Abstract
Selenium (Se) is an essential micronutrient for many organisms, but is also a toxin and environmental pollutant at elevated levels. Due to its chemical similarity to sulphur, most plants readily take up and assimilate Se. Se accumulators such as Brassica juncea can accumulate Se between 0.01% and 0.1% of dry weight (DW), and Se hyperaccumulators such as Stanleya pinnata (Brassicaeae) contain between 0.1% and 1.5% DW of Se. While Se accumulation offers the plant a variety of ecological benefits, particularly protection from herbivory, its potential costs are still unexplored. This study examines the effects of plant Se levels on reproductive functions. In B. juncea, Se concentrations >0.05–0.1% caused decreases in biomass, pollen germination, individual seed and total seed weight, number of seeds produced, and seed germination. In S. pinnata there was no negative effect of increased Se concentration on pollen germination. In cross-pollination of B. juncea plants with different Se levels, both the maternal and paternal Se level affected reproduction, but the maternal Se concentration had the most pronounced effect. Interestingly, high-Se maternal plants were most efficiently pollinated by Se-treated paternal plants. These data provide novel insights into the potential reproductive costs of Se accumulation, interactive effects of Se in pollen grains and in the pistil, and the apparent evolution of physiological tolerance mechanisms in hyperaccumulators to avoid reproductive repercussions.
Keywords: Hyperaccumulation, Indian mustard, phytoremediation, pollen germination, prince's plume
Introduction
The element selenium (Se) naturally occurs in seleniferous soils arising from shale originating from the Cretaceous and early Tertiary periods (∼130–40 million years ago; White et al., 2004). Seleniferous areas, for example in the Western USA and Hubei province in China, have soil Se levels that may exceed 10 mg Se kg−1, whereas other areas such as the Northeastern USA, Finland, and New Zealand have little or no Se in soils (Reeves and Baker, 2000; Hartikainen, 2005). Se is necessary for animal and human health, but like most trace elements can be toxic at higher levels (Rayman, 2000; Brown and Arthur, 2001; Goldhaber, 2003). In higher plants there is no demonstrated need for Se; however, Se can be beneficial to plant growth at low levels and detrimental at high levels (Pilon-Smits et al., 2009). Plants readily take up Se due to its similarity to sulphur (S) (Anderson, 1993; Arvy, 1993). This capacity may be used both to fortify crops with Se and thereby help alleviate Se deficiency in humans and livestock (Gissel-Nielsen, 1998; Gomez-Galera et al., 2010), and to remove excess Se from seleniferous areas (Banuelos et al., 2005, 2007; Bañuelos and Lin, 2010).
Not all plant species accumulate or metabolize Se in the same way. Most plant species are Se non-accumulators and typically accumulate 10–100 mg Se kg−1 dry weight (DW) when growing on seleniferous soil. Plants can also be classified as Se accumulators, also called secondary accumulators, which typically accumulate 100–1000 mg Se kg−1 DW. Thirdly, there is a small group of plant species that can accumulate 1000–15000 mg Se kg−1 DW from seleniferous soil; these plants are called Se hyperaccumulators (Neuhierl and Böck, 1996; Terry et al., 2000; White et al., 2004; Seppänen et al., 2010). Hyperaccumulators such as Astragalus bisulcatus (Fabaceae) and Stanleya pinnata (Brassicaceae) accumulate Se mainly in the organic form methyl-selenocysteine (MeSeCys), while non-accumulators and secondary accumulators accumulate mainly inorganic Se (Neuhierl and Böck, 1996; Pilon-Smits et al., 1999; Terry et al., 2000). In addition, hyperaccumulators show relatively higher shoot-to-root Se accumulation and Se-to-S ratios, and store Se in specialized epidermal storage areas (Freeman et al., 2006a, 2010). Both Se accumulators and hyperaccumulators readily accumulate Se in the reproductive tissues (Terry et al., 2000; Galeas et al., 2007; Dhillon and Dhillon, 2009a, b), and within hyperaccumulator flowers Se levels can be particularly high in ovules and pollen (Quinn et al., 2011).
Several studies have shown that Se accumulation and hyperaccumulation can serve as a form of defence against herbivores such as caterpillars (Pieris rapae), grasshoppers and crickets (Orthoptera), aphids (Myzus persicae), thrips and spidermites, prairie dogs, and two pathogenic fungi (Fusarium and Alternaria) (Hanson et al., 2003, 2004; Freeman et al., 2007, 2009; Quinn et al., 2010). Se concentrations as low as 10 mg kg−1 DW were already effective in reducing herbivory of aphids. Thus, this elemental defence can offer plants an evolutionary benefit even at low levels, and herbivores and pathogens may exert further positive selection pressures toward higher plant Se accumulation. This leads to the question of whether there are evolutionary selection pressures that would act against Se accumulation. One possible negative ecological effect of Se accumulation is deterrence of pollinators by volatile selenocompounds, or toxicity to pollinators after ingestion of Se-rich nectar or pollen. Also, physiological processes involved in plant growth and reproduction may be affected by high Se levels. There is no evidence that Se deters pollinators: the European honeybee (Apis mellifera) foraged without preference on high- and low-Se plants from either the Se accumulator Brassica juncea (Brassicaceae) or the Se hyperaccumulator S. pinnata (Quinn et al., 2011). Moreover, hyperaccumulators in their natural habitat appeared to be pollinated by native bumble bees, which contained significant Se levels in their tissues and may be Se tolerant.
