Abstract
The acid-base relations across the two surfaces of the epithelium of the turtle bladder were examined. By means of the 5,5-dimethyl-2,4-oxazolidinedione (DMO) technique the intracellular OH- concentration was measured in the presence and absence of a transepithelial pH gradient.
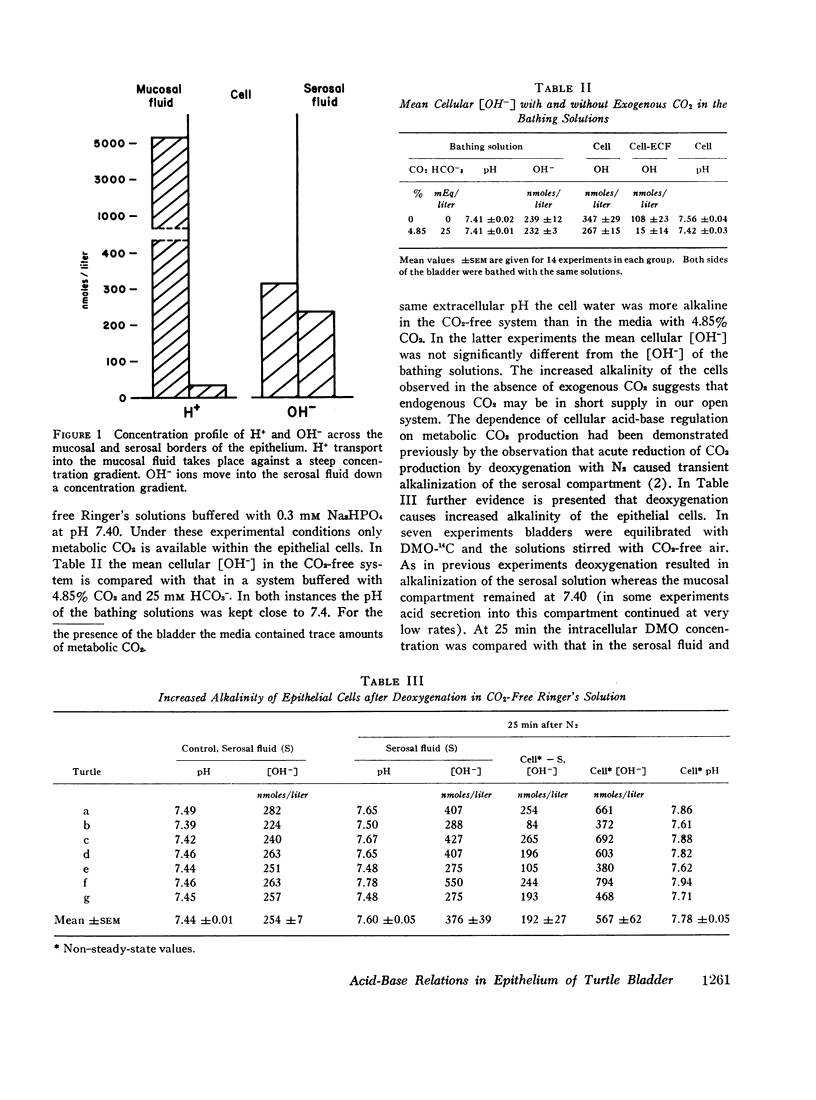
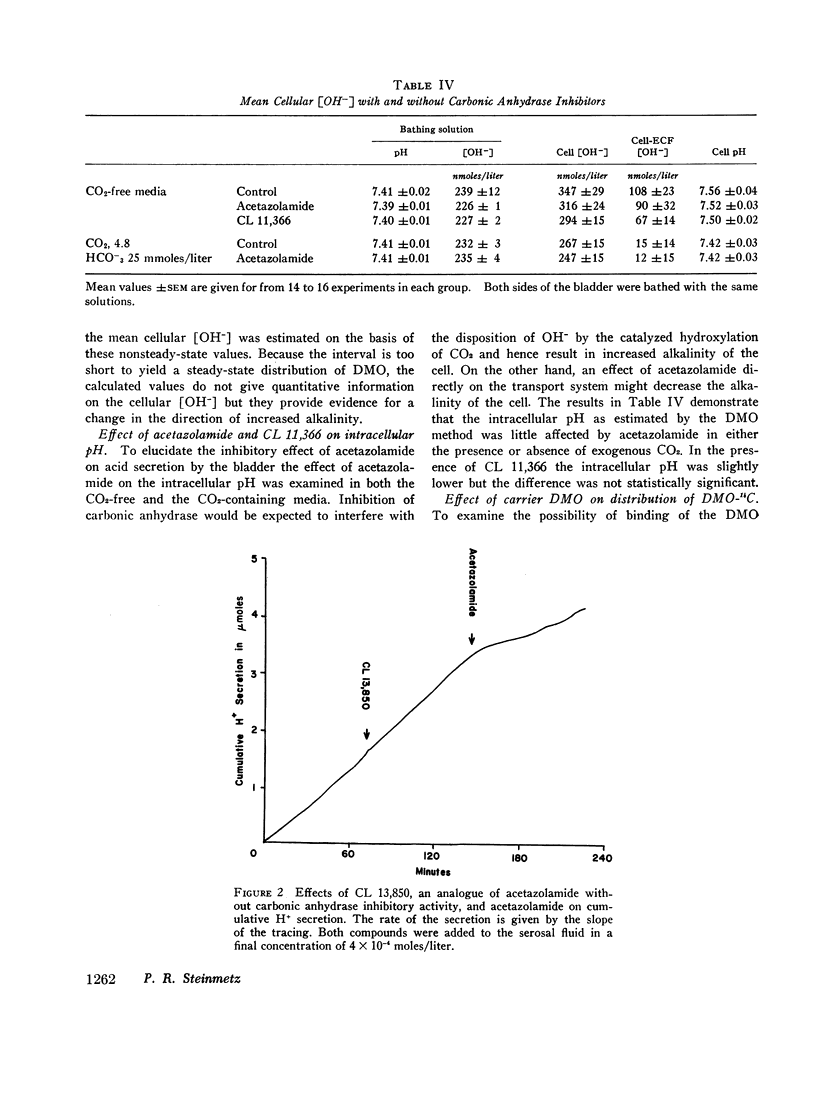
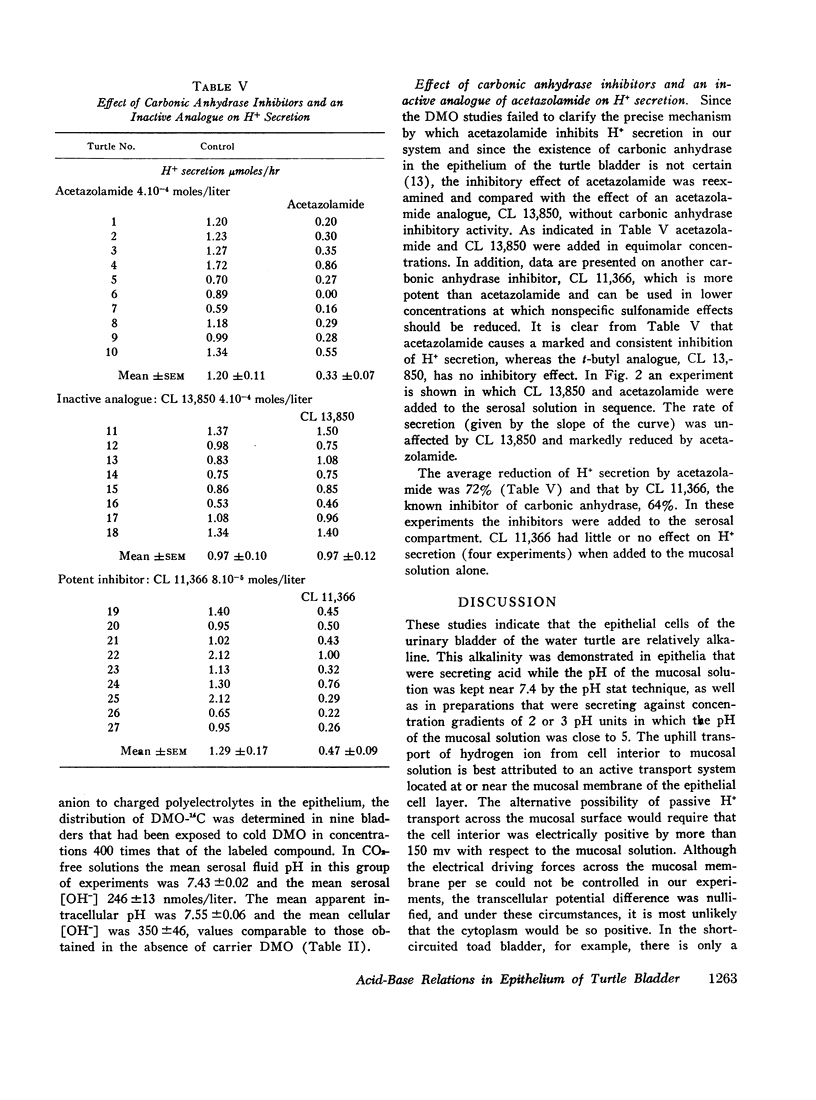
When both sides of the bladder were bathed with solutions free of exogenous CO2 and bicarbonate at pH 7.41 ([OH-] = 239 nmoles/liter), the epithelial cells were alkaline, the mean intracellular [OH-] being 347nmoles/liter. This alkalinity of the cells was preserved in bladders that secreted H+ against a gradient of over 2 pH units. In bathing solutions stirred with 4.85% CO2 and buffered with 25 mM HCO3- at pH 7.41 the intracellular [OH-] was lower than in CO2-free solutions and close to the extracellular [OH-]. In the CO2-free system anaerobiosis caused increased alkalinity of the cells and inhibition of H+ secretion presumably by decreased metabolic CO2 production. Carbonic acid inhibitors reduced H+ secretion, but had no significant effect on the alkalinity of the cells. An inactive analogue of acetazolamide had no effect on H+ secretion. The results indicate that the active step in acidification is located near the mucosal surface of the epithelium and that the alkali formed within the epithelial cells moves passively into the serosal solution along an