Abstract
The contamination of food crops by cadmium (Cd) is a major concern in food production because it can reduce crop yields and threaten human health. In this study, knockout rice plants (Oryza sativa) tagged with the gene trap vector pGA2707 were screened for Cd tolerance, and the tolerant line lcd was obtained. The lcd mutant showed tolerance to Cd on agar plates and in hydroponic culture during early plant development. Metal concentration measurements in hydroponically grown plants revealed significantly less Cd in the shoots of lcd plants compared with wild-type (WT) shoots. When cultured in the field in soil artificially contaminated with low levels of Cd, lcd showed no significant difference in the Cd content of its leaf blades; however, the Cd concentration in the grains was 55% lower in 2009 and 43% lower in 2010. There were no significant differences in plant dry weight or seed yield between lcd and wild-type plants. LCD, a novel gene, is not homologous to any other known gene. LCD localized to the cytoplasm and nucleus, and was expressed mainly in the vascular tissues in the roots and phloem companion cells in the leaves. These data indicate that lcd may be useful for understanding Cd transport mechanisms and is a promising candidate rice line for use in combating the threat of Cd to human health.
Keywords: Accumulation, cadmium, rice, tolerance
Introduction
Cadmium (Cd) is a heavy metal that has been widely released into the environment. Once released into the environment, Cd enters the biogeochemical cycle and tends to accumulate in soils and sediments, where it becomes available to rooted plants. Cd uptake is harmful to most plants; it can reduce plant growth, and even kill plants in extreme cases.
Critical problems related to environmental exposure to Cd were reported in Japan in the early 20th century; people consuming contaminated food and water developed ‘itai-itai disease’, which caused renal abnormalities and weak bones (Horiguchi et al., 2010). To suppress Cd pollution, most industrialized nations have set strict limits on Cd exposure, and in 2004 the Joint FAO/WHO Expert Committee on Food Additives, in association with the Codex Alimentarius Commission, established allowable limits for Cd levels in food; for rice, the limit was set at 0.4 mg kg−1. The mechanisms that control Cd accumulation in the edible parts of plants are poorly understood. For the general population, the major route of Cd exposure is through food (Nordic Council of Ministers, 2003). Rice grains contaminated with Cd represent a major risk to the health of more than half of the world’s population, which depends on rice as a basic staple.
Several mechanisms related to Cd tolerance in plants have been identified, one of which involves complexing with metal-chelating peptides, such as metallothionein (MT), glutathione (GSH), and phytochelatins (PCs) (Grill et al., 1995; Howden et al., 1995; Cobbett et al., 1998). In addition, transporter proteins play major roles in Cd tolerance and accumulation in many organisms. In Arabidopsis thaliana, the P-type ATPases AtHMA2/AtHMA4 and the ABC transporter AtPDR8, from the pleiotropic drug resistance (PDR) family, which was previously reported to be involved in pathogen resistance, are involved in Cd detoxification by mediating Cd efflux from cells (Das et al., 1997; Li et al., 1997; Eren et al., 2004; Kobae et al., 2006; Stein et al., 2006). In rice, the ZIP family transporters OsIRT1 and OsIRT2, which are iron (Fe) transporters, and OsZIP1, which is a zinc (Zn) transporter, have been reported to transport Cd (Korshunova et al., 1999; Nakanishi et al., 2006). Positron-emitting tracer imaging system (PETIS) experiments have shown the importance of the phloem system in Cd distribution in rice and transport to the grains (Fujimaki et al., 2010).
With the goal of improving food safety, the current study aimed to identify genes related to Cd tolerance and accumulation in rice through the screening of a library of T-DNA-tagged rice lines. In this study, the discovery of a Cd-tolerant knockout mutant that accumulates lower concentrations of Cd in its grains without significant differences in plant biomass and grain yield is reported. In this mutant, the T-DNA was inserted into the first intron of OsLCD; the gene’s possible roles in the mutant are discussed. A non-transgenic mutant in which OsLCD was knocked out was also found; this showed reduced Cd concentrations in its grains. This mutant is a promising candidate for the mitigation of the Cd threat to human health.
Materials and methods
Plant materials
Oryza sativa var. japonica cv. Hwayoung was used for Cd tolerance analyses, metal concentration measurements, and microarray analyses of the lcd mutant. To screen for Cd tolerance, seed stocks of mutant lines in which pGA2707 T-DNA was inserted were germinated and grown for 10–15 d in a growth chamber (16 h of light/8 h of dark at 28 °C) on Murashige and Skoog (MS) medium containing 50 mg l−1 hygromycin and 1.0 mM CdSO4. In the Cd sensitivity analyses, mutant and wild-type (WT) seeds were germinated on MS medium with or without hygromycin, respectively, for 3 d in a growth chamber (16 h of light/8 h of dark at 28 °C) then transferred to MS medium containing 1.0 mM CdSO4 without hygromycin and grown for another 10 d under the same conditions.