The goal of the study presented here was to obtain insight into potential physiology-based evolutionary selection pressures against Se accumulation. To date, little is known regarding physiology-based evolutionary selection pressures affecting hyperaccumulation. In one study using lead hyperaccumulators, there was an overall decrease in seed germination rate with increasing lead concentration (Xiong 1998). In another study by Searcy and MacNair (1990), copper (Cu)-tolerant plants had decreased seed production when grown on increasing Cu concentrations, but only when cross-pollinated with a Cu-sensitive plant. In the study described here, B. juncea and S. pinnata were examined with respect to the effects of Se accumulation on plant productivity and reproduction. The parameters examined include pollen germination, seed weight and number, seed germination, and vegetative and reproductive biomass. The findings from this study give further insight into the evolution of Se accumulation and have broader implications for cultivation of Se-rich plants for phytoremediation or as fortified foods.
Materials and methods
Plant material
Stanleya pinnata plants were grown together in the greenhouse (24/20 °C day/night, 16/8 h light/dark photoperiod, 300 μmol m−2 s−1 photosynthetic photon flux). The plants used were maintained in greenhouse conditions for 3 years on a 50:50 mix of Pro mix BX (Premier Horticulture, Quakertown, PA, USA) and Turface MVP (Profile Products LLC, Buffalo Grove, IL, USA). The plants were split into a high-Se treatment and a control (low-Se) treatment (n=15). The high-Se treatment received 80 μM Na2SeO4 twice a week and 1 g l−1 fertilizer (Miracle-Gro Excel, 15:5:15 Cal-Mag, The Scotts Co., Marysville, OH, USA) once a week. The control treatment was watered with fertilizer but no Se. To induce flowering in S. pinnata, plants were placed in a cold room (4 °C day and night, 16/8 h light/dark photoperiod, 75 μmol m−2 s−1 photosynthetic photon flux) for 4 weeks. Se treatments were continued throughout the cold treatment, and the plants began flowering within 4 weeks of returning to regular greenhouse conditions. Leaves and flowers were sampled for Se analysis, and pollen was collected for pollen germination studies, all as described below.
Brassica juncea seeds were germinated in 10×10 cm pots (T.O. Plastics, Clearwater, MN, USA) filled with Pro mix BX (Premier Horticulture, Quakertown, PA, USA), thinned to one plant per pot, and placed together in the greenhouse (conditions described above). Water was supplied until the first true leaves appeared, then Se treatments began. All plants received fertilizer once a week (1 g l−1 of Miracle-Gro Excel, 15:5:15 Cal-Mag, The Scotts Co.), and were supplied three times a week with one of five Se concentrations: 0, 20, 40, 60, or 80 μM Na2SeO4 (n=2×36 in two consecutive greenhouse studies).
Brassica juncea plants that were not used in cross-pollination studies were harvested and dissected into vegetative (shoots and leaves) and reproductive (flowers, siliques, and seeds) parts. The tissues were dried for 3 d at 60 °C, weighed for dry biomass, and analysed for Se concentration as described below. Other plants from the same treatments were used for cross-pollination experiments and to collect pollen for pollen germination experiments, as described below.
Pollen germination
Semi-solid pollen germination medium (PGM) was prepared [18% sucrose, 0.01% boric acid, 1 mM CaCl2, 1 mM Ca(NO3)2, 1 mM MgSO4, and 5% agar] (Carlson et. al 2009) and three Se concentrations: 0, 100, or 1000 mg l−1 Na2SeO4, were added to simulate stigma/style Se concentrations in the plant. Anthers from newly opened B. juncea flowers from plants treated with 0, 20, 40, 60, or 80 μM Na2SeO4 and S. pinnata flowers from plants treated with 0 or 80 μM Na2SeO4 were collected and placed on all three PGM Se treatment plates. The pollen grains were germinated on the plates in a moist environment for 3 h (for B. juncea) or 24 h (for S. pinnata) based on preliminary trials, photographed under a compound light microscope, then counted with respect to the number of pollen tubes that had broken the pollen coat, and the percentage pollen germination calculated.
