Abstract
N-Methyl-D-aspartate (NMDA) receptors are tetrameric ion channels containing two of four possible GluN2 subunits. These receptors have been implicated for decades in neurological diseases such as stroke, traumatic brain injury, dementia, and schizophrenia. The GluN2 subunits contribute substantially to functional diversity of NMDA receptors and are distinctly expressed in development and among brain regions. Thus, subunit-selective antagonists and modulators that differentially target the GluN2 subunit might provide an opportunity to pharmacologically modify the function of select groups of neurons for therapeutic gain. A flurry of clinical, functional, and chemical studies have together reinvigorated efforts to identify subunit-selective modulators of NMDA receptor function, resulting in a handful of new compounds that appear to act at novel sites. Here we review the properties of new emerging classes of subunit-selective NMDA receptor modulators, which we predict will mark the beginning of a productive period of progress for NMDA receptor pharmacology.
Historical overview of NMDA receptor pharmacology
Nearly three decades ago, pharmacological advances in subtype-selective glutamate receptor antagonists fueled an explosive increase in our understanding of NMDA receptor function in the central nervous system. D-APV and MK-801 (Box 1, Table 1) showed strong selectivity for NMDA receptors over AMPA and kainate receptors, providing neuroscientists studying physiology, behavior, development, and neurological disease tools with which to dissect the role NMDA receptors play in a myriad of processes. Use of both competitive antagonists and NMDA receptor channel blockers provided key insights about the receptor composition of the excitatory postsynaptic current, and the role of various glutamate receptors in synaptic plasticity, neuronal death during CNS injury, and developmental biology. This stimulating period in excitatory amino acid research was closely followed by the cloning of cDNAs that revealed NMDA receptors consist of a glycine-binding GluN1 subunit and glutamate-binding GluN2 subunits [1], providing even deeper insight into NMDA receptor function, and ushering in a molecular era for those working on glutamate receptors. The identification of four different GluN2 gene products as integral components of NMDA receptors (Figure 1) offered the promise that subunit-selective agonists, antagonists, and modulators could be found that would allow NMDA receptor function to be regulated in region-specific manner. This hope initially seemed to be well-founded, as extensive pharmacology was developed around the GluN2B subunit, triggered by the discovery that the antihypertensive agent ifenprodil was a subunit-selective antagonist for NMDA receptors containing the GluN2B subunit [2]. However, shortly after this period of sustained progress, discovery of new ligands and pharmacological tools stalled. For over ten years there seemed to be little advance in development of subunit-selective antagonists and modulators for NMDA receptors comprised of subunits other than GluN2B (Figure 1). Moreover, this pause in discovery coincided with the realization that the clinical utility of NMDA receptor antagonists in neurological diseases such as stroke, traumatic brain injury, and dementia was more complex than initially appreciated, as a multitude of clinical trials involving a wide range of mechanistically distinct NMDA receptor antagonists repeatedly failed [3,4]. With the gradual realization that NMDA receptor on-target actions often constitute dose-limiting side effects for many indications, reports on the development of new ligands by the pharmaceutical industry diminished. Nevertheless, academic interest in subunit-selective NMDA receptor antagonists persisted, as a need for tools to dissect subunit contribution to region-specific processes was acutely appreciated by those working on systems involving excitatory amino acids. This sustained need motivated multiple laboratories to search for subunit-selective compounds with which to answer important questions about the role of NMDA receptors in normal and neuropathological functions.
Box 1. Discovery of NMDA Receptor Ligands.
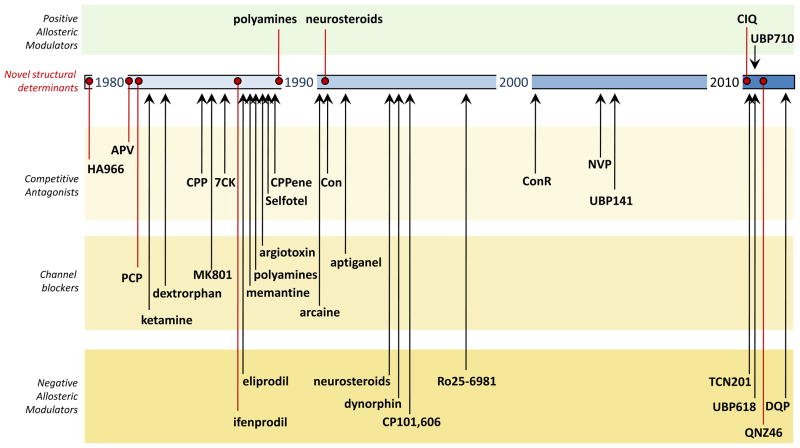
An active period for discovery of new ligands as well as new sites and mechanisms of action on the NMDA receptor occurred following the description of D-APV as a highly selective competitive NMDA receptor antagonist [68]. During the following dozen or so years, antagonists acting at the glycine binding site, the glutamate binding site, the channel pore, the amino terminal domain, and a region of the ligand binding domain encoded by the S2 region of the cDNA (e.g. neurosteroids) were described. The pace of discovery slowed over the next dozen years, with relatively few new prototypical compounds being described between 1995 and 2009, even though significant advances in medicinal chemistry continued to refine and embellish existing classes of compounds (such as GluN2B antagonists), as well as identify new scaffolds acting at known sites. In 2010, however, discovery of new ligands accelerated as a number of new compounds acting on the receptor with unique structural determinants were reported. These included a novel GluN2A-selective antagonist (TCN 201 [7]), a highly selective GluN2C/D potentiator with unique structural determinants of action in the M1 transmembrane region (CIQ [6]), GluN2C/D inhibitors with structural determinants of action in the lower lobe of the GluN2 ligand binding domain (QNZ46 [5,37]; DQP-1105 [9]), as well as a host of intriguing napthyl and phenanthryl antagonists (e.g. UBP618, UBP710, see [8] for additional analogues). These new advances in understanding the pharmacology and structure of this receptor class bodes well for future availability of subunit-selective pharmacological tools with which to elucidate the role of NMDA receptor subtypes in normal and neuropathological processes. Moreover, testing of these new compounds and the forthcoming analogues in animal models of disease may lead to new therapeutic strategies to address unmet clinical needs.
