Abstract
We recently developed a Janus kinase 2 (Jak2) small-molecule inhibitor called G6 and found that it inhibits Jak2-V617F-mediated pathologic cell growth in vitro, ex vivo, and in vivo. However, its ability to inhibit Jak2-V617F-mediated myeloproliferative neoplasia, with particular emphasis in the bone marrow, has not previously been examined. Here, we investigated the efficacy of G6 in a transgenic mouse model of Jak2-V617F-mediated myeloproliferative neoplasia. We found that G6 provided therapeutic benefit to the peripheral blood as determined by elimination of leukocytosis, thrombocytosis, and erythrocytosis. G6 normalized the pathologically high plasma concentrations of interleukin 6 (IL-6). In the liver, G6 eliminated Jak2-V617F-driven extramedullary hematopoiesis. With respect to the spleen, G6 significantly reduced both the splenomegaly and megakaryocytic hyperplasia. In the critically important bone marrow, G6 normalized the pathologically high levels of phospho-Jak2 and phospho-signal transducer and activator of transcription 5 (STAT5). It significantly reduced the megakaryocytic hyperplasia in the marrow and completely normalized the M/E ratio. Most importantly, G6 selectively reduced the mutant Jak2 burden by 67%on average, with virtual elimination of mutant Jak2 cells in one third of all treated mice. Lastly, clonogenic assays using marrow stem cells from the myeloproliferative neoplasm mice revealed a time-dependent elimination of the clonogenic growth potential of these cells by G6. Collectively, these data indicate that G6 exhibits exceptional efficacy in the peripheral blood, liver, spleen, and, most importantly, in the bone marrow, thereby raising the possibility that this compound may alter the natural history of Jak2-V617F-mediated myeloproliferative neoplasia.
Introduction
Polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are myeloproliferative neoplasms (MPNs) lacking the Philadelphia chromosome. They are caused by the transformation of an early hematopoietic stem cell resulting in abnormal hematopoiesis [1,2]. These disorders are categorized according to the syndromes caused by the terminally differentiated hematopoietic cells such as increased production of red blood cells (PV), platelets (ET), and neutrophils with concomitant fibrosis of the bone marrow tissue (PMF). Clinically, these diseases are characterized by pathologic peripheral blood syndromes such as leukocytosis, erythrocytosis, and thrombocytosis. These syndromes predispose patients to vascular diseases such as thrombosis, atherosclerosis, coronary heart disease, and cerebral ischemia [3–7]. In addition, patients with MPNs often have high levels of circulating inflammatory cytokines, such as interleukin 6 (IL-6), which have been associated with symptoms such as cachexia and listlessness [8–12]. Moreover, MPNs can often transform to acute myeloid leukemia [12]. Although these disorders can be fatal with a life expectancy that can be as few as 5 years [13], currently available treatments are limited.
The discovery of the Janus kinase 2 (Jak2)-V617F mutation in most patients with MPN spurred the development of small-molecule Jak2 inhibitors through molecularly targeted drug discovery. In preclinical experiments, many of these small molecules exhibited potent inhibition of Jak2-mediated pathologic cell growth. Some have subsequently progressed to clinical trials where they exhibited some benefit by reducing clinical symptoms associated with the MPN phenotype [14]. However, none of these inhibitors have been reported to be curative because they have little to no efficacy in the bone marrow and there is often a relapse of the clinical disease manifestations after withdrawal of treatment. Thus, current Jak2 inhibitors are largely palliative because they are unable to eradicate the Jak2 mutant burden in the bone marrow, which is the primary predilection site of the MPN disease pathogenesis.