Cross-pollination experiments
Flowering B. juncea plants used as the maternal plant were emasculated, and unopened flowers were removed. Anthers with mature pollen were removed from the paternal plant and rubbed on the stigma of the maternal flowers. Most Se treatments were used as both maternal and paternal plants for a total of 23 cross-pollinations for every possible combination of Se treatments (e.g. the 40 μM×60 μM cross was conducted 23 different times). The 80 μM plants, however, did not make enough flowers to complete all crosses, resulting in lower numbers of crosses. Glassine pollination bags were placed over the cross-pollinated inflorescences (Ward et al., 2009) and daily hand pollination was used to ensure pollination was successful. Mature seeds were harvested from each treatment and the average individual seed weight was determined. The total number of successful crosses, average seed number per silique, and total seed weight per silique are given in Supplementary Table S1 available at JXB online.
For seed germination studies, seeds from all crosses were placed on sterile filter paper moistened with ddH2O inside culture plates (Lifeline Sciences, Frederick, CO, USA). The dishes were closed and sealed with Parafilm® (Pechiney Plastic Science, Menasha, WI, USA) and placed under fluorescent lights (24 °C, 12/12 h light/dark photoperiod). Every day the number of germinated seeds was recorded and the seed germination percentage calculated.
To quantify fecundity, seeds were collected from 10 B. juncea plants from each Se treatment, and dried for 3 d at 60 °C. The total number of seeds produced per plant and the total seed biomass were determined.
Elemental analysis
Brassica juncea and S. pinnata leaves, flowers, and seeds were analysed for Se as described previously (Freeman et al., 2006a). In short, 0.1 g of dried material was acid-digested in nitric acid as described by Zarcinas et al. (1987) and analysed for Se analysis via inductively coupled plasma atomic emission spectroscopy (ICP-AES) as described by Fassel (1978).
Statistical analysis
All statistical analyses were performed using JMP-IN (version 3.2.6, SAS Institute, Cary, NC, USA) or SAS software (9.2, SAS Institute). Tests for normality and equal variances were conducted. One-way analyses of variance (ANOVAs) were used to compare several means, multiple linear regression analysis of covariance (ANCOVA) models were used to analyse the effect of several variables and the interactions, χ2 tests were done to analyse percentages, and Student's t-tests were used to compare two means. All ANOVAs and ANCOVAs were post-hoc evaluated using Tukey–Kramer tests for significance at α=0.05.
Results
Plant material
In B. juncea, treatments with increasing Se concentration resulted in a significantly increased plant Se concentration in both leaf and reproductive tissues (P <0.0001, Fig. 1A; and P <0.0001, Fig. 1D). Also in S. pinnata, the Se concentration in leaf and reproductive tissues of the high-Se treatment were significantly higher than those of the low-Se treatment (P <0.0001, Fig. 1C; and P=0.0017, Fig. 1F). Increased plant Se concentration decreased the dry biomass of B. juncea in both vegetative and reproductive tissues (P <0.0001, Fig. 1B; and P <0.0001, Fig. 1E). The effect, however, was not significant until the 60 μM SeO4 treatment. The S. pinnata plants used in the pollinator studies and pollen germination experiments were not harvested and dry biomass data not collected.
Fig. 1.
Biomass (g) and tissue Se concentration (mg Se kg−1 DW) of B. juncea and S. pinnata treated with different Se concentrations (0–80 μM Na2SeO4). (A) Se concentration in B. juncea leaf (n=8). (B) Dry weight of B. juncea vegetative tissues (n=16). (C) Se concentration in S. pinnata leaf (n=15). (D) Se concentration of B. juncea reproductive tissues (n=8). (E) Dry weight (n=16) of B. juncea reproductive tissues. (F) Se concentration of S. pinnata reproductive tissues (n=15). Values are means ±SE; different letters above the bars represent a significant difference (α=0.05).
Pollen germination
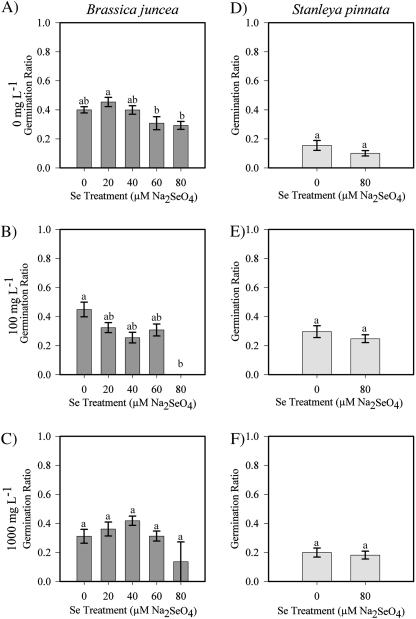
With respect to pollen germination in B. juncea, the Se level in the plant and the Se level in the media interacted and had different effects on pollen germination (F=2.93, P=0.0035, Fig. 2). On pollen germination medium containing no Se, pollen collected from plants that had received the 60 μM or 80 μM Na2SeO4 treatment germinated less often (ratios of 0.30 and 0.29, respectively) than pollen from plants that received other Se treatments (0.40, 0.45, and 0.40, P=0.0480, Fig. 2A). On the 100 mg l−1 Na2SeO4 pollen germination medium, plants with higher Se levels showed a decrease in pollen germination, with germination ratios ranging from 0.45 in the no-Se treatment to 0.0 in the 80 μM Se treatment (P=0.0035, Fig. 2B). On the 1000 mg l−1 Na2SeO4 pollen germination medium, there were no significant differences in pollen germination between Se treatments (Fig. 2C). The Se concentration in the media had a significant effect on pollen germination, with the average ratio in the no-Se treatment being the highest at 0.37 compared with the 100 mg l−1 Se treatment, which was the lowest at 0.27 (F=7.47, P=0.0007). The 1000 mg l−1 medium treatment was not significantly different from either the no-Se or moderate Se media treatments.