The figure provides a time line illustrating several different ligand classes acting by different mechanisms to inhibit or potentiate NMDA receptor function; a more comprehensive list of antagonists with Ki and IC50 values can be found elsewhere (see Tables 11, 13, and 15 in [4]). Identification of a new site of action is marked by the red circles within the time line. Representative examples for competitive antagonists (including both glycine site antagonists and glutamate site antagonists), channel blockers (acting at the ion channel pore), and negative allosteric modulators (acting at the amino terminal domain of GluN2B-containg receptors) are shown, and include D-aminophosphonovaleric acid (APV [68]), the low efficacy agonist HA-966 [69], phencyclidine (PCP [70]), ketamine [70], dextrorphan [71], CPP [72], MK801 [73], 7-chlorokynurenic acid (7CK [74]), ifenprodil [75,76], eliprodil [75], memantine [77], argiotoxin [59], selfotel [78], CPPene [79], polyamines[12,80], arcaine [14], conoantokins-G and -T (Con, [81,82]), aptiganel (CNS-1102, [83]), neurosteroids [84], CP101,606 [85], Ro25-6981 [62], Conantokin-R [86], NVP-AAM077 [42], UBP141 [56]. In addition, representative members of the few known classes of positive allosteric potentiators are shown, including polyamines [13,87] and neurosteroids [25]. Some of these ligands were exceptionally potent, highly-selective for NMDA receptors over kainate and AMPA receptors, and in some cases (ifenprodil and analogues acting at GluN2B) highly subunit-selective.
Table 1.
Subunit-selectivity of compounds acting on recombinant NMDA receptors
| Compound | GluN2A | GluN2B | GluN2C | GluN2D | Ref |
|---|---|---|---|---|---|
| Competitive Antagonists | Ki (μM) | Ki (μM) | Ki (μM) | Ki (μM) | |
| APV, (R)-AP5 | 0.28 | 0.46 | 1.6 | 3.7 | [55] |
| Selfotel (CGS-19755) | 0.15 | 0.58 | 0.58 | 1.1 | [55] |
| (R)-CPP | 0.041 | 0.27 | 0.63 | 1.99 | [55] |
| NVP-AAM077 | 0.015 | 0.078 | -- | -- | [44] |
| UBP-141 | 14 | 19 | 4.2 | 2.8 | [56] |
| Conantokin-G | 10 | 0.1 | 1 | 1 | [57] |
| Conantokin-R | 1 | 1 | 7 | 10 | [57] |
| 7-Cl-kynurenatea | 0.6 | 0.2 | -- | -- | [58] |
| Channel blockers | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | |
| Argiotoxin636 | 0.009 | 0.005 | 0.46 | -- | [59] |
| (+)MK-801 | 0.015 | 0.009 | 0.024 | 0.038 | [53] |
| Memantineb | 13 | 10 | 1.6 | 1.8 | [60] |
| Ketamineb | 5.4 | 5.1 | 1.2 | 2.9 | [60] |
| Phencyclidine | 0.82 | 0.16 | 0.16 | 0.22 | [53] |
| Dextrorphan | 1.3 | 0.33 | 0.15 | 0.74 | [53] |
| CNS-1102 (aptiganel) | 0.13 | 0.068 | 0.087 | 0.14 | [53] |
| Noncompetitive Antagonists | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | |
| Ifenprodil | 39 | 0.15 | 29 | 76 | [61] |
| Ro 25-6981 | 52 | 0.0090 | -- | -- | [62] |
| CP-101,606 | >100 | 0.039 | >100 | >100 | [63] |
| QNZ46 | 229 | >300 | 6 | 3 | [5] |
| DQP-1105 | 206 | 121 | 9 | 3 | [9] |
| TCN 201 | 0.109 | >30 | -- | >30 | [7] |
| UBP-618 | 1.8 | 2.4 | 2.0 | 2.4 | [8] |
| Dynorphin A (1–13) | 19 | 29 | 228 | 57 | [64] |
| Pregnanolone sulfate (3α5βS) | 50 | 44 | 26 | 30 | [65] |
| Allosteric Potentiators | EC50 (μM) | EC50 (μM) | EC50 (μM) | EC50 (μM) | |
| CIQ | NE | NE | 2.8 | 3 | [6] |
| Spermine | NEc | 163 | NE | NE | [66,67,21] |
| Pregnenolone sulfate | 21 | 33 | NEc | NEc | [26] |
| UBP-710 | >30 | >30 | NEc | NEc | [8] |
NE indicates no detectable effect, and -- indicates that data is not available.
KB values calculated from Cheng-Prusoff correction of IC50 values.
IC50 values determined in extracellular Mg2+, which alters subunit selectivity.