Recently, we developed a stilbenoid small-molecule Jak2 inhibitor, G6, which exhibits potent inhibition of Jak2-V617F-mediated pathologic cell growth in vitro and ex vivo [15,16]. We subsequently reported that G6 has therapeutic potential in a NOD-SCID mouse model of Jak2-V617F-mediated hyperplasia because it eliminated the burden of tumorigenic Jak2-V617F cells from the host bone marrow [17]. However, its ability to inhibit Jak2-V617F-mediated myeloproliferative neoplasia, with particular emphasis in the bone marrow, is not known. Therefore, we hypothesized here that G6 would be efficacious against Jak2-V617F-mediated myeloproliferative neoplasia and would provide significant efficacy to a number of tissues including the bone marrow. To test this, we used a transgenic mouse model of Jak2-V617F-mediated myeloproliferative neoplasia and found that G6 treatment greatly alleviated the phenotype by providing significant therapeutic benefit to the peripheral blood, liver, spleen, and, most notably, the bone marrow. As such, G6 may alter the natural history of Jak2-V617F-mediated myeloproliferative neoplasia, thereby raising the possibility that this compound may have curative potential.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida. Animals were maintained in accordance with National Institutes of Health standards established in the Guidelines for the Care and Use of Experimental Animals. Transgenic male mice expressing the Jak2-V617F mutated enzyme in the hematopoietic system driven by the vav promoter and generated on a C57BL/6 background strain were used in these experiments; their creation and characterization have previously been described [18]. The transgenic mice were identified by polymerase chain reaction (PCR) using primers 5′-TACAACCTCAGTGGGACAAAGAAGAAC and 5′-CCATGCCAACTGTTTAGCAACTTCA, which cover a 594-bp region in the coding sequence of Jak2-V617F [18]. At 3 months of age, a baseline peripheral blood sample was obtained from each mouse through submandibular bleeding. The mice then received vehicle (n = 6) or 10-mg/kg per day (n = 6) injections of G6 for 28 days. Blood samples were subsequently obtained after 14 and 28 days of vehicle or G6 treatment.
Analysis of Peripheral Blood Cells
Blood samples were obtained (∼50 µl) through submandibular bleeding into tubes containing potassium salt of ethylenediamine tetra-acetic acid. Complete blood cell (CBC) counts were obtained using a HESKA Vet ABC-Diff Hematology analyzer (Heska, Loveland, CO). Blood samples were also smeared onto glass slides and stained using DipQuick (Jorgensen Laboratories, Inc, Loveland, CO).
IL-6 Analysis
Blood samples were obtained (∼50 µl) through submandibular bleeding into tubes containing potassium salt of ethylenediamine tetraacetic acid. Plasma was centrifuged at 10,000g for 10 minutes and then stored at -80°C for subsequent analysis. Plasma levels of IL-6 were measured using a commercially available mouse ELISA kit (Ray Biotech, Norcross, GA) according to the manufacturer's instructions.
Histologic Analysis
Tissues (liver, spleen, and femurs) were fixed overnight in buffered formalin. Femurs were decalcified for 16 hours. The tissues were subsequently dehydrated through a graded ethanol series, paraffin embedded, and sectioned (4 µm). The tissues were then stained with hematoxylin and eosin, and they were examined for normal histologic appearance as well as any lesions through standard light microscopy by two independent veterinary pathologists who were blinded to treatment groups. Bone marrow analysis was done according to established guidelines [19]. The bone marrow was evaluated for necrosis, fibrosis, hemorrhage, overall cellularity, M/E ratios, and megakaryocytic counts. All cell counts were made at 600x high-power field (HPF). Spleen sections were examined for evidence of extramedullary hematopoiesis (EMH), and a quantitative analysis of megakaryocytes was made. Liver sections were examined for evidence of EMH at 40x.
Immunohistochemistry
Immunohistochemistry (IHC) on bone marrow samples was carried out as previously described [17]. Briefly, 5-µm sections mounted on gelatin-coated slides were dewaxed in ethanol, rehydrated, and then blocked in 3% H2O2 followed by 5% normal goat serum. Sections were exposed to either anti-phospho-Jak2 (Ab32101; Abcam, Cambridge, MA) or anti-phospho-signal transducer and activator of transcription 5 (STAT5, Ab32364; Abcam) primary antibody overnight at 4°C. The sections were washed and then treated with the biotinylated secondary antibody. After secondary antibody incubation, the samples were washed, exposed to the avidin-peroxidase reagent (Vectastain Elite; Vector Laboratories, Burlingame, CA), and reacted with diaminobenzidine to produce a brown reaction product. The sections were dehydrated in ethanol, mounted with Permount (Fisher Scientific, Pittsburgh, PA), and observed by light microscopy.
Determination of Jak2-V617F Allele Burden in the Bone Marrow
RNA was isolated from bone marrow using the RNeasy Mini Kit (QIAGEN, Valencia, CA), and DNA contamination was removed using the RNase-free DNase set (QIAGEN). Complementary DNA (cDNA) was synthesized using the high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) from 2 µg of RNA. Real-time PCR was performed in a Multicolor Real-time PCR Detection System using TaqMan gene expression assays (Applied Biosystems). PCR amplifications were performed in duplicate for human Jak2 (Hs01078124_m1) and mouse Jak2 (Mm00434577_m1) along with parallel measurements of mouse β-actin cDNA as an internal control. The copy number of the human Jak2 sequence relative to β-actin was calculated using a standard curve technique. The allele burden was computed by calculating the ratio of human Jak2-V617F to endogenous mouse Jak2-wild-type (WT) transcripts.
