrated that Y-27632, a specific inhibitor for Rho-dependent protein kinase (ROCK), improves hESC survival by blocking dissociation-induced cell death [4][5][6]. Other small molecules that inhibit the Rho-ROCK pathway also enhance hESC survival [7].
Spontaneous differentiation of hESCs into different cell types can be triggered by formation of 3-dimensional (3D) embryoid bodies (EBs) [8]. Although the EB is far less organized than an embryo, it can partially mimic the spatial organization of cells in an embryo [9], allowing the assessment of cell-cell interactions and the developmental niche in vitro. However, the formation of EBs from hESCs is inefficient because of low survival of hESCs, and usually requires an entire colony of hESCs, resulting in variable sizes of EBs, thus rendering poor reproducibility of the differentiation procedure. We and others have developed systems to induce hESC differentiation directly for investigating the roles of extracellular molecules in lineage-specific differentiation [10][11][12][13][14]. However, we were unable to use direct differentiation of hESCs to assess the effect of cell-cell interaction during hESC differentiation.
The assumption that apoptosis is involved in hESC single-cell survival is plausible. Diverse groups of molecules are involved in the apoptotic pathway. One set of mediators functioning in apoptosis are asparate-specific cysteine proteases or caspases. Sequential activation of caspase cascades has a pivotal role in the execution-phase of cell apoptosis. Wang X et al. recently reported that inhibition of caspase-mediated anoikis is critical for FGF2-sustained culture of hESCs and iPS cells [15]. The B-cell lymphoma-2 (Bcl-2) family, consisting of 25 pro- and anti-apoptotic members, regulates a caspase apoptotic cascade [16] and maintains a balance between newly-formed cells and old, dying cells [17]. When anti-apoptotic Bcl-2 family members are overexpressed, the ratio of pro- and anti-apoptotic Bcl-2 family members is disrupted and apoptotic cell death can be prevented [18]. Mouse ES cells overexpressing Bcl-2 proliferate in serum-free and feeder-free conditions when supplemented with LIF [19], indicating that attenuation of apoptosis is critical for ES cell survival and self-renewal. An anti-apoptotic protein of the Bcl-2 family, Bcl-xL, contains all four Bcl-2 homology domains [17, 20].
Bcl-2 and Bcl-xL are expressed in undifferentiated hESCs and differentiating EBs [15, 21]. To improve the efficiency of hESC growth and differentiation, we investigated the protective role of Bcl-xL in dissociation-induced hESC death. Here, we demonstrated that activated caspase-3+ apoptotic cells, as well as gene expression of other apoptotic-related genes, were significantly increased when hESCs were dissociated into single cells. Ectopic expression of Bcl-xL prevented hESCs from undergoing apoptosis following enzymatic dissociation into single cells, resulting in both an increase of hESC colonies and an increase of differentiation efficiency to form EBs. However, hESC self-renewal was not altered by overexpression of Bcl-xL. Our study demonstrated that Bcl-xL overexpression not only decreased apoptotic caspase-3+ cells, but also downregulated pro-apoptotic TNF signaling mediators. In addition, Bcl-xL regulated gene expression of adhesion molecules, suggesting an enhancement of attachment and cloning efficiency of single hESCs.
Results
Rapid increase of caspase-3+ apoptotic cells after hESC dissociation
One limiting factor for hESC and iPS cell expansion is poor cell survival during subcultures. To verify that hESCs underwent apoptosis after enzymatic dissociation, we assessed apoptotic onset at different time points after hESC dissociation into single-cells. Caspase-3 acts as a key mediator of apoptosis in mammalian cells, and activation of caspase-3 is one of the penultimate steps in apoptotic cell death pathways [22–24]. We used specific antibodies for the subunit of cleaved caspase-3 (activated caspase-3) to determine caspase-3 activation following enzymatic dissociation of hESCs (Figure 1A). Flow cytometry has been used to quantify the apoptotic cells containing activated caspase-3 [25, 26]. Our data of flow cytometry indicated that the caspase-3+ population rapidly increased following enzymatic dissociation of hESCs (Figure 1B). Approximately 18% of the cells were caspase-3+ in the first 3 hours, whereas a moderate increase of caspase-3+ cells was observed between 3 and 6 hours. Concurrently, the number of the non-viable cells, which stained for 7-AAD, increased steadily over time (Supplementary figure 1). Parallel analysis by quantitative PCR (qPCR) showed that after hESC dissociation into single cells, the expressions of anti-apoptotic genes, such as Bcl-2A1 and Bcl-xL, were downregulated; whereas, the expressions of several pro-apoptotic related-genes, including tumor necrosis factor receptor (TNFR) superfamily member-9 (TNFRSF9), tumor necrosis factor (TNF) superfamily member 8 (TNFSF8), and TNF ligand family member LTA, were upregulated (Figure 1C). However, qPCR array analysis indicated that trancription of the caspase genes were not affected in dissociated hESCs (Supplementary figure 2). These data demonstrated that (i) hESC-dissociation induced rapid and extensive apoptotic response in hESCs, thereby leading to subsequent cell death, and (ii) the caspase-3 activity in dissociated hESCs was regulated at the post-transcriptional level.
Figure 1. Increase of apoptotic cells after hESC dissociation into single cells.
HESCs were treated by Accutase to generate single-cell suspensions. (A) Activated caspase-3 was detected by immunostaining after 3 hours of hESC dissociation. Cells were collected on a glass slide by cytospinning to take photos under a fluorescence microscope. (B) Apoptotic cells, in which caspase-3 is activated, were analyzed by flow cytometry at different time points after hESC dissociation into single cells. Data are representative of three independent experiments. * p<0.05 is considered statistically significant. (C) After 6 hours of hESC dissociation into single cells, the expressions of the pro-apoptotic genes (LTA, TNFSF8 and TNFRSF9) and the anti-apoptotic genes (Bcl2A1 and Bcl-xL) were analyzed by qPCR. A single-cell suspension at 0 hour was used as a control.