Fig. 2.
Pollen germination ratios for B. juncea and S. pinnata treated with different Se concentrations (0–80 μM Na2SeO4). (A) B. juncea pollen germination on medium without Se (n=30 plants). (B) B. juncea pollen germination on medium containing 100 mg l−1 Na2SeO4 (n=12 plants). (C) B. juncea pollen germination on medium containing 1000 mg l−1 Na2SeO4 (n=12 plants). (D–F) S. pinnata pollen germination ratios on medium containing 0, 100, and 1000 mg l−1 Na2SeO4, respectively (n=10 plants). Values are means ±SE; a different letter above the bars represents a significant difference (α=0.05).
Stanleya pinnata showed different results from B. juncea. At each of the three Se concentrations in the pollen germination medium, there was no significant difference between plant Se treatments (P=0.1549, P=0.3096, P=0.7103, Fig. 2D–F). There was a significant effect of Se in the media, and S. pinnata pollen germinated better on media containing Se than they did on media without Se. The average germination ratio for the no-Se treatment was 0.13, which was significantly lower compared with the high-Se media treatment at 0.19 and the moderate-Se media treatment at 0.27 (F=9.82, P <0.0001, Fig. 2D–F).
Cross-pollinations
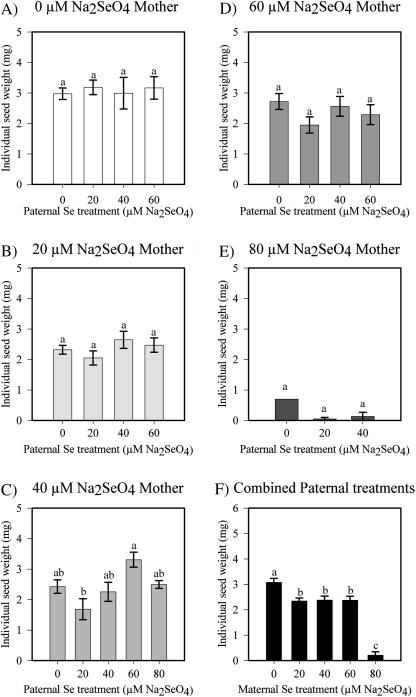
Cross-pollinations were performed between B. juncea plants treated with different Se concentrations. Individual seed weight was determined from each of the crosses to measure if there is any effect on seed vigour with increasing Se concentration. The paternal Se treatment had no effect on the individual seed weight (P=0.9538, Fig. 3A–E). The maternal Se treatment did have a significant effect on seed weight, especially at leaf concentrations >1000 mg Se kg−1 DW (P <0.0001, Figs 3F, 1A). The 20, 40, and 60 μM Se maternal treatments resulted in a 20% reduction in seed weight compared with the 0 μM Se treatment, and the 80 μM-treated plants even showed a 90% reduction in seed weight. However, the heaviest seeds were obtained from the 40 μM×60 μM Se cross, which resulted in an average weight of 3.31 mg as compared with 2.98 mg for the 0 μM×0 μM Se cross (Fig. 3C). The number of seeds produced in each cross was not significantly different between Se treatments, except at the Se treatment of 80 μM. (Supplementary Table S1 at JXB online).
Fig. 3.
Average individual seed weight (mg) from crosses of B. juncea parents treated with 0, 20, 40, 60, or 80 μM Na2SeO4. A–E each represent plants from one maternal Se treatment that were pollinated with paternal plants that received different Se levels. F shows the average for each maternal Se treatment when all paternal Se treatments are pooled. Values are means ±SE; a different letter above the bars represents a significant difference (α=0.05). See Supplementary Table S1 at JXB online for details on the number of seeds weighed.
Seeds from each of the cross-pollinations were collected and analysed for their Se concentration. Due to the high number of seeds required for Se analysis and the need for seeds for seed germination studies, only one replicate for each cross was obtained and analysed. For each maternal Se concentration, the paternal Se concentration did not affect the seed Se concentration (P=0.9966). The increasing maternal treatment concentration, however, significantly increased seed Se concentration (P <0.0001). Average seed Se concentration for increasing maternal Se treatments pooled across paternal Se treatments were 8, 86, 153, and 792 mg kg−1.