Partially inhibits with IC50 value of 100 μM or greater.
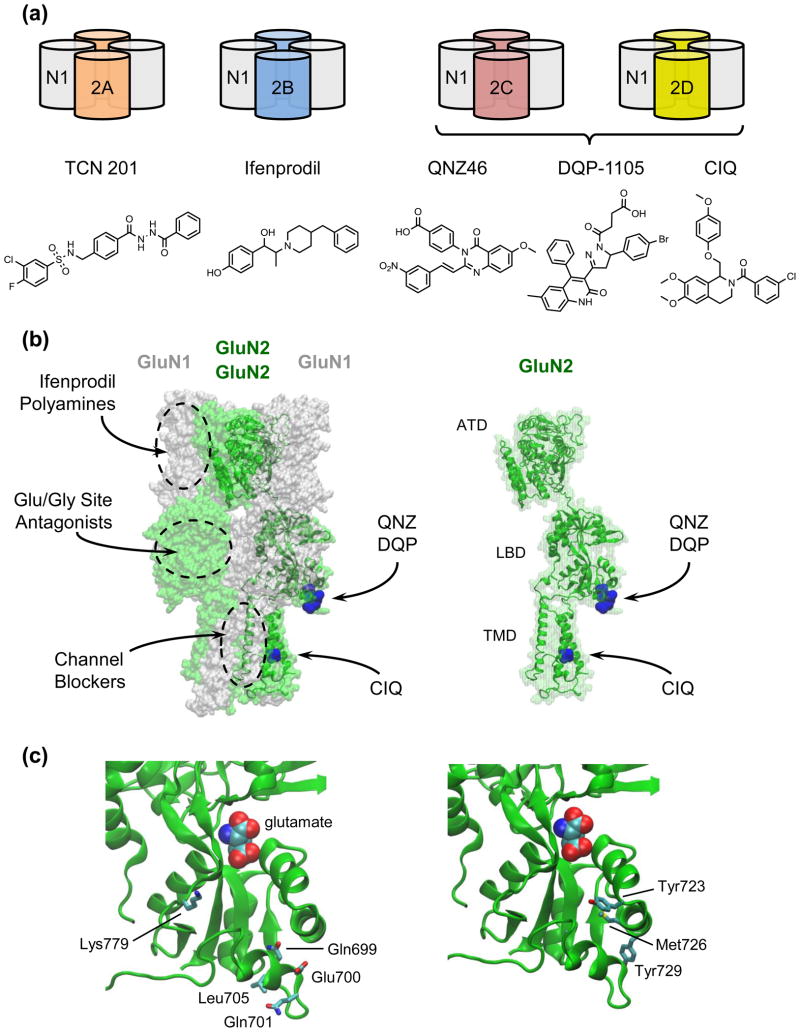
Figure 1. NMDA Receptor Architecture and Ligand Binding Sites.
NMDA receptors are tetrameric ion channels canonically comprised of two GluN1(labeled N1) subunits and two GluN2 subunits (2A, 2B, 2C, or 2D) in a 1-2-1-2 arrangement (a). Receptors containing different GluN1 splice variants and/or a GluN3 subunit have distinct functional properties. Additionally, much of the diversity among receptor subtypes arises from the GluN2 subunits, which are critical in determining biophysical and pharmacological properties of the receptor. Representative compounds acting with greater than 50-fold selectivity at individual GluN2-containing receptors are shown below the receptor subtypes. (b) Major ligand binding sites on the NMDA receptor are depicted on a GluN1/GluN2D homology model based on X-ray structures of a GluA2 tetramer (PDB 3KG2), GluN1 amino terminal domain (PDB 3Q41), GluN1 ligand binding domain (PDB 2A5T), GluN2B ATD (PDB 3JPY), and GluN2D LBD (PDB 3OEN) (Modeller 9.9). Ifenprodil and related GluN2B-selective molecules bind to the amino terminal domain (ATD) as do polyamines. Competitive antagonists of glycine and glutamate bind to the ligand binding domain (LBD) of GluN1 and GluN2, respectively. Channel blockers bind in the transmembrane domain (TMD). Residues critical for the activity of QNZ46, DQP1105, or CIQ are shown in blue. Right, an individual GluN2 subunit from a tetrameric complex is shown with the sites for QNZ46, DQP-1105, and CIQ highlighted in blue. (c) The lower portion of the GluN2D LBD harbors residues critical for the actions of QNZ46. Residues Gln699, Glu700, Gln701, Leu705 and Lys779 (left) are not conserved between GluN2D and GluN2A, while residues Tyr723, Met726, and Tyr729 (right) are conserved. Mutating Gln699, Glu700, Met726 and Tyr729 to the corresponding residue in GluN2A increased QNZ inhibition, while mutating Gln701, Leu705, Lys779, and Tyr729 to the corresponding GluN2A residue reduced QNZ inhibition.
In the last few years, there has been an acceleration in our understanding of the pharmacology of the NMDA receptor (Box 1), as the discovery of a handful of new binding sites on the receptor for positive and negative allosteric modulators has stimulated new thinking about NMDA receptor regulation and function [5–9]. This advance, fueled both by translational programs at the USA National Institutes of Health (NIH) and other funding agencies, as well as cross-cutting academic collaborations between neuropharmacologists and medicinal chemists, correlates with renewed clinical interest in NMDA receptor pharmacology driven by several intriguing clinical trials (e.g. [10,11]). Thus, it appears that the glutamate receptor field may again be poised to witness a rapid set of advances as proof-of-concept compounds acting at new sites are refined through medicinal chemistry into potent compounds with high degrees of subunit-selectivity that can serve as reliable new subunit-selective probes. Here we review the recent advances in identifying new NMDA receptor potentiators and inhibitors.