Attenuation of apoptosis by overexpression of Bcl-xL in hESCs
The caspase cascade is mediated by the Bcl-2 family of proteins in mitochondria-dependent apoptosis [16]. We next investigated whether attenuation of apoptosis by ectopic expression of Bcl-xL in an inducible lentiviral system enhances hESC survival. Expression of the human Bcl-xL gene was controlled by a tetracycline inducible promoter in the lentiviral vector pLentiGFPtc, and GFP expression was driven by the human EF-1alpha promoter (Figure 2A). Bcl-xL-expressing hESCs (H1-Bcl-xL) and vector control hESCs (H1-GFP) were established after several runs of manual selection of GFP+ hESC colonies. Without doxycycline induction, Bcl-xL was expressed at base levels in hESCs. Bcl-xL expression in H1-Bcl-xL hESCs was induced by doxycycline in a dose-dependent manner (Figure 2B).
Figure 2. Attenuation of apoptosis by Bcl-xL overexpression in hESCs.
(A) Schematic diagram of inducible strategy in a lentiviral vector. The human Bcl-xL gene was driven by a tetracycline inducible promoter. The constitutive expression of GFP was driven by a human EF-1alpha promoter. (B) Western blot analysis of Bcl-xL in H1-Bcl-xL cells. Different concentrations of doxycycline were tested to induce Bcl-xL expression in the H1-Bcl-xL cells. H1-GFP cells were used as control hESCs. (C) Decrease of apoptotic cells in H1-Bcl-xL cells. HESCs were dissociated into single cells. Activated caspase-3+ cells in the presence of doxycycline (500 ng/ml) were determined by FACS every three hours. Data are representative of three independent experiments. * p<0.05 is considered statistically significant.
To test the anti-apoptotic effect of Bcl-xL upon hESC dissociation, we measured caspase-3 activity in H1-Bcl-xL hESCs by flow cytometry. Compared with H1-GFP control cells, the number of caspase-3+ cells was decreased in H1-Bcl-xL hESCs upon doxycycline induction (Figure 2C). However, transcription of the caspase genes was not altered by Bcl-xL expression before (0 hour) and after (6 hours) hESC dissociation (Supplementary figure 3), suggesting that caspase-3 activity triggered by single-cell dissociation are regulated at the post-transcriptional level in Bcl-xL-expressing hESCs. It is unclear whether the anti-apoptotic function of Bcl-xL in hESCs is mediated specifically through inhibition of the pro-apoptotic effects of caspase-3.
Bcl-xL increased hESC single-cell cloning efficiency without affecting self-renewal
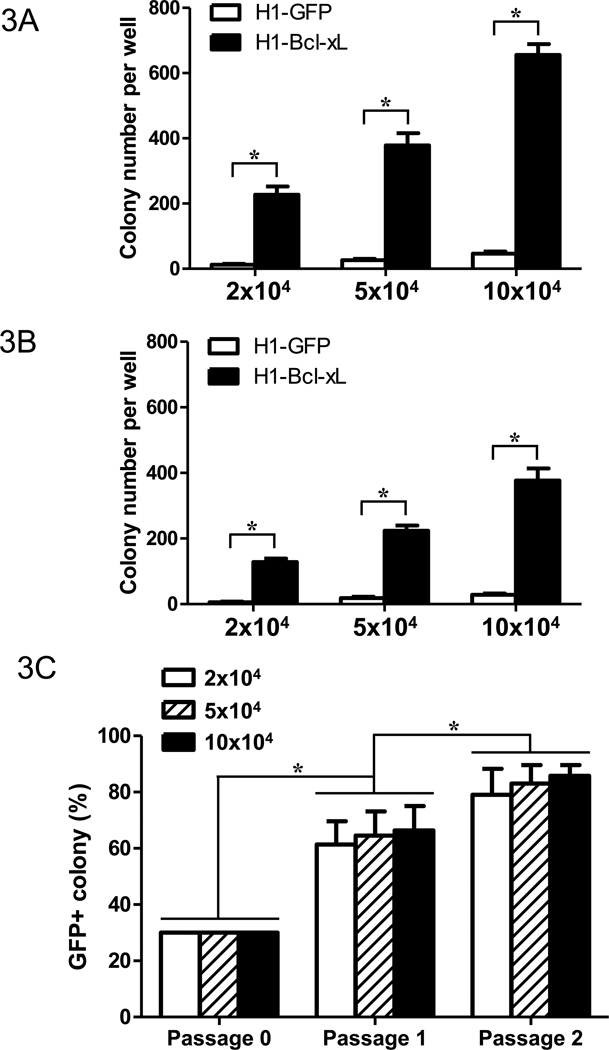
HESCs in single-cell culture have poor survival rates, resulting in fewer colonies than hESCs from small clusters [3, 27]. To test whether overexpression of Bcl-xL enhances single-cell survival, we cultured single-cell suspension of hESCs on MEF feeder cells or Matrigel-coated wells, and determined hESC colony numbers with or without Bcl-xL ectopic expression. Compared with the H1-GFP control, the numbers of hESC colonies increased significantly in H1-Bcl-xL cells upon induction of Bcl-xL expression (Figure 3A and 3B). Culture on MEF feeder cells gave rise to more hESC colonies (Figure 3A) than those on Matrigel-coated wells (Figure 3B). However, the sizes of hESC colonies were similar with or without doxycycline induction of Bcl-xL expression (Supplementary figure 4), suggesting that Bcl-xL increased hESC single-cell cloning efficiency without affecting self-renewal. After 6 days of culture, the average cell number per colony of H1-Bcl-xL cells was approximate 500 cells with or without doxycycline induction (Supplementary figure 4).
Figure 3. Increase of single-cell survival by overexpression of Bcl-xL in hESCs.
Single-cell suspensions of hESCs (2, 5, or 10 × 104 cells per well) were subcultured on MEF (A) or on Matrigel-coated wells (B) in 12-well plates in hESC growth medium in the presence of doxycycline (500 ng/ml). The hESC colony numbers in either MEF (A) or Matrigel-coated wells (B) were counted from triplicate wells after six days of culture. * p<0.05 is considered statistically significant. (C) Competitive growth of H1-Bcl-xL cells (30%) and parent hESCs. The mixture of single-cell suspensions of H1-Bcl-xL cells (30%) and parent hESCs (70%) were cultured on MEF for six days. The hESC colonies were counted under a fluorescence microscope after the first and second passage. The percentage of GFP+ hESC colonies was calculated for the frequencies of H1-Bcl-xL colonies.