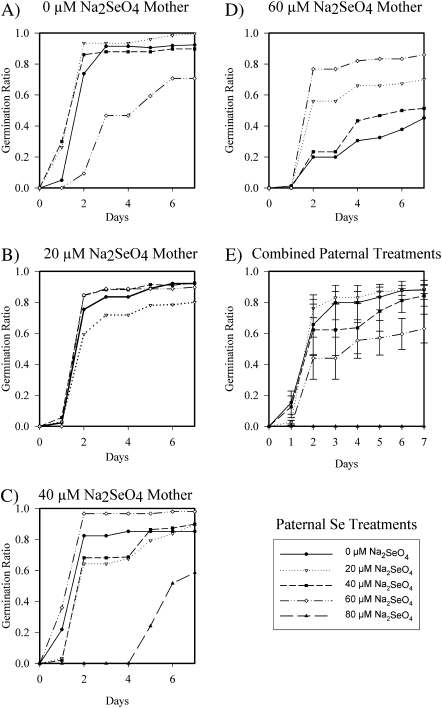
In the B. juncea seed germination study, all cross-pollinations done with an 80 μM Na2SeO4 plant as the maternal or paternal plant did not produce enough seeds for adequate statistical power and are not included in the statistical analyses. The study was terminated after 7 d because germination rates of most treatments were beginning to plateau (Fig. 4E). χ2 analyses were done on the frequency of germination at the end of the 7 d germination period, for an estimation of total seed germination percentage. All P-values are reported in the text, and χ2 values and P-values are reported in Fig. 4. When considering both maternal and paternal Se treatments together, the combination of treatments significantly affected the total seed germination (P <0.0001, Fig. 4). When the maternal Se treatment was analysed across all paternal Se treatments, the maternal Se treatment significantly affected the total percentage of seed germination (P <0.0001, Fig. 4E). The seed germination ratio decreased with increasing levels of maternal Se treatment. The paternal Se treatment, when analysed across all maternal Se treatments, also had a significant effect on the total percentage of seed germination (P=0.0100), but there was no consistent relationship between increasing paternal Se treatment and seed germination.
Fig. 4.
Progressive seed germination rates over a period of 7 d (seeds germinated/total seeds sown) of B. juncea seeds obtained from crosses of parents treated with different Se concentrations. In A–D different lines correspond to different paternal Se treatments (μM Na2SeO4) as labelled in the panel on the right. (A) Maternal plants treated with 0 μM Na2SeO4. (B) Maternal plants treated with 20 μM Na2SeO4. (C) Maternal plants treated with 40 μM Na2SeO4. (D) Maternal plants treated with 60 μM Na2SeO4. (E) Average for each maternal Se treatment when all paternal Se treatments are pooled. Each line corresponds to a maternal Se treatment, as described in the panel on the right. Values are the means ±SE; n ≥150 seeds.
When the interactions of the maternal and paternal Se treatments were analysed, the Se treatment showed a significant effect on the percentage of seed germination by day 5 (P <0.0001). The maternal and paternal Se treatment effects were also analysed separately, and both treatments yielded a significant effect on seed germination (P <0.0001 and P <0.0001). χ2 values and probabilities within each maternal treatment show a significant effect of the paternal Se concentration (Fig. 5A–D). Increasing paternal Se concentration had a significant negative effect when the maternal Se treatment was 0 μM Na2SeO4 (P=0.0052, Fig. 4A). When the maternal Se treatment was 20 μM and 40 μM Na2SeO4, the paternal Se treatment did not have a significant effect (P=0.2380, P=0.5193, Fig. 4B, C). Then, at the maternal Se treatment of 60 μM Na2SeO4, the increasing Se treatment of the paternal plant significantly increased seed germination (P=0.0013, Fig. 4D). At the maternal Se treatment of 80 μM Na2SeO4, none of the seeds germinated over the 7 d period. Thus, the effect of the paternal Se treatment on seed germination depended on the Se level of the mother plant.
Fig. 5.
Fecundity parameters of B. juncea plants from all Se treatments not used in cross-pollinations (i.e. seed number and weight that resulted from selfing). (A) Total seed weight (g) per plant (n=16 plants). (B) Total number of seeds per plant (n=16 plants). Values are means ±SE; different letters above the bars represent a significant difference (α=0.05).
The total weight of seeds produced from each B. juncea plant not used in cross-pollination experiments (via selfing) was significantly decreased with increased seed Se concentration (F=15.7589, P <0.0001, Fig. 5A). Plants treated with 20 μM Se had on average 20% lower total seed weight, 40 μM Se treatment resulted in 60% lower total seed weight, and plants treated with 60 μM or 80 μM Se produced no seeds. Similarly, when the total number of seeds was counted, there was a significant effect of increasing Se concentration (F=17.9588, P <0.0001, Fig. 5B). Plants not treated with Se produced more seeds than plants treated with increasing Se levels. This effect became significant above 20 μM Na2SeO4, corresponding to a leaf and flower Se concentration >500 mg Se kg−1 DW (Fig. 1A, D).