Positive modulators
Throughout the early 1990s, several endogenous compounds including polyamines and other biogenic amines were recognized as NMDA receptor positive modulators, i.e. compounds that bind to the receptor and cause an increase in its maximal response or agonist affinity (or both), but do not act as agonists[12–19]. Spermine can enhance the response of GluN2B-containing neurons through interactions with an extracellular site on the receptor [12–17] and has been suggested to bind at the GluN1-GluN2B amino terminal domain dimer interface, potentially shielding ionic interactions between residues that influence receptor function [20]. Polyamine binding to NMDA receptors enhances the sensitivity of the receptors to glycine [16] and relieves tonic proton inhibition of the receptors [21]. However, the potency with which polyamines potentiate native NMDA receptor responses (EC50 of 10–125 μM) is higher than estimates of the basal extracellular spermine concentrations (4 μM; [22]).
There are extensive reports documenting interactions of dynorphin and the NMDA receptor system. Interestingly, dynorphin can both inhibit NMDA receptor-mediated currents, as well as potentiate responses in low glycine with an EC50 value for dynorphin A(1–13) of 2.8 μM [23] (Table 1). Arachidonic acid can also enhance native NMDA receptor responses, presumably through direct interactions with a fatty acid binding domain on the receptor [24]. Furthermore, certain neurosteroids potentiate NMDA receptor function with some subunit selectivity [25]. Pregnenolone sulfate (PS), for example, potentiates GluN1/GluN2A and GluN1/GluN2B, but inhibits GluN1/GluN2C and GluN1/GluN2D [26,27] (Table 1). PS acts by increasing the peak open probability [28] in a manner controlled by phosphorylation [29]. Moreover, portions of the S2 region between the third and fourth transmembrane helices are critical for PS activity [30, 27]. Although some neurosteroids are likely to have important roles in regulating receptor function in tissue, the neurosteroid scaffold has yet to be exploited for the development of potent and highly subunit-selective molecules that can discriminate between NMDA receptors that contain different GluN2 subunits.
Recent evaluation of a series of tetrahydroisoquinoline analogues lead to the identification of (3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1 H)-yl)methanone), or CIQ, which is a small drug-like molecule that is a highly selective allosteric potentiator of recombinant and native NMDA receptors that contain the GluN2C or GluN2D subunits [6]. This compound has no detectable potentiating activity on AMPA or kainate receptors or on GluN2A- or GluN2B-containing NMDA receptors, but can enhance by over 2-fold the response amplitude of GluN2C- and GluN2D-containing NMDA receptors. Potentiation of GluN2D-containing receptors occurs with an EC50 value of 3 μM, whereas this compound is inactive at GluN2A- and GluN2B-containing receptors at 20 μM. CIQ has no agonist activity, does not alter agonist EC50 values, and is uninfluenced by agonist concentration [6]. Single channel analysis demonstrates that CIQ enhances channel opening frequency without impacting lifetime of the open state, suggesting that CIQ reduces the activation energy for channel opening.
A chimeric strategy using GluN2A and GluN2D revealed that the structural determinants of action involve membrane spanning elements as well as extracellular linker regions, and ensuing site-directed mutagenesis identified a single residue residing in the first transmembrane helix that can control CIQ function [6]. This residue (Thr592 in GluN2D) is conserved between GluN2C and GluN2D but divergent in GluN2A and GluN2B, suggesting it may interact directly with CIQ, although further experiments are needed to verify this (see [31] for an example where a crystal structure revealed a ligand binding site that was not predicted by chimera and mutagenesis data). Nevertheless, Thr592 and the nearby pre-M1 helix, a short helix running almost parallel to the membrane and acting as a ‘cuff’ at the extracellular region of the ion channel pore [32], are ideally positioned to influence the gating process [33] and an interaction between CIQ and Thr592 would be consistent with the effects of CIQ at the single channel level. It is noteworthy that this region of the NMDA receptor has not previously been implicated in either gating or pharmacology, suggesting tetrahydroisoquinolines such as CIQ influence function in a manner that involves novel structural elements. Figure 1 illustrates the position of the residues that can control the actions of CIQ in homology model of GluN1/GluN2D. The discovery of a subunit-selective NMDA receptor potentiator and identification of its molecular determinants of action provides new opportunities to investigate mechanisms of NMDA receptor allosteric regulation as well as to explore the nature of channel gating at the single channel level.
CIQ can enhance the response of native NMDA receptors to exogenous application of NMDA in neurons in the subthalamic nuclei [6], which has been suggested to express GluN2D [34]. By contrast, CIQ appears to have little effect on the response to NMDA of CA1 pyramidal cells, which have typically been thought to express GluN2A and GluN2B. Thus, CIQ can be used to probe the subunit composition of native NMDA receptors. Moreover, the existence of a small molecule allosteric potentiation provides an opportunity to test a number of hypotheses about the effects that enhancement of NMDA receptor function might have on circuits, behaviors, and in disease models.