The self-renewal and survival of hESCs may be mediated by para/autocrine signals [28]. To test whether hESCs overexpressing Bcl-xL provide paracrine signals for cell growth, we mixed GFP+ H1-Bcl-xL cells (30%) with GFP− parent hESCs (70%). The ratio of H1-Bcl-xL cells (GFP+ colonies) versus parent hESCs (GFP− colonies) was measured in the subsequent culture. As shown in Figure 3C, the ratio of GFP+ versus GFP− colonies increased to approximately 60% and 80% after one and two subcultures, respectively. Similar elevation of GFP+ versus GFP− colonies was observed in the cultures at low, medium or high cell density (2×104, 5×104, or 10×104 cells) (Figure 3C), indicating that cell density had no significant effect on the ratio of GFP+ versus GFP− colonies. Our study suggested that overexpression of Bcl-xL in hESCs increases single-cell survival during hESC growth in a paracrine signal-independent manner.
To determine whether overexpression of Bcl-xL affects hESC pluripotency, we examined pluripotent gene expression in H1-Bcl-xL cells that were cultured for 6 days with doxycycline induction. Immunohistochemistry and flow cytometric analysis showed that hESC pluripotent markers, including SSEA-4, TRA-1-60, and TRA-1-81, were expressed in undifferentiated H1-Bcl-xL cells with or without doxycycline induction (Figure 4A), similar to the behavior of the parent hESCs and H1-GFP control cells (data not shown). To examine whether Bcl-xL alters the kinetics of pluripotent gene expression during hESC differentiation, we induced hESC differentiation in EBs for 21 days in the presence of doxycycline. RT-PCR analysis at different time points showed that Oct4 and Nanog expression patterns were similar in H1-Bcl-xL cells and H1-GFP cells (Figure 4B). This result was further confirmed by qPCR (Figure 4C). Our data suggested that the kinetics of pluripotent gene expression is not altered by Bcl-xL overexpression during hESC differentiation.
Figure 4. Pluripotency of H1-Bcl-xL hESCs.
(A) Analyses of immunohistochemistry and flow cytometry for pluripotent genes, SSEA-4, TRA-1-60, and TRA-1-81. H1-Bcl-xL cells were cultured with or without doxycycline (500 ng/ml) six days prior to immunostaining. Flow data are representative of three independent experiments. (B) RT-PCR analysis of pluripotent genes during hESC differentiation. H1-Bcl-xL hESC colonies were differentiated to form EBs in the presence of doxycycline (500 ng/ml) on ultra-low attachment plates. RNA samples were harvested at different time points for RT-PCR analysis. The H1-GFP cells were used as a control. (C) The gene expressions of OCT4, NANOG, and SOX2 were analyzed by qPCR. Undifferentiated hESCs were used as controls. (D) The effect of Bcl-xL on hESC growth. H1-Bcl-xL hESCs and H1-GFP hESCs were subcultured from small clusters after collagenase treatment. The hESC colony numbers were counted after 6 days of hESC culture in the presence of doxycycline.
To determine whether ectopic expression of Bcl-xL affects hESC proliferation, we cultured H1-Bcl-xL hESCs as small clusters. In contrast to the result observed with hESC cultures initiated with single cells, overexpression of Bcl-xL had no significant effect on hESC colony number (Figure 4D) and size (Figure 4A) when H1-Bcl-xL cells were subcultured as clusters. The growth potential of H1-Bcl-xL hESCs that were cultured as clusters were not significantly different from H1-GFP control cells at passage 5, 15, and 25 (Figure 4D). Our data suggest that Bcl-xL increases clonal survival of dissociated hESCs by enhancing the attachment and survival of single hESCs.
Overexpression of Bcl-xL increased the efficiency of EB formation in vitro and teratomas in vivo
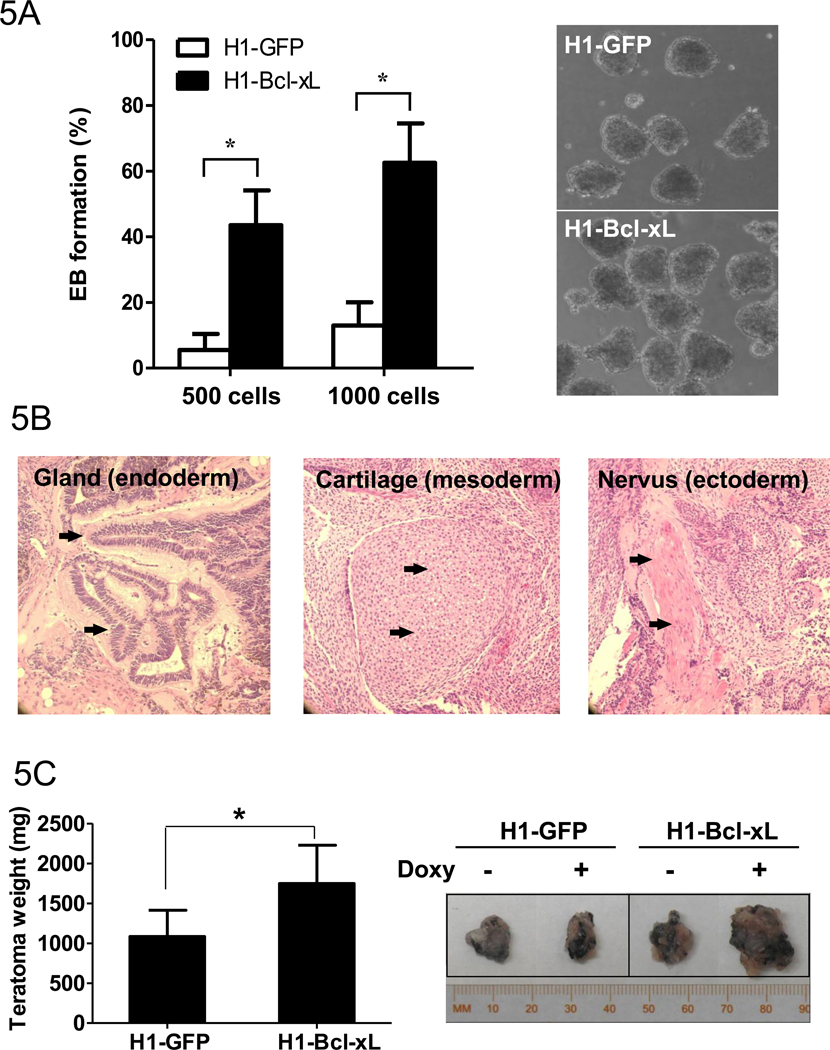
Differentiation of hESCs is conventionally induced from large hESC colonies to circumvent the restriction of low EB formation efficiency after single-cell dissociation [3, 27]. As a consequence, the resulting EBs vary in sizes, making it difficult to control hESC differentiation. To examine the effect of Bcl-xL on the efficiency of EB formation, we employed the hanging-drop method with defined cell numbers to generate uniform EBs. Compared to H1-GFP control cells, the efficiency of EB formation increased significantly in H1-Bcl-xL cells grown under Bcl-xL induction conditions (Figure 5A). When 500 cells in each drop were used, approximately 40% of the drops formed EBs in H1-Bcl-xL cells, compared to approximately 5% of the EB containing drops from H1-GFP control cells. When 1000 cells per drop were used to form EBs, approximately 60% of the drops contained EBs from H1-Bcl-xL cells, compared to approximately 15% EB containing drops from H1-GFP cells. Further increase of cell numbers up to 2000 cells had a modest effect on EB formation from either H1-Bcl-xL cells or H1-GFP cells (data not shown). The qPCR analysis indicates that PAX6 and MAP2 gene expression during hESC differentiation by Bcl-xL overexpression were upregulated, whereas RUNX1, PITX and FOXA2 gene expression were downregulated (Supplementary figure 5).