Clonogenic Assay
Bone marrow cells from Jak2-V617F transgenic mice were harvested from the femur through aspiration. After 2 days of culture ex vivo, the cells were exposed to 25 µM of G6 for the indicated periods and then washed extensively. Duplicate cultures were then plated in MethoCult medium (STEMCELL Technologies, Vancouver, Canada) lacking erythropoietin and thrombopoietin. Five days later, the number of granulocyte-macrophage colony-forming units and erythroid burst-forming units were counted and plotted as a function of treatment condition.
Statistical Analysis
All results were expressed asmean ± SEM. Statistical comparisons were performed by Student's t test. Changes in peripheral blood cell counts were analyzed by a repeated-measures analysis of variance followed by Bonferroni and Student-Newman-Keuls post hoc test for multiple comparisons. P < .05 (2-tailed) were considered statistically significant.
Results
G6 Provides Therapeutic Benefit in the Peripheral Blood of Jak2-V617F MPN Mice
Here, we used a previously established transgenic mouse model of Jak2-V617F-mediated myeloproliferative neoplasia [18]. These mice express the human Jak2-V617F cDNA under the control of the hematopoietic promoter, vav. These mice exhibit a number of phenotypes that recapitulate those observed in human MPN including constitutive Jak/STAT signaling, myeloid neoplasia, leukocytosis, thrombocytosis, erythrocytosis, and splenomegaly. CBC counts were first performed on 3-month-old male mice to confirm the MPN phenotype. Mice fully manifesting the MPN phenotype were randomly assigned to one of two groups (n = 6 per group) and then began receiving either 10 mg/kg per day of G6 or vehicle control solution. CBCs were subsequently collected on days 14 and 28 of treatment through mandibular vein bleeding, and after 28 days of treatment, all the mice were euthanized and prepared for analysis.
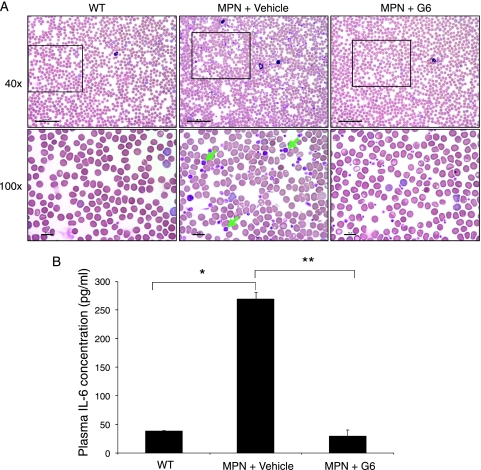
The CBC values were first examined by a repeated-measures analysis of variance to determine whether there were any significant differences between treatment conditions (Table 1). Values from nontransgenic control mice are also shown for comparison. We found that G6 provided significant therapeutic improvement in the red blood cell count, hematocrit, mean corpuscular volume, red blood cell distribution width, hemoglobin, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, mean platelet volume, platelet distribution width, white blood cell count, neutrophil count, lymphocyte count, and monocyte count. To confirm these observations using an alternate means, terminal peripheral blood smears from all mice were examined in parallel. In addition to validating many of the CBC values, examination of the slides revealed an increased appearance of large platelets in the vehicle-treated MPN mice that were lacking in the nontransgenic control mice (WT) and G6-treated mice (Figure 1A).
Table 1.
Summary of Peripheral Blood Analyses of Nontransgenic and Vehicle- or G6-Treated Jak2-V617F Transgenic Mice.