In addition to CIQ, several structurally unrelated molecules (e.g. UBP710 and UBP646) have recently been reported to potentiate NMDA receptors [8] without agonist activity. At concentrations above 30 μM, UBP646 potentiated responses from GluN2A, 2B, 2C, and 2D. By contrast, UBP710 only potentiated GluN2A- and GluN2B-containing receptors. Recordings from chimeras of GluN2A and GluN2C suggest the entire S2 region between the third and fourth transmembrane helices is important for potentiation by UBP710. The existence of at least two structurally unrelated classes of subunit- selective NMDA receptor potentiators in addition to other potentiators with intriguing patterns of subunit-selectivity [8] suggests that either multiple potentiating sites exist or a single site can accommodate a variety of different scaffolds.
Non-competitive antagonists
The structure-activity relationship of GluN2B-selective antagonists that interact with the amino terminal domain has been extensively studied (for reviews see [35, 36]). An X-ray crystal structure of the GluN1/GluN2B amino terminal domain dimer has revealed the binding site for the GluN2B- selective antagonists ifenprodil and Ro 25-6891 [31], which will no doubt aid in development of a clinically well-tolerated GluN2B-selective antagonist. In contrast to the abundant GluN2B-selective antagonists, no other highly subunit-selective non-competitive antagonists have been described. However, novel non-competitive antagonists of both GluN2A and GluN2C/D have been recently reported. Bettini et al. [7] described a series of sulfonamides with high selectivity for GluN2A- containing receptors. For example, 3-chloro-4-fluoro-N-[(4-{[2-(phenylcarbonyl)hydrazino] carbonyl}phenyl)methyl]benzenesulfonamide (compound 1, referred to as TCN 201) is an apparent non- competitive antagonist whose actions cannot be surmounted by glutamate. It does partially displace glutamate site antagonists in radioligand binding assays in a manner similar to displacement of glutamate site ligands by glycine site antagonists. In contrast to glutamate, glycine can surmount the inhibition by TCN 201 in functional Ca2+ imaging assays, even though TCN 201 is minimally effective in displacing glycine site antagonists in radiolabelled binding studies. These data lead to the hypothesis that TCN 201 acts at a site allosterically modulated by glycine as well as the prediction that TCN 201 may act at the GluN1/GluN2A ligand binding domain interface [7]. A recent study by Costa et al. [8] has identified several phenanthrene and naphthyl analogues that act as non-competitive antagonists of GluN2C- and GluN2D-containing receptors. UBP618 shows robust inhibition with IC50 values of 1.8–2.4 μM (Table 1) that are minimally influence by glutamate and glycine concentration [8]. These compounds suggest a new negative modulatory site exists that could be exploited for the development of novel probes.
Another series of novel non-competitive antagonists were recently discovered through medicinal chemistry efforts to increase the potency of a quinazolin-4-one that was identified as an NMDA receptor inhibitor through a fluorescence-based multi-well screen [5]. These antagonists had IC50 values ranging from 600 nM to 3 μM. A gain-of-function chimeric strategy utilizing one analogue (QNZ46) with greater than 50-fold selectivity for recombinant GluN2D over GluN2A identified unique structural determinants of action of these compounds [37]. The selectivity of QNZ46 for GluN2D can be transferred to GluN2A by residues within a loop region on the lower lobe of the ligand-binding bilobed clamshell (Figure 1). Subsequently, a handful of individual residues were identified that control the actions of these compounds. Interestingly, this class of antagonist shows a unique mechanism of action, with an unusual use-dependence that involves a 20-fold enhancement of IC50 when glutamate (but not glycine) binds to its recognition site. This enhancement suggests a simple mechanism of action that leads to an inhibition of opening frequency upon binding of inhibitors after glutamate binding. The dependence on glutamate (but not glycine) binding is consistent with the modular nature of glutamate receptor structure, as well as previous suggestions that different subunits may control independent conformational changes [38].
In addition to quinazoline-4-ones, structurally unrelated compounds that possess a pyrazoline scaffold appear to act at a similar site, as determined by the coordinated study of GluN2A-GluN2D chimeric receptors together with site-directed mutagenesis [9]. These compounds, exemplified by DQP-1105, show an unusual property in that their potencies at the GluN2A subunit may be enhanced by desensitization that occurs following dialysis of the intracellular solution. Thus, in conventional patch mode, these antagonists do not appear as selective as they do in perforated patch recording mode. The mechanistic basis of this observation remains to be determined.
Competitive antagonists and channel blockers
The identification of D-APV as a competitive antagonist that is highly selective for NMDA receptors over AMPA and kainate receptors led to significant advances in our understanding of the role of NMDA receptors in numerous biological phenomena. D-APV has for years been the definitive antagonist to show NMDA receptor involvement in a neurological process or disease state [39–41]. However, D-APV shows Ki values that vary less than ten-fold among NMDA receptors comprised of different GluN2 subunits, a level of selectivity generally considered too low to provide trustworthy dissection of subunit contribution, particularly in native tissue. Moreover, efforts to identify competitive antagonists with GluN2 subunit-selectivity have yet to improve much on D-APV (e.g. see table 11 in [4]). Considerable enthusiasm around NVP-AAM077, a proposed GluN2A-selective competitive antagonist [42], was short-lived as estimates of Ki values from IC50 values [43] and later determination of the Ki values by Schild analysis [44,45] revealed that selectivity was less than 10-fold. Similarly, a handful of phenanthrene derivatives show 7- to 10-fold differences in estimated Ki values [46], favoring inhibition of GluN2D over GluN2A.