Figure 5. Increase of EB formation in vitro and teratomas in vivo by overexpression of Bcl-xL in hESCs.
(A) Efficiency of EB formation and representative photos of EBs from H1-GFP control cells and H1-Bcl-xL cells. HESCs were dissociated into single cells by Accutase to form EBs (500 or 1000 cells per hanging drop) in differentiation medium with doxycycline (500 ng/ml) for three days. The percentages of EB formation were calculated based on the number of EBs from 100 drops in three independent experiments. (B) Teratomas formation in vivo of H1-Bcl-xL cells. Tissues of three germ layers in the teratomas from H1-Bcl-xL cells were analyzed after eight weeks of cell implantation in nude mice. Photos were taken under 200× magnification. (C) Increase of teratoma sizes by Bcl-xL. Left panel: The weight of teratomas generated from H1-GFP and H1-Bcl-xL cells was determined after eight weeks of injection with doxycycline. *p<0.05 is considered statistically significant. Right panel: Examples of teratomas generated from H1-GFP and H1-Bcl-xL cells with or without doxycycline administration.
We further examined whether Bcl-xL expression affects teratoma formation in nude mice. As shown in Figure 5B, tissues derived from three germ layers including gland, cartilage, and neural cells, were observed in teratomas that originated from H1-Bcl-xL hESCs, suggesting that H1-Bcl-xL hESCs remain pluripotency. Interestingly, the teratomas generated from Bcl-xL overexpressing cells were significantly larger than those from H1-GFP control cells (Figure 5C), suggesting that Bcl-xL enhances hESC survival and growth in vivo.
Upregulation of adhesion molecules and downregulation of TNF signaling mediators by Bcl-xL
Adhesive interactions between cells-cells and cells-extracellular matrix proteins (ECM) are critical to many biological processes, including cell proliferation and cell survival [29–31]. Adhesion molecules, such as EpCAM and E-cadherin, are involved in maintenance of murine and human embryonic stem cell phenotypes [7, 32, 33]. To investigate the potential adhesive interaction involved in hESC survival, we analyzed gene expression of adhesion molecules in H1-Bcl-xL hESCs. By analyzing the expression profile of 84 adhesion molecules using a qPCR array, we found that 18 of these adhesion molecule genes were upregulated by more than a two-fold increase in H1-Bcl-xL hESCs (Supplementary table 3). The upregulation of extracellular matrix protein 1 (ECM1), fibronectin 1 (FN1), CD44, integrin-α3 (ITGA3), collagen VI-α2 (COL6A2), thrombospondin 1 (THBS1), and TIMP inhibitor 1 (TIMP1) were confirmed by qPCR (Figure 6A). Upregulation of adhesion molecules by Bcl-xL expression suggests that Bcl-xL may promote hESC survival in part by enhancing the hESC adhesion potential to feeder cells or Matrigel. Consistent with a previous study, E-cadherin transcripts were not altered during hESC dissociation [7]. The functional roles of individual adhesion molecules are currently under investigation.
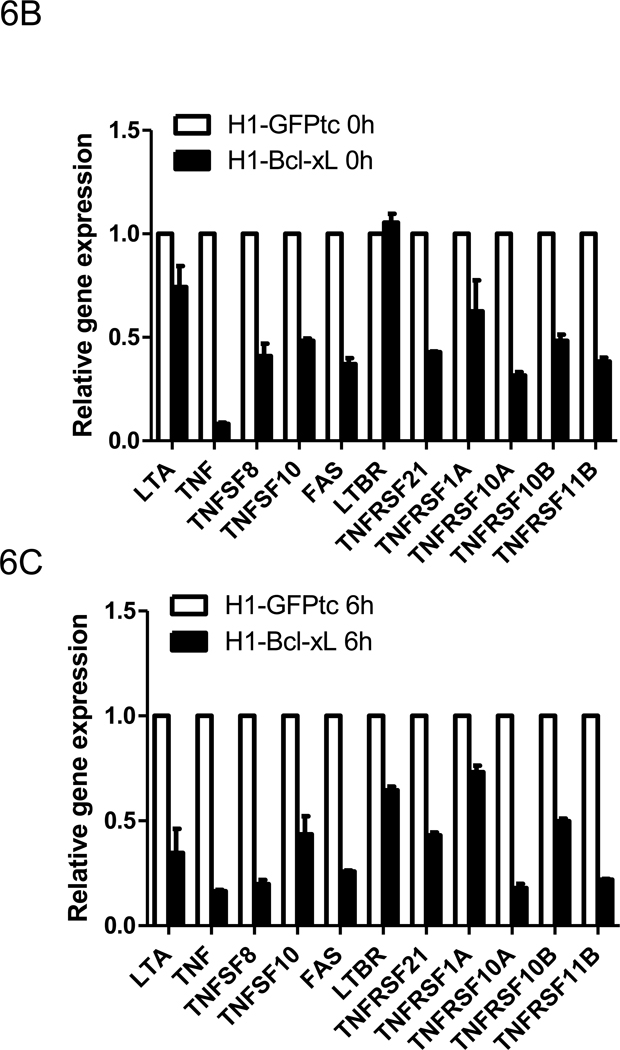
Figure 6. Regulations of adhesion molecules and TNF signaling mediators by Bcl-xL.