electro-chemical gradient. The inhibitory effect of certain sulfonamides on H+ secretion by the bladder is directly correlated with their known carbonic anhydrase inhibitory activity, but not associated with a measurable change in the mean intracellular [OH-].
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian C., Hogben M. The chloride effect of carbonic anhydrase inhibitors. Mol Pharmacol. 1967 Jul;3(4):318–326. [PubMed] [Google Scholar]
- BRADFORD N. M., DAVIES R. E. The site of hydrochloric acid production in the stomach as determined by indicators. Biochem J. 1950 Apr;46(4):414–420. doi: 10.1042/bj0460414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky W. A., Schilb T. P. Mechanism of acidification in turtle bladder. Fed Proc. 1967 Sep;26(5):1314–1321. [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. pH values of the yeast cell. Biochem J. 1950 Sep;47(3):355–360. doi: 10.1042/bj0470355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH of skeletal muscle with pH-sensitive glass microelectrodes. J Clin Invest. 1967 Jun;46(6):920–933. doi: 10.1172/JCI105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. P. THE MEASUREMENT OF CARBONIC ANHYDRASE ACTIVITY. Methods Biochem Anal. 1963;11:307–327. doi: 10.1002/9780470110294.ch7. [DOI] [PubMed] [Google Scholar]