Crystal structures of the ligand binding domain among glutamate receptors offer an explanation for the difficulty in obtaining subunit-selective competitive antagonists. The key agonist contact residues are conserved almost universally across the glutamate receptor family and strongly conserved within the glutamate binding pocket across GluN2 subunits [47–49], although the hinge region in the GluN2 ligand binding domain shows agonist-dependent diversity [50]. Whereas the glutamate binding pockets show some differences [51] that can be exploited to develop agonists with limited preference for different GluN2 subunits [52], in general, the highly conserved nature of the agonist binding domain diminishes the prospect that strong subunit selectivity can be achieved through conventional modifications of competitive antagonist structure.
Uncompetitive antagonists, which are typically channel blockers, target perhaps the most highly conserved portion of the NMDA receptor, and thus there is little selectivity (<10 fold) for various channel blockers across NMDA receptor subunits [53]. The lone exception is a class of synthetic polyamines that contain a long chain with various aromatic headgroups [54]. Some of these compounds can achieve up to 50-fold selectivity between GluN2A and GluN2D, raising the possibility that these scaffolds could be modified in a manner that allows interaction with less conserved portions of the receptor. This may occur as the length of the blocker engages atomic interactions beyond the conserved ion permeation pore, potentially sensing more distinctive elements of the receptor. In addition, given the unusually large and diverse collection of molecules that can act as NMDA receptor blockers, it seems possible that some subunit selectivity might yet be encountered through extensive screening among GluN2 subunits; however, this awaits further study.
Concluding remarks
Our knowledge of NMDA receptor biology has been inextricably linked to the diverse array of small molecules acting on the receptors. For example, identification of NMDA receptors as a mediator of excitatory neurotransmission and some forms of synaptic plasticity relied heavily upon agonists and antagonists that were selective for NMDA over AMPA and kainate receptors. These pharmacological tools have been used extensively to test important hypotheses about the contribution of the NMDA receptor family to neurological diseases including stroke, traumatic brain injury, dementia, epilepsy, and neuropathic pain. However, the numerous roles of NMDA receptors in normal brain function make it difficult to selectively target this receptor class in a disease state while preserving normal function in patients, which has limited the utility of NMDA receptor antagonists as therapeutics.
A promising approach to achieve the selective modulation of aberrant NMDA receptor function is to target the specific GluN2 subtypes, which are differentially expressed in the CNS. Additionally, there has been a growing appreciation that various NMDA receptor subtypes are likely to play unique roles in the neurophysiology of different brain regions. However, apart from the GluN2B subunit, it has been impossible to assess contribution of specific NMDA receptor subtypes to brain function or evaluate the potential therapeutic utility of targeting particular NMDA receptor subtypes because potent, subunit-selective antagonists that distinguish between NMDA receptors comprised of different GluN2 subunits simply have been unavailable. This mismatch finally is being resolved, as a handful of novel subunit-selective NMDA receptor modulators have been described recently. It is no accident that the newly discovered GluN2 subunit-selective molecules are largely allosteric modulators. Linker regions, the amino terminal domain, and portions of the ligand binding domain distal to the residues that contact the agonist are not well conserved among NMDA receptor subunits. Moreover, the highly modular arrangement of extracellular domains creates numerous potential regulatory sites at various protein-protein interfaces, and provides an opportunity to inhibit specific conformational changes that might take place within individual subunits en route to channel opening. We predict that new subunit-selective compounds acting at sites distinct from agonist binding pocket or the channel pore will continue to be found for the NMDA receptor. Almost certainly, medicinal chemistry efforts applied to the initial proof-of-concept molecules will improve the potency and selectivity of these new families of modulators. Availability of the resulting new pharmacological tools will stimulate experimental work that dissects the contribution of the different GluN2 subunits to various neurological processes and disease states, with potential for addressing unmet clinical need. Thus, our understanding of NMDA receptors seems poised again for explosive growth, triggered by the discovery of pharmacological tools.
Box Figure.
Acknowledgments
We thank Mark L. Mayer, Kasper B. Hansen, Katie M. Vance, and Timothy M. Acker for valuable comments on the manuscript. This work was supported by NINDS (NS036654, NS065371 SFT), NIGMS (GM008602 KKO), and NIDA (DA015040 KKO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 3.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Current Opinion in Pharmacology. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Traynelis SF, et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosley CA, et al. Quinazolin-4-one Derivatives: A Novel Class of Noncompetitive NR2C/D Subunit-Selective N-Methyl-d-aspartate Receptor Antagonists. J Med Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullasseril P, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettini E, et al. Identification and Characterization of Novel NMDA Receptor Antagonists Selective for NR2A- over NR2B-Containing Receptors. J Pharmacol Exp Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 8.Costa BM, et al. A Novel Family of Negative and Positive Allosteric Modulators of NMDA Receptors. J Pharmacol Exp Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acker T, et al. Mechanism for Non-competitive Inhibition by Novel GluN2C/D NMDA Receptor Subunit-selective Modulators. Mol Pharmacol. 