For the metal concentration measurements and microarray analyses, mutant and WT seeds were germinated and grown for 7 d on MS medium with or without hygromycin, respectively, then transferred to 20.0 l plastic containers containing a nutrient solution composed of 2 mM Ca(NO3)2, 0.5 mM MgSO4, 0.7 mM K2SO4, 0.1 mM KCl, 0.1 mM KH2PO4, 0.1 mM Fe(III)-EDTA, 10 μM H3BO3, 0.5 μM MnSO4, 0.5 μM ZnSO4, 0.2 μM CuSO4, and 0.01 μM (NH4)6Mo7O25. The culture solution was adjusted to pH 5.0–5.5 with 1 N KOH. After 7 d, the plants were transferred to containers containing the same nutrient solution with or without the addition of 10 μM CdSO4 and grown for another 14 d.
Inverse PCR (iPCR) (Hui et al., 1998; Spertini et al., 1999) was performed to isolate the T-DNA flanking sequences. A 1 μg aliquot of genomic DNA was digested with 10 U of PstI in 50 μl for 10 h. Following heat inactivation of the enzyme, the samples were precipitated with ethanol and dissolved in 50 μl of ligation buffer, then ligated at 8 °C for 12 h using 1 U of T4 DNA ligase (Boehringer Mannheim/Roche, Basel, Switzerland). Nested PCR was performed to amplify the right and left flanking sequences of the T-DNA. For the first PCR, ∼2% of the ligated DNA was incubated with 5 μM primer (GUS2R, TTGGGGTTTCTACAGGACGTAAC, and HPH4R, CCATGTAGTGTATTGACCGATTC for the right border; HPH1F, GATCGTTATGT-TTATCGGCACTT, and Ptub3R, GGTGAATGGCATCGTTTGAA for the left border) in a 25 μl solution containing dNTPs and 0.1 U of ExTaq polymerase (Takara, Shiga, Japan). PCR was performed with an initial 5 min denaturation at 94 °C, followed by 35 cycles of 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 4 min, followed by a final 10 min at 72 °C. A 0.1 μl aliquot of the product was then used as template for the second PCR with the primers GUS1R, CAAGTTAGTCATGTAATTAGCCAC, and HPH3R, TCGTCTGGCTAAGATCGGCCGCA for the right border; and HPH2F, AGTGCTTGACATTGGGGAATTCAG, and Ptub2R, ACAAGCCGTAAGTGCAAGTG for the left border under the same conditions. The products were separated by 1.0% (w/v) agarose gel electrophoresis, eluted with a Geneclean III Kit (Q-Biogene, Carlsbad, CA, USA), and sequenced using a 3130 Genetic Analyzer (Applied Biosystems).
RT-PCR
Total RNA was isolated from lcd, lcd-tos17, and their respective WT plants grown under normal conditions. Total RNA was treated with RNase-free DNase I (Takara Bio, Otsu, Japan) to remove contaminating genomic DNA. First-strand cDNA was synthesized from 2 μg of RNA using SuperScript II reverse transcriptase (Toyobo) by priming with oligo-d(T)30, the final volume was adjusted to 100 μl, and 2 μl from each sample was used for subsequent reactions. Fragments were amplified by PCR. The LCD primers used for RT-PCR were as follows: LCD forward 5′-CTATGATTTCATCGGATCTACCGACTG-3′ and LCD reverse 5′-CTAAGAACCAAAAACTCCTAACAGGAG-3′. For LCD RT-PCR, the annealing temperature was set to 65 °C (20 s). The α-tubulin primers used for RT-PCR were as follows: α-tubulin forward 5′-TCTTCCACCCTGAGCAGCTC3′ and α-tubulin reverse 5′-AACCTTGGAGACCAGTGCAG-3′. For α-tubulin RT-PCR, the annealing temperature was set to 55 °C (30 s).
Metal concentration measurements
Roots and shoots were harvested and dried overnight at 80 °C. Dried samples (50–100 mg of roots; 100–200 mg of shoots) were digested in 3 ml of 13 M HNO3 at 220 °C for 20 min with MARS Xpress (CEM, Matthews, NC, USA). After digestion, the samples were diluted to a volume of 5 ml and analysed by inductively coupled plasma atomic emission spectrometry (model SPS1200VR; Seiko, Tokyo, Japan) at the following wavelengths: 226.502 (Cd), 238.204 (Fe), 213.856 (Zn), 293.930 (manganese, Mn), and 324.754 (copper, Cu) nm.