| RBC (M/µl) | HCT (%) | MCV (fl) | RDW (%) | |||||
| Nontransgenic | 9.7 ± 0.1 | 42.4 ± 0.2 | 43.7 ± 0.3 | 18.3 ± 0.5 | ||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 |
| Baseline | 13.5 ± 0.4 | 13.7 ± 0.7 | 53.0 ± 1.3 | 53.5 ± 1.9 | 39.3 ± 0.3 | 39.3 ± 0.5 | 20.4 ± 0.3 | 20.5 ± 0.2 |
| Week 2 | 13.7 ± 0.6 | 10.7 ± 1.3*,† | 52.2 ± 2.0 | 42.1 ± 4.8*,† | 38.3 ± 0.5 | 39.6 ± 0.9 | 20.5 ± 0.3 | 19.5 ± 0.5 |
| Week 4 | 12.6 ± 0.5 | 10.3 ± 0.5*,† | 47.2 ± 1.4 | 43.0 ± 0.5† | 37.2 ± 0.6 | 42.2 ± 1.4*,† | 20.2 ± 0.4 | 18.3 ± 0.4*,† |
| HB (g/dL) | MCH (pg) | MCHC (g/dL) | ||||||
| Nontransgeinc | 11.9 ± 0.1 | 12.3 ± 0.2 | 28.1 ± 0.4 | |||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | ||
| Baseline | 14.3 ± 0.3 | 14.4 ± 0.6 | 10.6 ± 0.1 | 10.6 ± 0.1 | 27.1 ± 0.2 | 26.9 ± 0.3 | ||
| Week 2 | 15.5 ± 0.7 | 12.5 ± 1.4* | 11.3 ± 0.1 | 11.8 ± 0.2† | 29.8 ± 0.2† | 29.8 ± 0.3† | ||
| Week 4 | 13.9 ± 0.4 | 12.1 ± 0.2 | 11.1 ± 0.2 | 11.9 ± 0.4*,† | 29.7 ± 0.2 | 28.2 ± 0.3*,† | ||
| PLT (K/µl) | MPV (fl) | PDW (%) | ||||||
| Nontransgenic | 1198 ± 151 | 4.0 ± 0.4 | 30.2 ± 0.6 | |||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | ||
| Baseline | 2879 ± 145 | 2802 ± 234 | 5.3 ± 0.1 | 5.3 ± 0.1 | 28.7 ± 0.4 | 28.9 ± 0.4 | ||
| Week 2 | 2732 ± 465 | 1708 ± 259*,† | 5.4 ± 0.1 | 5.0 ± 0.2* | 25.9 ± 1.2 | 28.1 ± 1.5 | ||
| Week 4 | 3311 ± 313 | 1180 ± 253*,† | 5.4 ± 0.2 | 4.1 ± 0.3*,† | 27.2 ± 1.4 | 31.7 ± 1.9* | ||
| WBC (K/µl) | NE (K/µl) | NE (%) | ||||||
| Nontransgenic | 9.1 ± 0.9 | 2.4 ± 0.3 | 23.4 ± 2.3 | |||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | ||
| Baseline | 16.8 ± 1.6 | 14.8 ± 0.9 | 5.3 ± 0.5 | 5.8 ± 0.9 | 34.1 ± 2.6 | 34.9 ± 2.9 | ||
| Week 2 | 19.8 ± 2.4 | 12.0 ± 3.1* | 6.2 ± 0.9 | 4.3 ± 1.1 | 31.3 ± 2.2 | 30.0 ± 2.6 | ||
| Week 4 | 21.3 ± 2.6 | 10.3 ± 0.9* | 7.5 ± 1.1 | 2.7 ± 0.3*,† | 35.1 ± 1.1 | 27.3 ± 3.6 | ||
| LY (K/µl) | LY (%) | MO (K/µl) | MO (%) | |||||
| Nontransgenic | 6.4 ± 0.7 | 62.4 ± 1.5 | 0.8 ± 0.1 | 7.9 ± 0.9 | ||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 |
| Baseline | 8.2 ± 0.6 | 8.1 ± 0.9 | 51.4 ± 1.8 | 51.7 ± 3.4 | 1.7 ± 0.2 | 1.4 ± 0.2 | 11.0 ± 1.1 | 9.2 ± 0.6 |
| Week 2 | 10.3 ± 1.3 | 6.7 ± 1.5* | 52.1 ± 2.9 | 57.6 ± 2.8 | 2.4 ± 0.4 | 1.3 ± 0.3* | 12.2 ± 1.2 | 9.3 ± 1.1* |
| Week 4 | 10.8 ± 1.3 | 6.1 ± 0.8* | 50.7 ± 0.9 | 59.0 ± 3.8* | 2.0 ± 0.3 | 0.8 ± 0.1* | 9.13 ± 0.3 | 8.0 ± 0.8 |
| EO (K/µl) | EO (%) | BA (K/µl) | BA (%) | |||||
| Nontransgenic | 0.6 ± 0.2 | 4.7 ± 0.6 | 0.1 ± 0.0 | 0.7 ± 0.4 | ||||
| Jak2-V617F | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 | Vehicle | G6 |
| Baseline | 0.6 ± 0.1 | 0.5 ± 0.1 | 3.4 ± 0.8 | 3.9 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.1 |
| Week 2 | 0.8 ± 0.1 | 0.5 ± 0.2 | 4.0 ± 0.6 | 2.6 ± 0.7 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.1 |
| Week 4 | 0.9 ± 0.2 | 0.5 ± 0.1 | 4.5 ± 1.0 | 4.7 ± 0.6 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.2 | 0.1 ± 0.0 |
BA indicates basophil; EO, eosinophil; HB, hemoglobin; HCT, hematocrit; LY, lymphocyte; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MO, monocyte; MPV, mean platelet volume; NE, neutrophil; PDW, platelet distribution width; PLT, platelet; RBC, red blood cell; RDW, red blood cell distribution width; WBC, white blood cell.