2011 doi: 10.1124/mol.111.073239. Epub 2 Aug 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preskorn SH, et al. An Innovative Design to Establish Proof of Concept of the Antidepressant Effects of the NR2B Subunit Selective N-Methyl-D-Aspartate Antagonist, CP-101,606, in Patients With Treatment-Refractory Major Depressive Disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 11.Mony L, et al. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ransom RW, Stec NL. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem. 1988;51:830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams K, et al. Characterization of polyamines having agonist, antagonist, and inverse agonist effects at the polyamine recognition site of the NMDA receptor. Neuron. 1990;5:199–208. doi: 10.1016/0896-6273(90)90309-4. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds IJ. Arcaine uncovers dual interactions of polyamines with the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1990;255:1001–1007. [PubMed] [Google Scholar]
- 15.Rock DM, Macdonald RL. The polyamine spermine has multiple actions on N-methyl-D-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992;41:83–88. [PubMed] [Google Scholar]
- 16.Benveniste M, Mayer ML. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol (Lond) 1993;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand GM, et al. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekkers JM. Enhancement by histamine of NMDA-mediated synaptic transmission in the hippocampus. Science. 1993;261:104–106. doi: 10.1126/science.8391168. [DOI] [PubMed] [Google Scholar]
- 19.Williams K. Subunit-specific potentiation of recombinant N-methyl-D-aspartate receptors by histamine. Mol Pharmacol. 1994;46:531–541. [PubMed] [Google Scholar]
- 20.Mony L, et al. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011;30:3134–3146. doi: 10.1038/emboj.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traynelis SF, et al. Control of Proton Sensitivity of the NMDA Receptor by RNA Splicing and Polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 22.Fage D, et al. Selective release of spermine and spermidine from the rat striatum by N-methyl-D-aspartate receptor activation in vivo. J Neurochem. 1992;58:2170–2175. doi: 10.1111/j.1471-4159.1992.tb10960.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, et al. Potentiation of NMDA receptor-mediated responses by dynorphin at low extracellular glycine concentrations. J Neurophysiol. 1997;78:582–590. doi: 10.1152/jn.1997.78.2.582. [DOI] [PubMed] [Google Scholar]
- 24.Miller B, et al. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 25.Wu FS, et al. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 26.Malayev A, et al. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horak M, et al. Subtype-dependence of N-methyl-d-aspartate receptor modulation by pregnenolone sulfate. Neuroscience. 2006;137:93–102. doi: 10.1016/j.neuroscience.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Horak M, et al. Molecular Mechanism of Pregnenolone Sulfate Action at NR1/NR2B Receptors. J Neurosci. 2004;24:10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovic M, et al. Pregnenolone sulfate modulation of N-methyl-d-aspartate receptors is phosphorylation dependent. Neuroscience. 2009;160:616–628. doi: 10.1016/j.neuroscience.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Jang MK, et al. A steroid modulatory domain on NR2B controls N-methyl-d-aspartate receptor proton sensitivity. Proc Natl Acad Sci USA. 2004;101:8198–8203. doi: 10.1073/pnas.0401838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakas E, et al. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobolevsky AI, et al. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talukder I, et al. Specific sites within the ligand-binding domain and ion channel linkers modulate NMDA receptor gating. J Neurosci. 2010;30:11792–11804. doi: 10.1523/JNEUROSCI.5382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Standaert DG, et al. Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- 35.Borza I, Domány G. NR2B selective NMDA antagonists: the evolution of the ifenprodil-type pharmacophore. Curr Top Med Chem. 2006;6:687–695. doi: 10.2174/156802606776894456. [DOI] [PubMed] [Google Scholar]
- 36.Beinat C, et al. Insights into structure-activity relationships and CNS therapeutic applications of NR2B selective antagonists. Curr Med Chem. 2010;17:4166–4190. doi: 10.2174/092986710793348572. [DOI] [PubMed] [Google Scholar]
- 37.Hansen KB, Traynelis SF. Structural and Mechanistic Determinants of a Novel Site for Noncompetitive Inhibition of GluN2D-Containing NMDA Receptors. J Neurosci. 2011;31:3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- 39.Herron CE, et al. Intracellular demonstration of an N-methyl-D-aspartate receptor mediated component of synaptic transmission in the rat hippocampus. Neurosci Lett. 1985;60:19–23. doi: 10.1016/0304-3940(85)90375-1. [DOI] [PubMed] [Google Scholar]
- 40.Hestrin S, et al. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol (Lond) 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris RG, et al. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, et al. Role of NMDA Receptor Subtypes in Governing the Direction of Hippocampal Synaptic Plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 43.Feng B, et al. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frizelle PA, et al. Equilibrium Constants for (R)-[(S)-1-(4-Bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic Acid (NVP-AAM077) Acting at Recombinant NR1/NR2A and NR1/NR2B N-Methyl-d-aspartate Receptors: Implications for Studies of Synaptic Transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- 45.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa BM, et al. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther. 2009;331:618–626. doi: 10.1124/jpet.109.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinarsky L, et al. Identification of subunit- and antagonist-specific amino acid residues in the N-Methyl-D-aspartate receptor glutamate-binding pocket. J Pharmacol Exp Ther. 2005;313:1066–1074. doi: 10.1124/jpet.104.082990. [DOI] [PubMed] [Google Scholar]