Subcellular localization of LCD
The full-length open reading frame (ORF) of LCD (951 bp) was amplifiend with forward and reverse primers as follows: 5′- CACCATGGTGGAAACCACAGTTG-3′ and 5′- TTTTTTACAAGGCCACTTCCAGA-3′. The full-length LCD ORF was cloned into pENTR/TOPO (Invitrogen) and then subcloned into pH7WGF2 (Karimi et al., 2002) under control of the Cauliflower mosaic virus (CaMV) 35S promoter using the LR recombination reaction (Invitrogen) (Ishimaru et al., 2009). Onion epidermal cells were transformed using a Biolistic PDS-1000/He particle delivery system (Bio-Rad).
Histochemical analysis
The 1.5 kb 5′ upstream region of the LCD gene was amplified using KOD plus polymerase at an annealing temperature of 65 °C by the forward primer 5′- GGGGGGAAGCTTCGGACTATAATATGTTGTGTGTA-3′ and the reverse primer 5′-GGGGGGTCTAGATTATAAGTTCCATTAAACAAGTT-3′ which contain HindIII and XbaI restriction sites. The amplified fragment was fused into the pBluescriptIISK+ vector, and its sequence was confirmed. The LCD promoter was digested with XbaI and HindIII, and the digested 1.5 kb fragment was subcloned upstream of the uidA ORF, which encodes β-glucuronidase (GUS), in the pIG121Hm vector. Oryza sativa L. cv. Tsukinohikari was transformed (Ishimaru et al., 2006). Rice plants were grown in culture solution in a greenhouse for 3 weeks after germination on MS medium, and the histochemical localization was observed in four different independent T2 plants. Roots and stems of transgenic plants were cut with a scalpel into ∼1 cm sections. The sections were embedded in 4% agar and then cut in sections of 80–130 μm using a DTK-100 vibrating microtome (Dosaka EM Co. Ltd, Kyoto, Japan). The sections were incubated at 37 °C for 15 min in GUS reaction buffer {1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide, 3 mM K4[Fe(CN)6], 0.5 mM K3[Fe(CN)6], 50 mM sodium phosphate (pH 7.0), and 20% (v/v) methanol}. Following staining, the sections were washed in 70% ethanol until observation. GUS staining was observed using an Axiophoto microscope (Carl Zeiss), following the manufacturer’s instructions.
Oligo-DNA microarray analysis
A rice 44k oligo DNA microarray (Agilent Technology, Tokyo, Japan) containing 45 151 60-mer oligonucleotides based on the sequence data of the rice full-length cDNA project (http://cdna01.dna.affrc.go.jp/cDNA/) was used. Total RNA was extracted from shoots and roots using an RNeasy Plant Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The yield and RNA purity were determined spectrophotometrically. The integrity of the RNA was checked using an Agilent 2100 Bioanalyzer. Total RNA (200 ng) was labelled with Cy-3 or Cy-5 using an Agilent Low RNA Input Fluorescent Linear Amplification Kit. Fluorescently labelled targets were hybridized to Agilent rice 44K oligo DNA microarrays. Microarray hybridization, scanning, and data analysis were performed following the manufacturer’s instructions. The reproducibility of the analyses was assessed by a dye swap in each experiment.
Results
Isolation of a Cd-tolerant mutant from a library of T-DNA-tagged lines
WT plants (cv. Hwayoung) were sown on MS medium at different concentrations of CdSO4 to find the optimum Cd2+ concentration in the medium for the selection of Cd-tolerant mutants. Addition of CdSO4 caused some complications in the MS culture medium. First, high concentrations of salts bind to agar, preventing it from hardening. To solve this issue, the concentration of agar in the medium was raised from 0.8% to 1.2% to make the MS medium hard enough for proper plant culture. Secondly, usually addition of salts to the culture displaces the Fe from Fe-EDTA, and then addition of high concentrations of CdSO4 would displace almost all the Fe from Fe-EDTA; however, this did not seem to affect the selection efficiency of the screening. A concentration of 1.0 mM CdSO4 was found to slow shoot and root growth development visibly in the WT plants and was consequently used to select for Cd-tolerant mutants (Supplementary Fig. S1 available at JXB online).
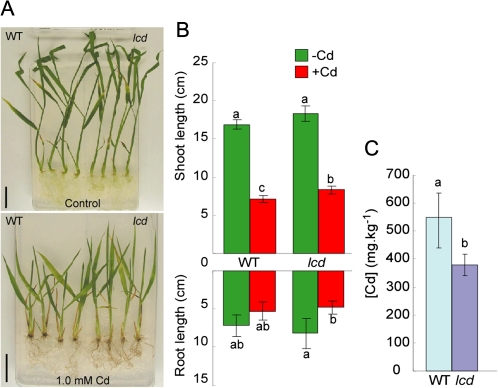
A pool of rice T-DNA mutants in which the plasmid pGA2707 (Spertini et al., 1999) was randomly inserted into the rice genome, resulting in knockout mutants, was screened for mutants exhibiting Cd tolerance. A Cd-tolerant mutant, named lcd (low Cd), was selected from 3993 independent T-DNA mutant lines among ∼9500 seeds. The lcd mutant grew better than WT plants on MS agar plates containing 1.0 mM CdSO4 and had longer shoots (Fig. 1A, B). The Cd concentration in the shoots of the mutant was ∼30% less than in the shoots of the WT plants (Fig. 1C).