P < .05 in reference to vehicle treated.
P < .05 in reference to baseline.
Figure 1.
G6 provides therapeutic benefit in the peripheral blood of Jak2-V617F MPN mice. (A) Representative peripheral blood smears from wild-type and Jak2-V617F MPN mice at low (upper panel) and high magnification (lower panel). The giant platelets are indicated by green arrows. (B) Plasma IL-6 concentrations. *P = 2.62 x 10-6, **P = 6.37 x 10-8.
A previous work has shown that MPN patients have significantly elevated levels of inflammatory cytokines in the peripheral blood, such as IL-6 [11]. To determine whether this was also the case in our MPN mice and whether G6 could correct this, terminal plasma IL-6 concentrations from the mice were determined. When compared with nontransgenic WT controls, we found that the plasma concentrations of IL-6 were markedly elevated in the vehicle-treated MPN mice, and this was completely normalized with G6 treatment (Figure 1B).
We next wanted to determine whether the efficacious parameters observed in the peripheral blood of G6-treated mice correlated with the presence of the drug in the plasma. For this, mass spectrometry analysis was performed on the terminal plasma samples and the levels of G6 were determined (Table 2). We found that G6 was detectable in plasma samples from the mice that received the drug but not in the plasma taken from mice that received vehicle control solution.
Table 2.
Mass Spectrometry Results Showing Plasma Concentrations of G6 at Euthanasia of Jak2-V617F Transgenic Mice.
| Animal ID | Plasma* (µg/ml) |
| Vehicle Treated | |
| 1 | <0 |
| 2 | <0 |
| 3 | <0 |
| 4 | <0 |
| 5 | <0 |
| 6 | <0 |
| G6 Treated | |
| 1 | 48.6 |
| 2 | 0.155 |
| 3 | 0.036 |
| 4 | 0.025 |
| 5 | 0.030 |
| 6 | ND |
Standard curve was quadratic (1/x2), r = 0.9902 with range from 0.005 to 5.00 µg/ml. Shown is the average of replicate injections. Calculations were done using Analyst Software 1.4.2 (AB Sciex, Foster City, CA). Less than zero (<0) indicates peak quantities below the 0 value of the standard curve. ND indicates that value was not determined because of insufficient sample quantity at the time of analysis.
Altogether, the data in Tables 1 and 2 as well as those in Figure 1 indicate that G6 corrected, or in some cases completely normalized, numerous cell compartments within the peripheral blood in a mouse model of Jak2-V617F-mediated myeloproliferative neoplasia. Furthermore, G6 treatment completely normalized the levels of IL-6 in the plasma of these mice. Finally, the improvement observed in the G6-treated mice correlated positively with the presence of G6 in the plasma of these animals.
G6 Reduces EMH in Jak2-V617F MPN Mice
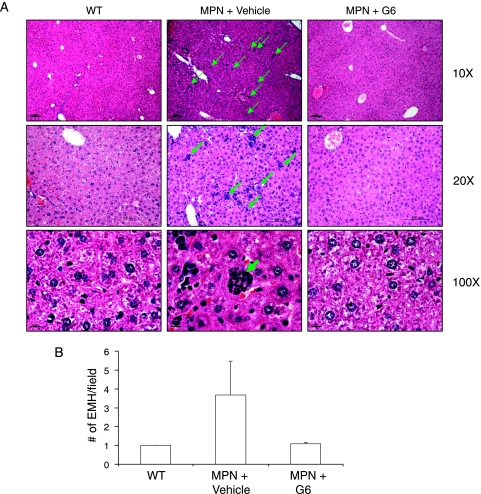
Another pathologic finding observed in the Jak2-V617F MPN mice is an abnormally high degree of EMH. Constitutive expression of the Jak2-V617F transgene drives hematopoiesis in a number of tissues including the liver. To determine whether G6 could reduce this Jak2-V617F-mediated pathogenesis, postmortem liver sections were examined by light microscopy and the levels of EMH were quantified. Figure 2A shows representative liver sections from all conditions and Figure 2B shows the quantitative values of EMH plotted as a function of condition. We found that when compared to wild-type mice, the MPN mice treated with vehicle control solution exhibited an increased level of EMH. However, this was corrected with G6 treatment. Overall, the data in Figure 2 indicate that G6 is efficacious in the liver given its ability to normalize the levels of EMH in Jak2-V617F MPN mice.