- 48.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 49.Risgaard R, et al. Partial Agonists and Subunit Selectivity at NMDA Receptors. Chemistry - A European Journal. 2010;16:13910–13918. doi: 10.1002/chem.201001366. [DOI] [PubMed] [Google Scholar]
- 50.Vance KM, et al. Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat Commun. 2011;2:294. doi: 10.1038/ncomms1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erreger K, et al. Subunit-Specific Agonist Activity at NR2A-, NR2B-, NR2C-, and NR2D-Containing N-Methyl-d-aspartate Glutamate Receptors. Mol Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- 52.Clausen RP, et al. N-Hydroxypyrazolyl Glycine Derivatives as Selective N-Methyl-d-aspartic Acid Receptor Ligands. J Med Chem. 2008;51:4179–4187. doi: 10.1021/jm800025e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dravid SM, et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igarashi K, et al. Benzyl-polyamines: novel, potent N-methyl-D-aspartate receptor antagonists. J Pharmacol Exp Ther. 1997;283:533–540. [PubMed] [Google Scholar]
- 55.Feng B, et al. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Morley RM, et al. Synthesis and Pharmacology of N1-Substituted Piperazine-2,3-dicarboxylic Acid Derivatives Acting as NMDA Receptor Antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- 57.Teichert RW, et al. Novel Conantokins from Conus parius Venom Are Specific Antagonists of N-Methyl-D-aspartate Receptors. J Biol Chem. 2007;282:36905–36913. doi: 10.1074/jbc.M706611200. [DOI] [PubMed] [Google Scholar]
- 58.Priestley T, et al. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- 59.Raditsch M, et al. Subunit-specific block of cloned NMDA receptors by argiotoxin 636. FEBS Lett. 1993;324:63–66. doi: 10.1016/0014-5793(93)81533-6. [DOI] [PubMed] [Google Scholar]
- 60.Kotermanski SE, Johnson JW. Mg2+ Imparts NMDA Receptor Subtype Selectivity to the Alzheimer’s Drug Memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hess SD, et al. Functional characterization of human N-methyl-D-aspartate subtype 1A/2D receptors. J Neurochem. 1998;70:1269–1279. doi: 10.1046/j.1471-4159.1998.70031269.x. [DOI] [PubMed] [Google Scholar]
- 62.Fischer G, et al. Ro 25–6981, a Highly Potent and Selective Blocker of N-Methyl-d-aspartate Receptors Containing the NR2B Subunit. Characterization in Vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 63.Mott DD, et al. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- 64.Brauneis U, et al. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subunits to inhibition by dynorphin. J Pharmacol Exp Ther. 1996;279:1063–1068. [PubMed] [Google Scholar]
- 65.Petrovic M, et al. 20-oxo-5beta-pregnan-3alpha-yl sulfate is a use-dependent NMDA receptor inhibitor. J Neurosci. 2005;25:8439–8450. doi: 10.1523/JNEUROSCI.1407-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams K, et al. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- 67.Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neurosci Lett. 1995;184:181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- 68.Evans RH, et al. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans RH, et al. Mg2+-like selective antagonism of excitatory amino acid-induced responses by alpha, epsilon-diaminopimelic acid, D-alpha-aminoadipate and HA-966 in isolated spinal cord of frog and immature rat. Brain Res. 1978;148:536–542. doi: 10.1016/0006-8993(78)90744-8. [DOI] [PubMed] [Google Scholar]
- 70.Anis NA, et al. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berry SC, Lodge D. Benz(f)isoquinolines as excitatory amino acid antagonists: an indication of their mechanism of action? Biochem Pharmacol. 1984;33:3829–3832. doi: 10.1016/0006-2952(84)90047-9. [DOI] [PubMed] [Google Scholar]
- 72.Davies J, et al. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986;382:169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 73.Wong EH, et al. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kemp JA, et al. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter C, et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II Evidence for N-methyl-D-aspartate receptor antagonist properties. J Pharmacol Exp Ther. 1988;247:1222–1232. [PubMed] [Google Scholar]
- 76.Reynolds IJ, Miller RJ. Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines. Mol Pharmacol. 1989;36:758–765. [PubMed] [Google Scholar]
- 77.Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur J Pharmacol. 1989;166:591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 78.Lehmann J, et al. CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988;246:65–75. [PubMed] [Google Scholar]
- 79.Aebischer B, et al. Synthesis and NMDA Antagonistic Properties of the Enantiomers of 4-(3-phosphonopropyl)piperazine-2-carboxylic acid (CPP) and of the unsaturated analogue (E)-4-(3-phosphonoprop-2-enyl)piperazine-2-carboxylic acid (CPP-ene) Helvetica Chimica Acta. 1989;72:1043–1051. [Google Scholar]
- 80.Williams K, et al. Effects of polyamines on the binding of [3H]MK-801 to the N-methyl-D-aspartate receptor: pharmacological evidence for the existence of a polyamine recognition site. Mol Pharmacol. 1989;36:575–581. [PubMed] [Google Scholar]
- 81.Haack JA, et al. Conantokin-T. A gamma-carboxyglutamate containing peptide with N-methyl-d-aspartate antagonist activity. J Biol Chem. 1990;265:6025–6029. [PubMed] [Google Scholar]
- 82.Mena EE, et al. Conantokin-G: a novel peptide antagonist to the N-methyl-D-aspartic acid (NMDA) receptor. Neurosci Lett. 1990;118:241–244. doi: 10.1016/0304-3940(90)90637-o. [DOI] [PubMed] [Google Scholar]
- 83.Minematsu K, et al. Effects of a novel NMDA antagonist on experimental stroke rapidly and quantitatively assessed by diffusion-weighted MRI. Neurology. 1993;43:397–403. doi: 10.1212/wnl.43.2.397. [DOI] [PubMed] [Google Scholar]
- 84.Park-Chung M, et al. 3 alpha-Hydroxy-5 beta-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–150. [PubMed] [Google Scholar]
- 85.Chenard BL, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 86.White HS, et al. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther. 2000;292:425–432. [PubMed] [Google Scholar]
- 87.McGurk JF, et al. Polyamines potentiate responses of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:9971–9974. doi: 10.1073/pnas.87.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]