Fig. 1.
Selection of the Cd-tolerant mutant line lcd. (A) WT and lcd mutants were grown in MS medium with or without 1.0 mM Cd for 10 d. (B) Root and shoot length of the WT and lcd in both conditions. (C) Cd concentration in shoots of the WT and lcd from plants grown in MS medium in both conditions. Bars=2 cm. Values followed by different letters are statistically different according to Student–Newman–Keuls test (n=4).
The T-DNA in lcd was inserted into the first intron of OsLCD, leading to knockout
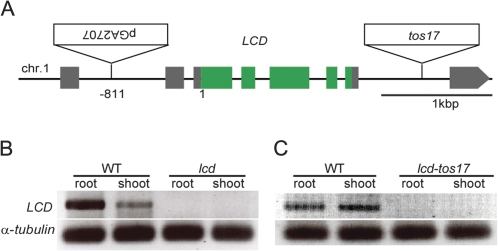
The T-DNA insertion in the lcd mutant was located using iPCR. A sequence analysis of the T-DNA-flanking region in lcd revealed that the T-DNA was inserted 811 bp upstream of the initiation codon in OsLCD (O. sativa LCD) on chromosome 1 (Fig. 2A). The gene was identified as locus Os01g0956700, according to the Rice Annotation Project (RAP; http://rapdb.dna.affrc.go.jp) of the International Rice Genome Sequencing Project (IRGSP; http://rapdb.dna.affrc.go.jp/E/IRGSP/index.html). T-DNA insertion usually leads to gene disruption. Therefore, RT-PCR was performed to confirm that OsLCD was knocked out in the mutants. OsLCD expression was observed in both the roots and shoots of WT (Hwayoung) plants, but not in lcd, confirming the knockout of OsLCD (Fig. 2B).
Fig. 2.
Insertion positions and LCD expression in the mutants. (A) Schematic representation of LCD and the insertion positions of the T-DNA and the tos17 fragment. (B and C) Total RNA was extracted from roots and shoots for lcd/lcd-tos17 and their respective WTs, and RT-PCR was performed.
Tos17 lines are mutants generated through retrotransposon insertion (Hirochika et al., 1996) available to order for research purposes (http://tos.nias.affrc.go.jp/). A tos17 mutant (cv. Nipponbare) with the retrotransposon fragment inserted into OsLCD was found. RT-PCR performed on both WT (Nipponbare) and lcd-tos17 mutant plants confirmed that OsLCD was expressed in the WT plants but was knocked out in the lcd-tos17 mutant plants (Fig. 2C).
Reduced Cd accumulation in the shoots of the lcd mutant
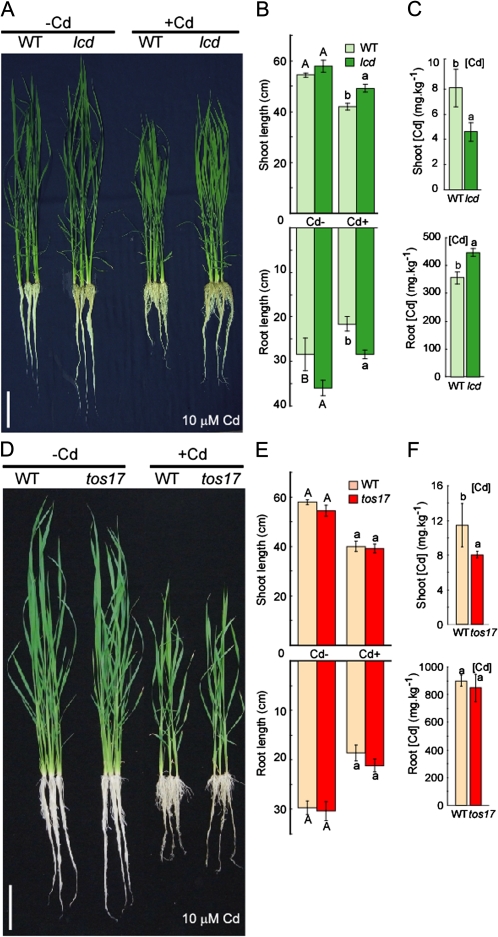
The selected mutants, lcd and lcd-tos17, and their WT versions, Hwayoung and Nipponbare, respectively, were germinated on MS plates then transferred to nutrient solution with or without 10 μM CdSO4. Under control conditions (no Cd), the lcd mutant had longer roots than did the WT (Hwayoung), but no visible differences were observed between the lcd-tos17 mutant and Nipponbare (Fig. 3A, B, D, E). When grown in the presence of Cd, the lcd mutant had longer roots and shoots compared with the WT (Hwayoung); this phenotype was not observed, however, in the lcd-tos17 mutant.