Figure 2.
G6 reduces EMH in Jak2-V617F MPN mice. (A) Liver sections showing EMH sites at the indicated magnifications (green arrows). (B) The average number of EMH sites plotted as a function of treatment group.
G6 Provides Therapeutic Benefit to the Spleen in Jak2-V617F MPN Mice
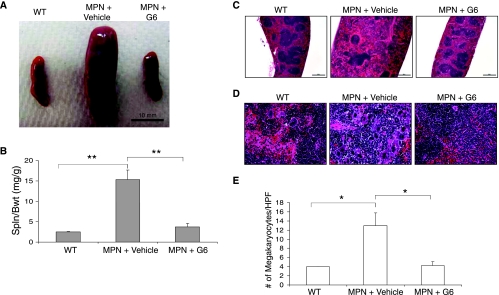
The Jak2-V617F mouse recapitulates many of the spleen pathologies observed in human MPN including splenomegaly and megakaryocytic hyperplasia. To determine the efficacy of G6 in the spleen, several parameters were measured. First, at euthanasia, spleens were immediately removed from the mice and gross spleen weights were determined. Figure 3A shows representative spleens from each condition and Figure 3B shows the quantitative spleen weight-to-body weight ratios for each group.We found that after 28 days of G6 treatment, the spleen size, which was significantly increased in Jak2-V617F MPN mice, was significantly reduced with G6 treatment.
Figure 3.
G6 provides therapeutic benefit to the spleen of Jak2-V617F MPN mice. (A) Representative spleens from each condition. (B) Spleen weight-to-body weight ratios graphed as a function of treatment group. (C) Histologic sections through the spleen at low magnification showing splenic architecture. (D) Histologic sections through the spleen at high magnification showing megakaryocytic hyperplasia in Jak2-V617F MPN mice and elimination of it with G6 treatment. (E) Number of megakaryocytes per HPF plotted as a function of treatment group. **P < .001 and *P < .05 versus vehicle treated.
Histologic sections through the spleen revealed a disorganized splenic architecture in the Jak2-V617F MPN mice treated with vehicle control solution and this was alleviated with G6 treatment (Figure 3C). Examination of the sections at higher power revealed a marked megakaryocytic hyperplasia in the Jak2-V617F MPN mice, which was absent in the G6-treated transgenic mice (Figure 3D). To quantitate this hyperplasia, the average numbers of megakaryocytes per HPF were plotted as a function of condition (Figure 3E). We found that G6 treatment returned the number of megakaryocytes to normal, nontransgenic levels. Collectively, the data in Figure 3 indicate that, in a mouse model of Jak2-V617F-mediated myeloproliferative neoplasia, G6 provides significant therapeutic benefit to the spleen as determined by a significantly reduced spleen weight-to-body weight ratio, a restoration of normal splenic architecture, and an elimination of megakaryocytic hyperplasia.
G6 Provides Therapeutic Benefit to the Bone Marrow in Jak2-V617F MPN Mice by Alleviating Megakaryocytic and Myeloid Hyperplasia
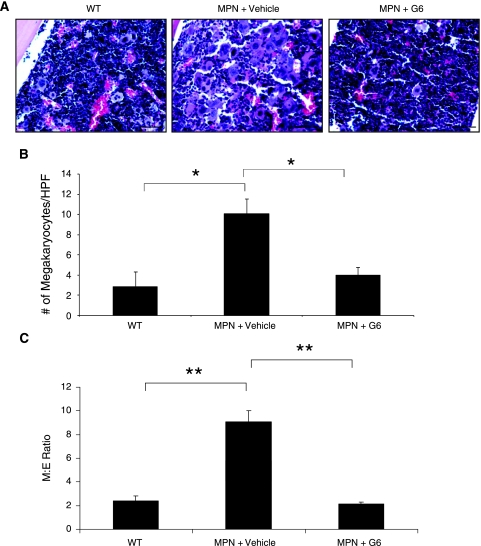
The ability of a drug to provide therapeutic benefit in the bone marrow of MPN patients is critically important because this is the site of initiation of disease pathogenesis. In addition, this has been the point of disappointment for current generation Jak2 inhibitors. To assess the efficacy of G6 in the bone marrow, we first examined marrow sections. Figure 4A shows representative histologic sections from each group. We found that when compared to nontransgenic controls, the vehicle-treated Jak2-V617FMPN mice had a hypercellular marrow because of the myeloid and megakaryocytic hyperplasia, and this corresponded with the increased platelet counts observed in the peripheral blood (Table 1). However, G6 seemed to restore the marrow to nondiseased conditions. To confirm this quantitatively, the average number of megakaryocytes per HPF was determined from all animals and plotted as a function of treatment group (Figure 4B). We found that, in the Jak2-V617F MPN mice, G6 significantly reduced the number of megakaryocytes in the marrow to near WT levels.