(A) Gene expression analysis of adhesion molecules from H1-Bacl-xL cells and H1-GFP cells by qPCR array. HESCs were dissociated into single cells by Accutase and cultured on ultra-low attachment plates in presence of doxycycline (500 ng/ml). After 0 hour (B) and 6 hours (C) of cell dissociation, gene expressions of the TNF-related ligands and receptors were analyzed by qPCR array.
To gain more insight into the apoptotic status, we next analyzed the expression of pro-apoptotic-related genes by qPCR array. Several members of TNF-related ligands and receptors that play important roles in regulating apoptosis [34] were downregulated in H1-Bcl-xL hESCs before (0 hr) and after (6 hr) hESC dissociation (Figure 6B and 6C). Comparing gene expression before (0 hr) and after (6 hr) hESC dissociation, we found that the downregulation of TNF-related genes by Bcl-xL was independent of cell dissociation (Supplementary figure 6). These data demonstrated that Bcl-xL enhancing hESC survival may be mediated by increase of cell-cell adhesion and by decrease of death signaling.
Discussion
Unlike mouse ES cells that are capable of forming colonies from single cells, hESC growth depends on cell-cell interactions [28]. As a result, single-cell subculture of hESCs leads to few colonies due to cell dissociation-induced cell death. Currently, hESCs are propagated by mechanical dissection of hESC colonies into small clusters [35, 36] or mild collagenase dissociation into clusters of cells [27, 37–39]. Those subculturing methods have disadvantages in (i) large-scale expansion, (ii) uniform colony size controlling, (iii) seeding and differentiation with defined cell number, and (iv) single-cell required experiments.
To investigate apoptosis onset in hESC propagation, we explored the possibility of apoptosis attenuation and its effect on hESCs survival. In the established H1-Bcl-xL hESCs, an anti-apoptotic gene, Bcl-xL, is ectopically expressed by an inducible expression system. Our studies demonstrated that H1-Bcl-xL cells maintained the pluripotent markers and differentiation potential in vitro and in vivo. When H1-Bcl-xL hESCs was subcultured by the traditional method of mechanical scraping and collagenase treatment into cell clusters, the colony numbers, colony size, colony morphology, and gene expression of pluripotent markers were not affected by Bcl-xL overexpression, suggesting that hESC self-renewal capability is not affected by Bcl-xL expression. Importantly, overexpression of Bcl-xL significantly increased colony numbers when H1-Bcl-xL hESCs were subcultured with single-cell suspensions. Moreover, the efficiency of EB formation in hanging-drops from single-cell suspension was significantly increased in H1-Bcl-xL cells. Our studies suggest that large-scale expansion of hESCs from signal cells after dissociation can be achieved by attenuation of apoptosis. During our manuscript preparation, a report by Ardehali R, et al. showed that ectopic expression of Bcl-2 significantly decreased hESC dissociation-induced apoptosis [40]. Therefore, attenuation of the apoptotic pathway by either overexpression of Bcl-xL or Bcl-2 enhances hESC survival.
Apoptosis can be initiated either by activation of death receptors on the cell surface membranes (extrinsic pathway) or through a series of cellular events primarily processed in the mitochondria (intrinsic pathway). Apoptosis involves cascades of caspases and Bcl-2 family members for its execution and regulation [41]. The Bcl-2 family delivers strong impacts on pivotal decisions regarding cell survival regulation [42]. As an anti-apoptotic member of the Bcl-2 family, Bcl-xL targets mitochondrial apoptotic pathways [18, 43]. Overexpression of Bcl-xL improves cell survival against apoptotic signals induced by a variety of treatments including viral infection, UV and γ-radiation, heat shock, and agents that promote formation of free radicals [18, 43]. Apoptotic signals trigger the caspase cascade in part through Bcl-xL, and eventually activate caspase-3 to cleave death substrates [16]. In our study, the antibodies that specifically recognize the large subunit (approximate 20kD) of activated caspase-3 [44, 45] were used to evaluate apoptosis in hESCs. The number of caspase-3+ cells quickly increased after trypsin or Accutase treatment aimed at single cell preparation from hESCs, indicating that disruption of cell-cell and cell-matrix interaction induced apoptosis. Indeed, the expression of many adhesion genes was elevated in H1-Bcl-xL hESCs. The upregulation of adhesion genes is independent of cell dissociation.
In addition, our gene expression analysis demonstrated that several TNF-related ligands and receptors were downregulated by overexpression of Bcl-xL in hESCs. A subgroup of the TNF-receptor superfamily is identified as death receptors with a predominant function in apoptosis induction [34]. TNF related-ligands bind to death receptors and induce receptor oligomerization, followed by the recruitment of an adaptor protein to the death domain through homophilic interaction. The adaptor protein then binds a proximal caspase, thereby connecting receptor signaling to the apoptotic effector machinery [34]. Our study demonstrated that the effect of Bcl-xL on hESC survival was executed through multiple pathways, including upregulation of adhesion molecular genes and downregulation of TNF-related death signals. How Bcl-xL regulates expression of adhesion and TNF-related molecules remains unknown.
Various cytokines and downstream signaling pathways, including FGF, BMP4 [46, 47], TGFβ [48, 49], p38 MAPK [50], JNK pathway [51], and ERK pathway [52, 53] regulate hESC self-renewal. Growth factors also influence apoptosis via PKC, PI3K, and Akt pathways [54]. Our study using inhibitors for specific signaling pathways indicated that Bcl-xL promoted single-cell survival of hESCs independent of those signaling pathways (Supplementary figure 7).
Improvement of hESC survival from single-cell culture should facilitate large-scale cultivation, and enable reliable differentiation and manipulation procedures of human pluripotent stem cells.