Fig. 3.
lcd and lcd-tos17 phenotype in response to Cd. (A and D) WT and lcd/lcd-tos17 were grown in nutrient solution for 1 week then grown in nutrient solution with or without 10 μM Cd for another week. (B and E) Root and shoot lengths of WT and lcd/lcd-tos17 in both conditions (n=3). (C and F) Cd concentration of roots and shoots (n=3). Bars=10 cm. Values followed by different letters are statistically different according to Student–Newman–Keuls.
Metal concentrations were measured in the roots and shoots of lcd, lcd-tos17, and WT plants after growth in the presence of Cd. Less Cd accumulated in the shoots of lcd and lcd-tos17 compared with their respective WT versions (Fig. 3C, F). More Cd accumulated in the roots of lcd than in the roots of Hwayoung; however, no significant difference in Cd accumulation was observed in the roots of lcd-tos17 compared with its WT (Fig. 3D, F). The concentrations of other metals, including Zn, Mn, Fe, and Cu, were also measured. In lcd shoots, decreased metal concentrations were observed in the Zn, Mn, and Fe concentrations, while in lcd roots, significant differences were observed only for increased Zn and decreased Fe. In the case of lcd-tos17, increased metal concentrations were found for the shoot Zn and Mn, while no differences were observed for root metal concentrations (Supplementary Fig. S2 at JXB online).
Subcellular localization of OsLCD
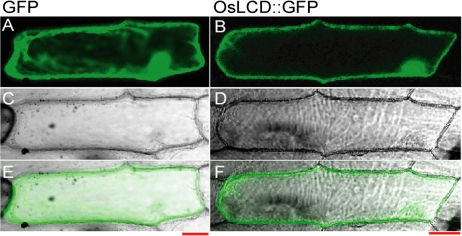
A fusion protein construct [OsLCD::green fluorescent protein (GFP)] was transiently expressed in onion (Allium cepa) bulb epidermal cells. OsLCD::GFP fluorescence was detected in the cytoplasm and nucleus (Fig. 4).
Fig. 4.
Subcellular localization of OsLCD. The OsLCD::GFP fusion protein was transiently expressed in onion (Allium cepa) epidermal cells and fluorescence was analysed. (A and B) GFP fluorescence. (C and D) Transient imaging. (E and F) Merged image. Bars=50 μm.
Tissue expression of OsLCD
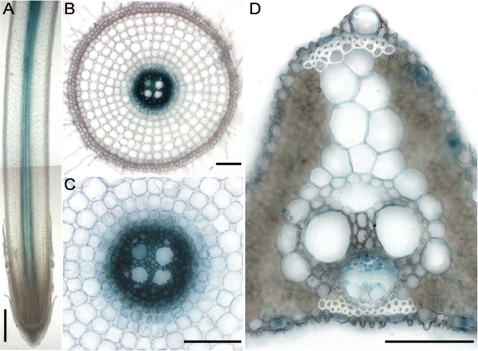
RT-PCR showed that OsLCD was expressed in both the roots and shoots of WT plants (Fig. 2B, C). To determine more accurately where OsLCD was expressed, GUS expression under control of the OsLCD promoter was examined in roots and shoots. In roots, GUS activity was localized to the central region (Fig. 5A, longitudinal section), which represents the root vascular bundle (Fig. 5B and C, transverse section). In leaves, GUS activity was observed mainly in the phloem companion cells (Fig. 5D). These data suggest that OsLCD affects Cd transport within plants as part of the plant transport system.
Fig. 5.
Tissue expression analysis of OsLCD. OsLCDp–GUS plants were germinated and cultured in nutrient solution for 2 weeks and analysed for GUS staining. (A) Longitudinal section of a root. (B) Cross-section. (C) Amplified view of the cross-section. (D) Leaf cross-section. Bars=500 μm in A, 100 μm in B and C, and 50 μm in D.