Figure 4.
G6 provides therapeutic benefit to the bone marrow of Jak2-V617F MPN mice by alleviating megakaryocytic and myeloid hyperplasia. (A) Representative bone marrow sections showing the effect of G6 on megakaryocytic hyperplasia. (B) Average number of megakaryocytes per HPF plotted as a function of treatment group. (C) M/E ratios plotted as a function of treatment group. *P < .05 and **P < .001 versus vehicle treated.
It is well accepted that an altered M/E ratio is often one of the characteristic signs of Jak2-V617F-mediated myeloproliferative neoplasia. To determine whether G6 could correct the abnormally high M/E ratio in the bone marrow of the Jak2-V617F MPN mice, we carried out a quantitative analysis of the myeloid and erythroid cells on the marrow sections (Figure 4C). We found that, when compared to the WT mice, there was a robust increase in the M/E ratio in the vehicle-treated Jak2-V617F MPN mice that was driven by myeloid neoplasia. However, G6 treatment returned the M/E ratio to wild-type levels. Altogether, the data in Figure 4 demonstrate that G6 has a marked therapeutic benefit in the bone marrow. Specifically, it reduced the pathologic increase in megakaryocytic and myeloid hyperplasia in the marrow, as a consequence of which, the M/E ratio was completely normalized.
G6 Provides Therapeutic Benefit to the Bone Marrow in Jak2-V617F MPN Mice by Reducing the Pathologic Levels of Phospho-Jak2 and Phospho-STAT5
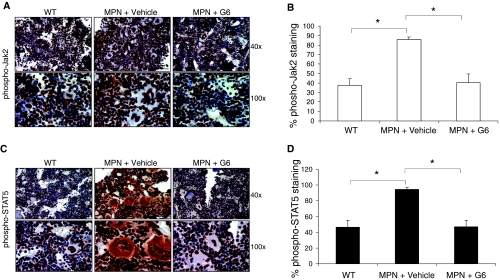
To determine whether the therapeutic benefit observed in the marrow with G6 treatment is a result of reduced Jak/STAT signaling, we carried out anti-phospho-Jak2 and anti-phospho-STAT5 IHC staining of the bone marrow sections. Figure 5A shows representative images of the anti-phospho-Jak2 IHC at two magnifications. Qualitatively, we found that bone marrow sections obtained from the Jak2-V617F MPN mice treated with vehicle control had a robust increase in phospho-Jak2 levels when compared to the wild-type mice. However, the phospho-Jak2 staining was reduced to wild-type levels in the Jak2-V617F MPN mice that were treated with G6. These qualitative observations were supported quantitatively when the numbers of anti-phospho-Jak2-stained cells were counted and plotted as a function of treatment group (Figure 5B).
Figure 5.
G6 reduces activation of Jak2 and STAT5 in Jak2-V617F MPN mice. (A) Representative anti phospho-Jak2 IHC in bone marrow sections from the indicated treatment groups shown at 40x and 100x. (B) Quantification of the anti-phospho-Jak2 staining plotted as a function of treatment group. (C) Representative anti-phospho-STAT5 IHC in bone marrow sections at 40x and 100x. (D) Quantification of the anti-phospho-STAT5 staining plotted as a function of treatment group. *P < .05 versus vehicle-treated MPN mice.