Materials and Methods
Maintenance of hESC cultures
The H1 and H9 hESCs were obtained from WiCell Research Institute (Madison, WI). Human foreskin fibroblasts, Hs27 cells, (ATCC, Manassas, VA) were used as feeder cells to maintain the hESCs. The hESCs (passages 29–60) were grown on mitotic-inactivated Hs27 cells in hESC growth medium containing DMEM/F-12 (Invitrogen, Carlsbad, CA), 20% knockout serum replacement (KSR, Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 2 mM L-glutamine (Mediatech, Inc, Herndon, VA), 0.1 mM beta-mercaptoethanol (Sigma, St. Louis, MO), and 4 ng/ml FGF2 (R&D, Minneapolis, MN or PeproTech, Rocky Hill, NJ).
Hs27 cells were cultured in hESC growth medium without FGF2, and were used for up to 15 passages as hESC feeder cells. For hESC culture, Hs27 cells (2×104 cells per cm2) were inactivated by mitomycin C (10 µg/ml) and seeded on 0.1% gelatin-coated plates. The hESCs were subcultured every seven days by collagenase type IV treatment (1 mg/ml, Invitrogen) followed by mechanical scrapping. The hESC growth media was changed daily as previously described [11, 55].
To remove feeder cells, hESCs were grown on Matrigel-coated plates in Hs27-conditioned media containing FGF2 (10 ng/ml). To induce ectopic expression of Bcl-xL, doxycycline (500 ng/ml, Sigma) was added into the growth medium 2 days before the experiments. To generate single-cell suspension, hESCs were treated with Accutase (Innovative Cell Technologies, CA) at 37°C for 5 minutes. The cells were dissociated with gentle agitation. Single-cell suspensions were prepared by passing dissociated cells through a 30 µm cell strainer.
Generation of H1-Bcl-xL cells by lentiviral transduction
The human Bcl-xL gene was cloned into a lentiviral vector pLentiGFPtc, in which Bcl-xL expression was driven by a mini-CMV inducible promoter, and constitutive expression of fluorescence marker GFP was driven by an individual EF-1alpha promoter. The lentiviral vector pLentiGFPtc-Bcl-xL and control vector pLentiGFPtc, were transfected into 293T cells respectively for lentivirus preparation. The lentivirus were concentrated by PEG-8000 and applied to transduce the hESCs, as previously described [11]. Using fluorescence microscopy, the GFP+ hESC colonies were manually picked up. After five passages of selection, the hESCs capable of induced expression of Bcl-xL (H1-Bcl-xL) and the control cells (H1-GFP) were established.
Differentiation of hESCs
To induce differentiation of hESCs, undifferentiated hESCs were maintained on Matrigel-coated plates for one week to remove feeder cells, then treated with Dispase (1 mg/ml, Invitrogen) at 37°C for 10 minutes to generate EBs, as previously described [11, 55]. EBs were formed with or without doxycycline (500 ng/ml, Sigma) in differentiation medium containing IMDM (Mediatech), 15% FBS (Hyclone, Logan, UT), 0.1 mM nonessential amino acids (Invitrogen), 2 mM L-glutamine (Mediatech), and 450 µM monothioglycerol (MTG, Sigma). The differentiation medium was changed every three days. The differentiated hESCs were harvested at different time-points (3, 6, 9, 12, 15, 18, and 21 days) for analyses.
Western blot analysis
Expression of Bcl-xL was monitored by Western blot analysis. To induce Bcl-xL expression, doxycycline of various concentrations (0, 500, and 1000 ng/ml) were added to the hESC growth medium for 2 days, and then the cells were lysed in RIPA buffer (1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 10 µM leupeptin, 150 mM NaCl, 50 mM Tris, pH 7.4) supplemented with 1% protease inhibitor cocktail (Sigma). Western blot analyses were performed with anti-Bcl-xL antibodies (BD Biosciences) as primary antibodies, and anti-rabbit IgG-HRP antibodies (Sigma) as secondary antibodies. The protein expression levels were quantified using Photoshop software based on band area and gray scale.
RT-PCR and real-time quantitative PCR (qPCR) analyses
Total RNAs from undifferentiated hESCs or differentiated hESCs at different time points were isolated using Trizol (Invitrogen). To eliminate DNA contamination, the RNA samples were treated with DNase (Invitrogen) and cleaned by RNeasy kit (QIAGEN) before the reverse transcription (RT) reaction. Total RNA (~100 ng) was used for each reverse transcription reaction with SuperScript III (Invitrogen). qPCR was performed on iQ5 thermal cycler (Bio-Rad). Samples were adjusted to yield equal amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Oligonucleotide primers and PCR conditions are listed in the Supplementary Table 1 (RT-PCR) and Table 2 (qPCR). The qPCR array analyses for adhesion molecules and apoptosis were performed by following the manufacturer's instructions (SABiosciences- QIAGEN, Frederick, MD).
Immunohistochemistry
For immunostaining, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 10 minutes, permeabilized with 0.1% Triton X-100 in PBS at room temperature for 10 minutes, and then incubated with 1% BSA for 30 minutes to block nonspecific binding. The cells were incubated for one hour with the primary antibodies SSEA-4, TRA-1-60, and TRA-1-81 (all from Chemicon International Inc.), washed three times, and then incubated with rabbit anti-mouse Alexa594 antibodies (Invitrogen) for one hour. The results were examined by a fluorescence microscope.
Flow cytometric analysis
HESCs were cultured on Matrigel-coated plates for four days, and treated with Accutase (Innovative Cell Technologies, CA) at 37°C for 5 minutes. The cells were dissociated with gentle agitation. Single-cell suspensions were prepared by passing dissociated cells through a 30 µm cell strainer. Single hESCs (1 × 105 per well) were cultured on 24-well ultra-low attachment plates in hESC growth medium. Caspases are synthesized as precursors that undergo proteolytic maturation in apoptosis, either autocatalytically or in a cascade by enzymes with similar specificity. An active caspase consists of two large and two small subunits that form two heterodimers which associate in a tetramer. To examine the apoptosis, the APO-ACTIVE 3 kit (Cell Technology, Inc., Mountain View, CA), which is highly specific for the subunit of cleaved caspase-3 (activated caspase-3), was used to detect activated caspase-3. Briefly, the cells were harvested at different time points (0, 3, 6, 9, and 12 hours), fixed by fixative solution, and then resuspended in PBS supplemented with 2% BSA to block nonspecific binding. The anti-caspase-3 antibodies and goat anti-rabbit IgG- phycoerythrin (PE) antibodies were used as primary and secondary antibodies respectively for flow cytometry. 7-Amino-Actinomycin D (7-AAD, BD Biosciences) was used to detect dead cells. Isotype-matched control antibodies (BD Biosciences) were used to determine the background staining. The cells were analyzed on FACSCalibur (BD Biosciences) with CellQuest software. Data analysis was performed using CellQuest or FlowJo Software.