- FRAZIER H. S. The electrical potential profile of the isolated toad bladder. J Gen Physiol. 1962 Jan;45:515–528. doi: 10.1085/jgp.45.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyassy P. F. Intracellular H+ concentration of the isolated urinary bladder of the toad. Nature. 1965 May 1;206(983):511–512. doi: 10.1038/206511a0. [DOI] [PubMed] [Google Scholar]
- IRVINE R. O., SAUNDERS S. J., MILNE M. D., CRAWFORD M. A. Gradients of potassium and hydrogen ion in potassiumdeficient voluntary muscle. Clin Sci. 1961 Feb;20:1–18. [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Depression of chloride transport by carbonic anhydrase inhibitors in the absence of carbonic anhydrase. Nature. 1967 May 20;214(5090):836–837. doi: 10.1038/214836a0. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Schilb T. P., Brodsky W. A. Acidification of mucosal fluid by transport of bicarbonate ion in turtle bladders. Am J Physiol. 1966 May;210(5):997–1008. doi: 10.1152/ajplegacy.1966.210.5.997. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Characteristics of hydrogen ion transport in urinary bladder of water turtle. J Clin Invest. 1967 Oct;46(10):1531–1540. doi: 10.1172/JCI105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Omachi R. S., Frazier H. S. Independence of hydrogen ion secretion and transport of other electrolytes in turtle bladder. J Clin Invest. 1967 Oct;46(10):1541–1548. doi: 10.1172/JCI105645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., HARDMAN H. F. Intracellular pH of isolated perfused turtle heart. Am J Physiol. 1960 Dec;199:1112–1114. doi: 10.1152/ajplegacy.1960.199.6.1112. [DOI] [PubMed] [Google Scholar]