Field-grown lcd grains accumulated less Cd
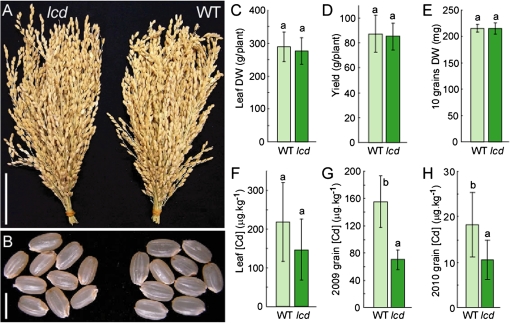
To determine the effects of knocking out OsLCD on field-grown plants, lcd (T-DNA) and Hwayoung (WT) plants were grown in a restricted experimental field where the soil was artificially contaminated (Cd-salt amendment for experiment purposes) with Cd (average of 3.6 ppm Cd) under continuous flooded conditions until harvest in 2009. The lcd mutant plants showed normal growth with no significant differences in panicle formation or seed appearance compared with the WT (Fig. 6A, B). There was also no difference in plant biomass, represented by leaf dry weight, grain yield, or grain weight (Fig. 6C–E). There was no significant difference in the Cd concentration in lcd mutant and WT leaves (Fig. 6F); however, the grains of the lcd plants accumulated 55% less Cd compared with the WT without significant changes in the Fe and Zn concentrations (Fig. 6G; Supplementary Fig. S3 at JXB online). To confirm these results, the field experiment was repeated in 2010 (average of 0.89 ppm Cd) with a greater number of plants. Again, the lcd mutant accumulated 43% less Cd in its grains than did the WT (Fig. 6H).
Fig. 6.
Field experiment. Photo of panicles (A) and grains (B). (C) Leaf dry weight (DW). (D) Grain yield per plant. (E) DW of 10 grains. (F) Leaf Cd concentration. (G) Grain Cd concentration in lcd and the WT from the field experiment in 2009 (n=6). (H) Grain Cd concentration in lcd and the WT from the field experiment in 2010 (n=25). Bars=5 cm in A and 5 mm in B. Values followed by different letters are statistically different according to Student–Newman–Keuls test.
Transcriptome analysis of the lcd mutant
To examine the mechanisms of Cd tolerance and accumulation in lcd and lcd-tos17, genome-wide mRNA levels were analysed in the mutants and their respective WT versions using a 44k oligonucleotide DNA chip. In lcd, 475 genes in both the roots and shoots showed a >2-fold change in expression relative to WT plants under normal growth conditions, while in the lcd-tos17 mutants, 42 genes showed a similarly high expression level. In the lcd plants, 325 genes, including LCD itself, showed a >50% decrease in expression relative to WT plants in both the roots and shoots under normal growth conditions, while in the lcd-tos17 mutants, only 70 showed such a decrease in expression.
Supplementary Table S1 at JXB online lists the genes that were down-regulated (ratio <0.5) in the roots and shoots of the lcd and lcd-tos17 mutants compared with their respective WT plants. Among these 24 genes, a dehydrin gene belonging to a group of genes previously reported to be related to abiotic stress (Nylander et al., 2000) showed very strong down-regulation, particularly in the roots. Supplementary Table S2 also lists the genes that were up-regulated (ratio >2) in both the lcd and lcd-tos17 mutants compared with their respective WT plants. Among the up-regulated genes, an ABC transporter (multidrug resistance protein 1 homologue; Os01g0533900) showed increased expression in both roots and shoots.
A previous report proposed that the transporter genes PDR, MDR, MRP, MATE, CDF, OsHMA5, and OsIRT were involved in the early response to Cd stress (3 h exposure at 1 μM Cd2+) (Ogawa et al., 2009). Table 1 shows the expression ratios in lcd and lcd-tos17 of the transporter genes proposed to be involved in the Cd stress response by Ogawa et al. (2009). WT plants exposed to 10 μM Cd2+ for 2 weeks showed enhanced expression levels of OsPDR9, OsPDR17, OsPDR16, MDR, MRP, and MATE efflux family proteins in response to Cd in the roots, in agreement with Ogawa et al. (2009). However, OsPDR1 showed reduced expression in the roots and enhanced expression in the shoots. Microarray analysis of these genes in lcd and lcd-tos17 revealed that none of these genes had significantly different expression levels relative to the WT.
Table 1.