Generation of teratoma in nude mice
To remove feeder cells, undifferentiated hESCs were maintained on Matrigel-coated plates for a week. The hESCs were treated with Accutase to generate single-cell suspensions as described above. The cells (5×106) were mixed with Matrigel (5 mg/ml) in a final volume of 50 µl, and injected into the hindlimb of 8-week-old male NIH III nude mice. To induce Bcl-xL expression, the mice were fed doxycycline (200 µg/ml) containing drinking water starting one week before cell injection. The drinking water was changed every three days. The mice were sacrificed eight weeks after the hESCs injection to analyze the teratomas.
Teratomas were harvested, fixed for 24 hours in 4% neutral buffered paraformaldehyde, transferred into 70% ethanol, and then examined by a routine wax-embedding histological procedure. Five-micrometer paraffin sections were mounted on slides and stained with hematoxylin and eosin.
Statistical analysis
The data were subjected to statistical analysis by the Student’s t-test. Results with values of p<0.05 were considered statistically significant.
Supplementary Material
Figure S1. Increase of non-viable cells from enzymatic dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium. The non-viable cells, in which the 7-AAD staining is positive, were analyzed by flow cytometry every 3 hours.
Figure S2. Gene expression of caspase families in dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium. After 6 hours of cell dissociation, gene expressions of the caspase families were analyzed by qPCR array. Single-cell suspension at 0 hour was used as a control.
Figure S3. Gene expression of caspase families in dissociated H1-Bcl-xL cells. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium with doxycycline (500 ng/ml). After of 0 hour and 6 hours of cell dissociation, gene expressions of the caspase families were analyzed by qPCR array. The H1-GFP cells were used as a control.
Figure S4. Bcl-xL has no significant impact on the size and cell number of hESC colonies. The H1-Bcl-xL hESCs were dissociated into single cells, and subcultured in hESC growth medium with or without doxycycline. The morphologies and cell numbers of hESC colonies were determined at day 6. The cell number per colony was calculated as the total number of cells by the total number of colonies.
Figure S5. Kinetics of germ layer gene expression during H1-Bcl-xL cell differentiation. The H1-Bcl-xL hESC colonies were treated to form EBs on ultra-low attachment plates with or without doxycycline (500 ng/ml). The RNA samples were harvested at different time points. The gene expressions of ectoderm (PAX6, MAP2), mesoderm (RUNX1, PITX), and endoderm (AFP, FOXA2) were analyzed by qPCR. The undifferentiated hESCs were used as a control.
Figure S6. Gene expression of TNF signaling mediators in dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium with doxycycline (500 ng/ml). After 0 hour and 6 hours of cell dissociation, the gene expressions of TNF-related ligands and receptors were analyzed by qPCR array. Single-cell suspension at 0 hour was used as a control.
Figure S7. The effects of apoptotic-independent pathways on the single-cell survival of H1-Bcl-xL cells. H1-Bcl-xL cells were dissociated into single cells and subcultured on MEF-coated 12-well plates (5 × 104 cells per well) in hESC growth medium containing doxycycline (500 ng/ml). The BMP4 inhibitor dorsomorphin (5 µM), TGFβ inhibitor SB431542 (5 µM), p38 MAPK inhibitor SB202190 (20 µM), JNK inhibitor SP600125 (20 µM), and ERK inhibitor A6355 (20 µM) were added to the cultures, respectively. Media were changed every 2 days. The colony numbers were counted at day 6. Data are representative of 3 independent experiments.
Acknowledgments
This work was supported by NIH NCRR 2P20RR018789, NIH/RC1 Challenge Grant RC1 HL100117, and American Society of Hematology Faculty Award (ZW). Additional technical core support was provided by NIH Center of Excellence in Stem Cell Biology and Regenerative Medicine core facilities (2P20RR18789) and Center of Excellence in Vascular Biology core facilities (2P20RR15555).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
HB: Collection and assembly of data, manuscript writing; KC, YXG, MA, YLX, and CM: Collection and assembly of data; YGY and WSW: Data analysis and interpretation; ZZW: Conception and design, data analysis and interpretation, and manuscript writing.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 3.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 5.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, Narumiya S, Niwa H, Sasai Y. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 10.Bai H, Gao Y, Arzigian M, Wojchowski DM, Wu WS, Wang ZZ. BMP4 regulates vascular progenitor development in human embryonic stem cells through a smad-dependent pathway. J Cell Biochem. 2010;109:363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, Kim WR, Lipton SA, Zhang K, Ding S. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu P, Pan G, Yu J, Thomson JA. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 8:326–334. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Lin G, Martins-Taylor K, Zeng H, Xu RH. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J Biol Chem. 2009;284:34054–34064. doi: 10.1074/jbc.M109.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanton E, Savory P, Cosulich S, Clarke P, Woodman P. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene. 1999;18:1781–1787. doi: 10.1038/sj.onc.1202490. [DOI] [PubMed] [Google Scholar]
- 17.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 18.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamane T, Dylla SJ, Muijtjens M, Weissman IL. Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc Natl Acad Sci U S A. 2005;102:3312–3317. doi: 10.1073/pnas.0500167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114:441–449. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rho JY, Yu K, Han JS, Chae JI, Koo DB, Yoon HS, Moon SY, Lee KK, Han YM. Transcriptional profiling of the developmentally important signalling pathways in human embryonic stem cells. Hum Reprod. 2006;21:405–412. doi: 10.1093/humrep/dei328. [DOI] [PubMed] [Google Scholar]
- 22.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 23.Pirnia F, Schneider E, Betticher DC, Borner MM. Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ. 2002;9:905–914. doi: 10.1038/sj.cdd.4401062. [DOI] [PubMed] [Google Scholar]