Discussion
The goal of this study was to analyse the effect of plant Se accumulation on reproductive functions in B. juncea and S. pinnata. The main finding of the study is that the Se accumulator B. juncea showed inhibited growth and reproduction with floral Se concentrations in excess of 500 mg Se kg−1 DW, in terms of biomass production, pollen germination, seed germination, and seed production. The Se hyperaccumulator S. pinnata, however, showed no negative effect of Se at floral concentrations of ∼4000 mg Se kg−1 DW. These results were reproducible in a consecutive greenhouse experiment where comparable results were obtained.
In the greenhouse trials, B. juncea with floral concentrations of ∼1500 mg Se kg−1 DW and leaf concentrations >1000 mg Se kg−1 DW showed a significant decrease in biomass. In contrast, the hyperaccumulator S. pinnata had floral concentrations close to 4000 mg Se kg−1, but this had no significant effect on growth.
Furthermore, in B. juncea, the 60 μM and 80 μM Se treatments led to decreased pollen germination rates, and increasing Se concentration in the pollen germination media decreased the pollen germination rate. Pollen germination in S. pinnata was not affected by high Se concentrations in the flower or pollen germination media; rather, S. pinnata pollen germinated better on media containing Se.
An interesting observation in the cross-pollination studies was that pollen from Se-rich B. juncea plants appeared to be better able to fertilize Se-rich maternal plants. In an earlier, somewhat similar study conducted by Searcy and Macnair (1990), Cu-tolerant plants fertilized with Cu-sensitive pollen showed decreased levels of fertilization. The authors hypothesized that the pollen tubes from Cu-sensitive plants did not survive and grow as well as the pollen from Cu-tolerant plants in the high-Cu environment of the style. Here, moderate levels of Se in the paternal plants improved seed germination and seed weight of Se-rich maternal plants. It is possible that plants treated with no or low Se produce pollen tubes that are inhibited by the high concentrations of Se in the style and ovule of a plant treated with higher Se concentrations. Pollen from a high-Se plant may have a physiological predisposition to cope with the toxic effects of Se and are not as vulnerable in a style with high Se concentration. It would be interesting to investigate this Se-dependent pollen–pistil interaction further and identify the underlying physiological mechanisms.
In B. juncea, the Se concentration of the maternal plant had the most pronounced effect on reproduction. The individual seed weight, seed germination, and seed production were all detrimentally affected by increasing maternal Se levels, with significant effects happening above the 40 μM or 60 μM Na2SeO4 treatment, which corresponded to 500–1000 mg Se kg−1 DW tissue Se concentrations. The maternal plant contributes more resources to seed production than the paternal plant, and thus it is to be expected that increasing Se concentration in the maternal plant would have a stronger effect on seed production compared with the paternal Se. One exception observed here is that the paternal Se concentration appeared additionally to affect the percentage of seed germination within the same maternal Se treatment.
The negative effects of Se on reproduction in B. juncea started around tissue concentrations of 1000 mg Se kg−1 DW; that is, the same as the defining Se concentration differentiating between secondary Se accumulators and Se hyperaccumulators. This appears to be an appropriate upper limit for defining secondary Se accumulators, since higher levels would probably not be sustainable in nature due to significant toxicity. Incidentally, common levels of Se in secondary Se accumulators used for phytoremediation in the field do not reach Se concentrations close to 1000 mg Se kg−1 DW and instead are more commonly ∼50 mg Se kg−1 DW Se (Bañuelos et al., 2005, Bañuelos 2006). Thus, negative effects of Se on reproduction and growth are not anticipated in current field settings.
Se levels in the hyperaccumulator S. pinnata are particularly high in pollen and ovules (Quinn et al., 2011), perhaps as a form of elemental defence. Yet, the plants appear to have evolved physiological mechanisms that enable them to prevent the toxic effects of floral Se on reproductive functions that were observed in B. juncea. Earlier research had shown that hyperaccumulators store more Se in organic form, particularly MeSeCys, which does not get incorporated into protein and therefore is relatively non-toxic (Neuhierl and Böck, 1996). This form was indeed found in both leaves and flowers of S. pinnata and may explain their extreme Se tolerance (Freeman et al., 2006a; Quinn et al., 2011).