Transporter genes possibly involved in Cd stress response
| Locus | Description | Signal (WT; HW) |
lcd/WT |
tos/WT |
Cd+/Cd– (WT; HW) |
||||
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | ||
| Os01g0609300 | OsPDR9 | 27 | 200 | 1.0 | 0.8 | 1.5 | 1.3 | 20.3 | 1.3 |
| Os08g0544400 | OaPDR1 | 261 | 20 | 1.0 | 1.0 | 0.8 | 0.7 | 0.1 | 4.5 |
| Os08g0384500 | OsPDR17 | 82 | 65 | 1.2 | 0.8 | 1.3 | 1.2 | 5.0 | 1.2 |
| Os01g0609900 | OsPDR8 | 8361 | 469 | 1.0 | 0.9 | 1.1 | 1.1 | 1.9 | 0.9 |
| Os01g0342700 | OsPDR16 | 45 | 32 | 1.1 | 0.6 | 1.0 | 1.5 | 2.1 | 1.4 |
| Os03g0667500 | OsIRT1 | 22 169 | 891 | 0.9 | 1.0 | 0.6 | 0.8 | 0.8 | 1.3 |
| Os07g0232900 | OsHMA3 | 10 083 | 949 | 1.1 | 1.0 | 0.8 | 0.8 | 0.4 | 1.3 |
| Os01g0695800 | MDR | 108 | 272 | 1.1 | 1.1 | 1.0 | 1.0 | 16.7 | 1.6 |
| Os04g0209200 | MRP | 186 | 156 | 1.3 | 1.3 | 1.1 | 1.0 | 2.5 | 0.7 |
| Os03g0571900 | MatE (PEZ1) | 252 | 702 | 0.9 | 0.9 | 1.2 | 0.9 | 4.1 | 1.2 |
Signal, signal intensity in WT for roots and shoots; lcd/WT, ratio of lcd in relation to WT (cv. Hwayoung) in roots and shoots; tos/WT, ratio of lcd-tos17 in relation to WT in roots and shoots; Cd+/Cd–, ratio between 10 μM Cd2+ treatment and control in the WT (cv. Hwayoung).
Discussion
Through screening of T-DNA insertion mutants, a Cd-tolerant mutant, lcd, was identified which accumulated less Cd in its shoots compared with WT plants. The T-DNA in lcd was inserted in the first intron of OsLCD (Os01g0956700). Currently, the function of OsLCD is unknown; thus, no information about putative domains and motifs could be found.
The lcd mutant, when grown in hydroponic culture, accumulated more Cd in its roots than did the WT, but accumulated less Cd in the shoots, suggesting that OsLCD is involved in Cd partitioning in rice. On the other hand, the lcd-tos17 mutant showed some phenotype discrepancies in root Cd accumulation and accumulation profiling of other metals compared with lcd, but a similar decrease in Cd accumulation in shoots, which might be the result of the differences in expression of genes related to metal homeostasis in the genetic backgrounds of each mutant (Supplementary Table S3 at JXB online). The Cd partioning hypothesis is supported by the OsLCDp–GUS analysis, which indicated that OsLCD was expressed in tissues related to transport, for example root vascular bundles and leaf phloem companion cells. A previous report (Fujimaki et al.. 2010) discussed the importance of phloem in Cd distribution within plants and subsequent transport to the grains. The present data support this previous report by showing that the knockout of a gene, the normal expression of which was localized to phloem companion cells, resulted in altered Cd distribution in the plant body and reduced the grain Cd concentration. Tian et al. (2010) reported that the knockout of a gene encoding a phloem companion cell-specific protein that is normally secreted into phloem sieve elements affected the distribution of certain metals in the plant, most probaby by altering the translocation of these metals. Although the function of LCD is unclear, it may be related to Cd transport in a similar manner, affecting its translocation/solubility inside plants.
In WT plants, exposure to Cd leads to less accumulation of Zn in the roots and shoots, suggesting that Cd interfered with Zn accumulation; this phenomenon was also observed in the lcd mutant (data not shown). Cd and Zn have similar physical and chemical properties; therefore, Cd may compete with Zn for various biological ligands (Azpiroz-Leehan et al., 1997), resulting in less Zn accumulation. In the lcd mutant, Zn showed the same distribution pattern as Cd; namely, greater accumulation in the roots and less accumulation in the shoots compared with the WT under control and high Cd conditions, which suggests that the natural function of LCD is related to Zn transport. However, when lcd plants were grown in soil as opposed to hydroponic culture with a low Cd concentration, there were no significant differences from the WT in the shoot Cd, Zn, Mn, and Fe concentrations. The grains of lcd mutant plants grown in soil had lower Cd concentrations relative to the WT, but showed no significant differences in Zn, Mn, and Fe relative to the WT. These observations suggest that the lcd mutant exhibits different behaviours with respect to Cd translocation in hydroponic versus soil culture. This may be a function of the bioavailability of Cd, which is lower in a soil culture system.
Sequence analysis of OsLCD showed no homology with other known proteins, suggesting that this protein may be specific to rice. Microarray analysis of lcd and lcd-tos17 mutant plants confirmed the knockout of OsLCD (Supplementary Table S1 at JXB online). Microarray analysis also revealed 24 genes that were down-regulated in both lcd and lcd-tos17, with strong down-regulation of a dehydrin gene, particularly in the roots. Dehydrins present a distinct group of stress response proteins, the so-called late embryogenesis abundant (LEA) proteins. Among the genes that were up-regulated in both lcd and lcd-tos17, an ABC transporter (multidrug resistance protein 1 homologue; Os01g0533900) showed increased expression in both roots and shoots (Supplementary Table S2). It was the only transporter protein found with a changed expression level in both mutants and may be related to the Cd accumulation phenotype of lcd and lcd-tos17; however, a more detailed analysis of the ABC transporter gene is required to understand the possible relationship(s) between its gene product, LCD, and the Cd phenotype. Ogawa et al. (2009) found that a number of transporters, including PDR, MDR, MRP, MATE, CDF, OsHMA3, and OsIRT, were induced under Cd stress and may be involved in the Cd stress response (Table 1). These transporters showed no significant changes in expression in lcd and lcd-tos17, indicating that the Cd phenotype presented by the mutants is not directly related to these previously reported transporters; therefore, another pathway remains to be elucidated.