- 24.Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garza AS, Miller AL, Johnson BH, Thompson EB. Converting cell lines representing hematological malignancies from glucocorticoid-resistant to glucocorticoid-sensitive: signaling pathway interactions. Leuk Res. 2009;33:717–727. doi: 10.1016/j.leukres.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson BR, Baccala R, Song J, Croft M, Kono DH, Theofilopoulos AN. Deficiency of the cyclin kinase inhibitor p21(WAF-1/CIP-1) promotes apoptosis of activated/memory T cells and inhibits spontaneous systemic autoimmunity. J Exp Med. 2004;199:547–557. doi: 10.1084/jem.20031685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa K, Fujioka T, Nakamura Y, Nakatsuji N, Suemori H. A method for the selection of human embryonic stem cell sublines with high replating efficiency after single-cell dissociation. Stem Cells. 2006;24:2649–2660. doi: 10.1634/stemcells.2005-0657. [DOI] [PubMed] [Google Scholar]
- 28.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 30.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 31.Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–1653. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- 32.Ng VY, Ang SN, Chan JX, Choo AB. Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells. 28:29–35. doi: 10.1002/stem.221. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez B, Denzel S, Mack B, Conrad M, Gires O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells. 2009;27:1782–1791. doi: 10.1002/stem.97. [DOI] [PubMed] [Google Scholar]
- 34.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 35.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 36.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 37.Sjogren-Jansson E, Zetterstrom M, Moya K, Lindqvist J, Strehl R, Eriksson PS. Large-scale propagation of four undifferentiated human embryonic stem cell lines in a feeder-free culture system. Dev Dyn. 2005;233:1304–1314. doi: 10.1002/dvdy.20459. [DOI] [PubMed] [Google Scholar]
- 38.Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, Wang S, Morton CC, McMahon AP, Powers D, Melton DA. Derivation of Embryonic Stem-Cell Lines from Human Blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 39.Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- 40.Ardehali R, Inlay MA, Ali SR, Tang C, Drukker M, Weissman IL. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc Natl Acad Sci U S A. 108:3282–3287. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 42.Schendel SL, Montal M, Reed JC. Bcl-2 family proteins as ion-channels. Cell Death Differ. 1998;5:372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Feldstein AE, McIntyre TM. Suppression of mitochondrial function by oxidatively truncated phospholipids is reversible, aided by bid, and suppressed by Bcl-XL. J Biol Chem. 2009 doi: 10.1074/jbc.M109.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi S, Zhang W, Wakamatsu K, Ito S, Hearing VJ, Kraemer KH, Brash DE. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc Natl Acad Sci U S A. 2004;101:15076–15081. doi: 10.1073/pnas.0403994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi X, Li T-G, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao G-Q. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. PNAS. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 48.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 49.Peiffer I, Barbet R, Zhou YP, Li ML, Monier MN, Hatzfeld A, Hatzfeld JA. Use of xenofree matrices and molecularly-defined media to control human embryonic stem cell pluripotency: effect of low physiological TGF-beta concentrations. Stem Cells Dev. 2008;17:519–533. doi: 10.1089/scd.2007.0279. [DOI] [PubMed] [Google Scholar]
- 50.Binetruy B, Heasley L, Bost F, Caron L, Aouadi M. Concise review: regulation of embryonic stem cell lineage commitment by mitogen-activated protein kinases. Stem Cells. 2007;25:1090–1095. doi: 10.1634/stemcells.2006-0612. [DOI] [PubMed] [Google Scholar]
- 51.Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5:204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, Stojkovic M, Lako M. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 54.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 55.Chen T, Bai H, Shao Y, Arzigian M, Janzen V, Attar E, Xie Y, Scadden DT, Wang ZZ. Stromal cell-derived factor-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401. doi: 10.1634/stemcells.2006-0145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Increase of non-viable cells from enzymatic dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium. The non-viable cells, in which the 7-AAD staining is positive, were analyzed by flow cytometry every 3 hours.
Figure S2. Gene expression of caspase families in dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium. After 6 hours of cell dissociation, gene expressions of the caspase families were analyzed by qPCR array. Single-cell suspension at 0 hour was used as a control.
Figure S3. Gene expression of caspase families in dissociated H1-Bcl-xL cells. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium with doxycycline (500 ng/ml). After of 0 hour and 6 hours of cell dissociation, gene expressions of the caspase families were analyzed by qPCR array. The H1-GFP cells were used as a control.
Figure S4. Bcl-xL has no significant impact on the size and cell number of hESC colonies. The H1-Bcl-xL hESCs were dissociated into single cells, and subcultured in hESC growth medium with or without doxycycline. The morphologies and cell numbers of hESC colonies were determined at day 6. The cell number per colony was calculated as the total number of cells by the total number of colonies.
Figure S5. Kinetics of germ layer gene expression during H1-Bcl-xL cell differentiation. The H1-Bcl-xL hESC colonies were treated to form EBs on ultra-low attachment plates with or without doxycycline (500 ng/ml). The RNA samples were harvested at different time points. The gene expressions of ectoderm (PAX6, MAP2), mesoderm (RUNX1, PITX), and endoderm (AFP, FOXA2) were analyzed by qPCR. The undifferentiated hESCs were used as a control.
Figure S6. Gene expression of TNF signaling mediators in dissociated hESCs. The hESCs were dissociated into single cells by Accutase, and cultured on ultra-low attachment plates in regular hESC growth medium with doxycycline (500 ng/ml). After 0 hour and 6 hours of cell dissociation, the gene expressions of TNF-related ligands and receptors were analyzed by qPCR array. Single-cell suspension at 0 hour was used as a control.
Figure S7. The effects of apoptotic-independent pathways on the single-cell survival of H1-Bcl-xL cells. H1-Bcl-xL cells were dissociated into single cells and subcultured on MEF-coated 12-well plates (5 × 104 cells per well) in hESC growth medium containing doxycycline (500 ng/ml). The BMP4 inhibitor dorsomorphin (5 µM), TGFβ inhibitor SB431542 (5 µM), p38 MAPK inhibitor SB202190 (20 µM), JNK inhibitor SP600125 (20 µM), and ERK inhibitor A6355 (20 µM) were added to the cultures, respectively. Media were changed every 2 days. The colony numbers were counted at day 6. Data are representative of 3 independent experiments.