In the past it has been shown that low levels of Se in both accumulator and hyperaccumulator species can act as a plant defence against a wide variety of herbivores as well as some fungi. Freeman et al. (2007) fed grasshoppers and crickets (Orthoptera) to S. pinnata plants with Se concentrations as low as 145 mg Se kg−1 DW and saw effective defence against these herbivores. Se was even more effective against aphids, where 10 mg Se kg−1 DW already protected B. juncea plants from phloem-feeding Myzus persicae (Hanson et al., 2004). Caterpillar (Pieris rapae) herbivory was deterred by B. juncea containing 600 mg Se kg−1 DW (Hanson et al., 2003). In field plants, an Se range of 50–750 mg Se kg−1 Se was sufficient in S. pinnata for deterring prairie dogs (Cynomys ludovicianus) (Freeman et al., 2009). As is to be expected for a plant defence, over time some herbivores appear to have evolved Se tolerance, and these can feed freely on hyperaccumulators. Freeman et al. (2006b) describe diamondback moth larvae (Plutella xylostella) that fed without ill effects on S. pinnata containing 2000 mg Se kg−1 DW. Together, these studies paint an interesting picture of the evolution of Se accumulation. Plants probably began to accumulate low levels of Se non-specifically through the S assimilation pathway, and even those low levels of Se aided in defence against herbivory. Continuous selection pressure in the form of herbivory may have favoured higher and higher plant Se accumulation, to the point where plants experienced toxicity. It appears that for secondary Se accumulators this level is between 500 mg Se kg−1 DW and 1,000 mg Se kg−1 DW, when physiological processes such as pollen germination and seed production become affected by the Se, resulting in an evolutionary disadvantage for further Se accumulation. In seleniferous habitats, some herbivores evolve tolerance to Se, leading to an increase in herbivory and a need for the plant to evolve even higher Se levels. Here, additional Se tolerance mechanisms and true hyperaccumulation may arise, leading to plants that experience no ill effect of Se on reproductive processes, and even more protection from herbivores. Finally, even at true hyperaccumulator levels, some herbivores will eventually disarm Se as an elemental defence. Along with the evolution of hyperaccumulation, insects with positive interactions with hyperaccumulators, such as native pollinators, may also have evolved tolerance to Se so that they can forage on hyperaccumulator plants without suffering toxicity.
In conclusion, the study reported here provides novel information regarding the effects of Se on reproductive parameters of Se hyperaccumulators and secondary accumulators, and is one of the first of its kind to be carried out for hyperaccumulators of any element. The results provide further insight into selective pressures in the evolution of Se hyperaccumulation and hypertolerance. In addition to increasing evolutionary insight, these findings may have applications for the cultivation of accumulators and hyperaccumulators in agriculture or for phytoremediation.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Brassica juncea cross-pollination parameters.
Acknowledgements
The authors thank Patricia Bedinger and Paul Covey for help with pollinator germination studies. Funding for these studies was provided by the National Science Foundation grant # IOS-0817748 to EAHPS.
Supplementary Material
References
- Anderson JW. Selenium interactions in sulfur metabolism. In: De Kok LJ, editor. Sulfur nutrition and assimilation in higher plants Regulatory, agricultural and environmental aspects. The Hague: SPB Academic Publishing; 1993. pp. 49–60. [Google Scholar]
- Arvy MP. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris) Journal of Experimental Botany. 1993;44:1083–1087. [Google Scholar]
- Bañuelos GS. Phyto-products may be essential for sustainability and implementation of phytoremedtion. Environmental Pollution. 2006;144:19–23. doi: 10.1016/j.envpol.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Bañuelos GS, LeDuc DL, Pilon-Smits EAH, Terry N. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environmental Science and Technology. 2007;41:599–605. doi: 10.1021/es061152i. [DOI] [PubMed] [Google Scholar]
- Bañuelos G, Lin ZQ. Cultivation of the Indian fig opuntia in selenium-rich drainage sediments under field conditions. Soil Use and Management. 2010;26:167–175. [Google Scholar]
- Bañuelos GS, Terry N, LeDuc DL, Pilon-Smits EAH, Mackey B. Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environmental Science and Technology. 2005;39:1771–1777. doi: 10.1021/es049035f. [DOI] [PubMed] [Google Scholar]
- Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health and Nutrition. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- Carlson AL, Telligman M, Swanson RJ. Incidence and post-pollination mechanisms of nonrandom mating in. Arabidopsis thaliana. Sexual Plant Reproduction. 2009;22:257–262. doi: 10.1007/s00497-009-0108-1. [DOI] [PubMed] [Google Scholar]
- Dhillon KS, Dhillon SK. Selenium concentrations of common weeds and agricultural crops grown in the seleniferous soils of northwestern India. Science of the Total Environment. 2009;407:6150–6156. doi: 10.1016/j.scitotenv.2009.08.051. [DOI] [PubMed] [Google Scholar]
- Dhillon KS, Dhillon SK. Accumulation and distribution of selenium in some vegetable crops grown in selenate-Se treated clay loam soil. Frontiers of Agriculture in China. 2009;3:366–373. [Google Scholar]