In summary, LCD is a soluble protein expressed in plant vascular tissues, the absence of which causes alterations in Cd transport within plants. Although the mechanisms involved in the lcd mutant phenotype are unclear, these data indicate that lcd might be a useful mutant line for research aimed at understanding Cd transport in plants and is a promising candidate rice line for use in combating the threat of Cd to human health.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. WT seeds were sown in MS medium containing Cd concentrations of 0, 0.25, 0.5, 1.0, 1.5, and 2.0 mM for 7 d.
Figure S2. Root and shoot metal concentration.
Figure S3. Field grain and leaf metal concentration.
Table S1. Down-regulated (ratio ≤0.5) genes in microarray analysis in both shoots and roots of lcd.
Table S2. Up-regulated (ratio ≥2) genes in microarray analysis in both shoots and roots of lcd.
Table S3. Differences in gene expression in different genetic backgrounds of rice.
Acknowledgments
This work was supported by the Programme for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, GMB0001).
References
- Azpiroz-Leehan R, Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: going back and forth. Trends in Genetcs. 1997;13:152–156. doi: 10.1016/s0168-9525(97)01094-9. [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. The Plant Journal. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plants: a review. Environmental Pollution. 1997;98:29–36. doi: 10.1016/s0269-7491(97)00110-3. [DOI] [PubMed] [Google Scholar]
- Eren E, Argüello JM. Arabidopsis HMA2, a divalent heavy metal-transporting PIB-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiology. 2004;136:3712–3723. doi: 10.1104/pp.104.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S. Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiology. 2010;152:1796–1806. doi: 10.1104/pp.109.151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1995;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M. Retrotransposons of rice involved in mutations induced by tissue culture. Proceedings of the National Academy of Sciences, USA. 1996;93:7783–7788. doi: 10.1073/pnas.93.15.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi H, Aoshima K, Oguma R, Sasaki S, Miyamoto K, Hosoi Y, Katoh T, Kayama F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. International Archives of Occupational and Environmental Health. 2010;83:953–970. doi: 10.1007/s00420-010-0510-x. [DOI] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Cobbet CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiology. 1995;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EK, Wang PC, Lo SJ. Strategies for cloning unknown cellular flanking DNA sequences from foreign integrants. Cellular and Molecular Life Sciences. 1998;54:1403–1411. doi: 10.1007/s000180050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Bashir K, Fujimoto M, An G, Itai R, Tsutsumi N, Nakanishi H, Mori S, Nishizawa NK. Rice-specific mitochondrial iron-regulated gene (MIR) plays an important role in iron homeostasis. Molecular Plant. 2009;2:1059–106. doi: 10.1093/mp/ssp051. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ The Plant Journal. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Kobae Y, Sekino T, Yoshioka H, Nakagawa T, Martinoia E, Maeshima M. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant and Cell Physiology. 2006;47:309–318. doi: 10.1093/pcp/pcj001. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with broad specificity. Plant Molecular Biology. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis (glutathionato) cadmium. Proceedings of the Nalional Academy of Sciences, USA. 1997;94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Science and Plant Nutrition. 2006;52:464–469. [Google Scholar]
- Nordic Council of Ministers. Cadmium review. Nordic Council of Ministers. 2003 [Google Scholar]
- Nylander M, Svensson J, Palva ET, Welin BV. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Molecular Biology. 2000;45:263–279. doi: 10.1023/a:1006469128280. [DOI] [PubMed] [Google Scholar]
- Ogawa I, Nakanishi H, Mori S, Nishizawa NK. Time course analysis of gene regulation under cadmium stress in rice. Plant and Soil. 2009;325:97–108. [Google Scholar]
- Spertini D, Béliveau C, Bellemare G. Screening of transgenic plants by amplification of unknown genomic DNA flanking T-DNA. Biotechniques. 1999;27:308–314. doi: 10.2144/99272st01. [DOI] [PubMed] [Google Scholar]
- Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. The Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Baxter IR, Lahner B, Reinders A, Salt DE, Ward JM. Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. The Plant Cell. 2010;22:3963–3979. doi: 10.1105/tpc.110.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.