- Fassel VA. Quantitative elemental analyses by plasma emission spectroscopy. Science. 1978;202:183–191. doi: 10.1126/science.202.4364.183. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Lindblom SD, Quinn CF, Fakra S, Marcus MA, Pilon-Smits EAH. Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytologist. 2007;175:490–500. doi: 10.1111/j.1469-8137.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Quinn CF, Lindblom SD, Klamper EM, Pilon-Smits EAH. Selenium protects the hyperaccumulator Stanleya pinnata against Black-tailed prairie dog herbivory in native seleniferous habitats. American Journal of Botany. 2009;96:1075–1085. doi: 10.3732/ajb.0800287. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Quinn CF, Marcus MA, Fakra S, Pilon-Smits EAH. Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense. Current Biology. 2006b;16:2181–2192. doi: 10.1016/j.cub.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Tamaoki M, Stushnoff C, et al. Molecular mechanisms of selenium tolerance and hyperaccumulation in. Stanleya pinnata. Plant Physiology. 2010;153:1630–1652. doi: 10.1104/pp.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Zang LH, Marcus MA, Fakra S, Pilon-Smits EAH. Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and. Stanleya pinnata. Plant Physiology. 2006a;142:124–134. doi: 10.1104/pp.106.081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related non-accumulators. New Phytologist. 2007;173:517–525. doi: 10.1111/j.1469-8137.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Gissel-Nielsen G. Effects of selenium supplementation of field crops. In: Frankenberger WT, Engberg RA, editors. Environmental chemistry of selenium. New York: Marcel Dekker Inc.; 1998. pp. 99–112. [Google Scholar]
- Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity. Regulatory Toxicology and Pharmacology. 2003;38:232–242. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Galera S, Rojas E, Sudhaker D, Zhu C, Pelacho AM, Capell T, Christou P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Research. 2010;19:165–180. doi: 10.1007/s11248-009-9311-y. [DOI] [PubMed] [Google Scholar]
- Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, Kramer K, Norton AP, Lawrence CB, Pilon-Smits EAH. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytologist. 2003;159:461–469. doi: 10.1046/j.1469-8137.2003.00786.x. [DOI] [PubMed] [Google Scholar]
- Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytologist. 2004;162:655–662. doi: 10.1111/j.1469-8137.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- Hartikainen H. Biogeochemistry of selenium and its impact on food chain quality and human health. Journal of Trace Elements in Medicine and Biology. 2005;18:309–318. doi: 10.1016/j.jtemb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Neuhierl B, Böck A. On the mechanism of selenium tolerance in selenium-accumulating plants. Purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisculatus. European Journal of Biochemistry. 2004;239:235–238. doi: 10.1111/j.1432-1033.1996.0235u.x. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N. Overexpression of ATP sulfurylase in indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiology. 1999;119:123–132. doi: 10.1104/pp.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Quinn CF, Tapken W, Malagoli M, Schiavon M. Physiological functions of beneficial elements. Current Opinion Plant Biology. 2009;12:267–274. doi: 10.1016/j.pbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Quinn CF, Freeman JL, Reynolds RJB, Cappa JJ, Fakra SC, Marcus MA, Lindblom SD, Quinn EK, Bennett LE, Pilon-Smits EAH. Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecology. 2010;10:1–11. doi: 10.1186/1472-6785-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CF, Galeas ML, Freeman JL, Pilon-Smits EAH. Selenium: dterrence, toxicity and adaptation. Integrated Environmental Assessment and Management. 2007:3,460–462. doi: 10.1002/ieam.5630030317. [DOI] [PubMed] [Google Scholar]
- Quinn CF, Prins CN, Gross AM, et al. Selenium accumulation in flowers and its effects on pollination. New Phytologist. 2011 doi: 10.1111/j.1469-8137.2011.03832.x. (in press) [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of Selenium to human health. The Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM. Metal accumulating plants. In: Raskin I, Ensley BD, editors. Phytoremediation of toxic metals: using plants to clean up the environment. New York: John Wiley; 2000. pp. 191–230. [Google Scholar]
- Searcy KB, Macnair MR. Differential seed production in Mimulus guttatus in response to increasing concentrations of copper in the pistil by pollen from copper tolerant and sensitive sources. Evolution. 1990;44:1424–1435. doi: 10.1111/j.1558-5646.1990.tb03836.x. [DOI] [PubMed] [Google Scholar]
- Seppänen MM, Kontturi J, Heras IL, Madrid Y, Cámara C, Hartikainen H. Agronomic biofortification of Brassica with selenium—enrichment of SeMet and its identification in Brassica seeds and meal. Plant and Soil. 2010;337:273–283. [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. Selenium in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- Ward SM, Fleischmann CE, Turner MF, Sing SE. Hybridization between invasive populations of Dalmatian toadflax (Linaria dalmatica) and yellow toadflax (Linaria vulgaris) Invasive Plant Science and Management. 2009;2:369–378. [Google Scholar]
- White PJ, Bowen HC, Parmaguru P, et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. Journal of Experimental Botany. 2004;55:1927–1937. doi: 10.1093/jxb/erh192. [DOI] [PubMed] [Google Scholar]
- Xiong ZT. Lead uptake and effects on seed germination and plant growth in a Pb hyperaccumulators Brassica pekinensis Rupr. Bulletin of Environmental Contamination and Toxicology. 1998;60:285–291. doi: 10.1007/s001289900623. [DOI] [PubMed] [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis. 1987;18:131–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.