The therapeutic effect within the bone marrow was further verified by the ability of G6 to reduce the levels of the proliferative marker, phospho-STAT5. STAT5 is an immediate downstream target of Jak2 and is hyperphosphorylated in Jak2-V617F expressing cells [16,17]. Figure 5C shows representative bone marrow pictures of the anti-phospho-STAT5 IHC-stained sections, and Figure 5D shows the quantification of all sections plotted as a function of treatment group. We similarly observed that when compared to wild-type mice, the Jak2-V617F MPN mice that were given vehicle control solution had pathologically high levels of phospho-STAT5. Again, however, G6 fully corrected this pathogenesis by returning the phospho-STAT5 levels to nontransgenic levels. In summary, the data in Figure 5 show that G6 has striking therapeutic efficacy in the bone marrow. Specifically, the Jak2-V617F MPN mice have significantly elevated levels of phospho-Jak2 and its proliferative downstream target, phospho-STAT5.However, G6 treatment normalizes these values to nondiseased levels.
G6 Provides Therapeutic Benefit to the Bone Marrow in Jak2-V617F MPN Mice by Significantly Reducing the Mutant Jak2 Burden
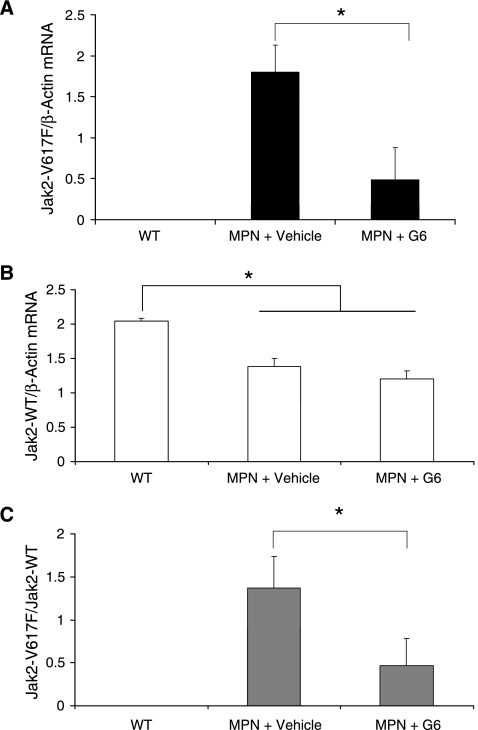
The greatest obstacle to current Jak2 inhibitors is the inability of these drugs to eliminate Jak2-V617F mutant clones from the bone marrow. To determine the efficacy of this parameter in our MPN model, we measured the mRNA levels of both the human Jak2-V617F mutant mRNA transcripts and endogenous mouse Jak2-WT transcripts. We found that whereas G6 treatment significantly reduced the levels of the mutant V617F transcripts (Figure 6A), endogenous wild-type transcripts were only slightly reduced by G6 treatment, and this change was not significant (Figure 6B). Furthermore, we found that the ratio of these two parameters (i.e., the mutant burden within the marrow) was reduced, on average, by ∼67% with G6 treatment when compared to Jak2-V617F MPN mice that received vehicle control injections (Figure 6C). Furthermore, one third of the G6-treated mice exhibited virtual elimination of all Jak2-V617F transcripts from the marrow. Thus, the data in Figure 6 demonstrate that G6 significantly reduces the burden of Jak2-V617F mutant cells from the bone marrow in a model of Jak2-V617F-mediated myeloproliferative neoplasia.
Figure 6.
G6 significantly reduces mutant burden in bone marrow of Jak2-V617F MPN mice. (A) The amount of Jak2-V617F mutant transcripts in the bone marrow of the indicated groups. (B) Amount of endogenous mouse Jak2-WT transcripts from the same groups. (C) The Jak2-V617F mutant burden (the ratio of mutant to wild type) transcripts plotted as a function of treatment group. *P < .05.
G6 Prevents Jak2-V617F-Mediated Clonogenic Growth
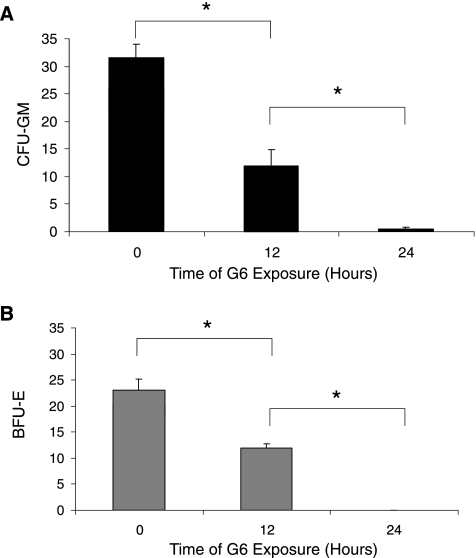
Lastly, we wanted to assess whether G6 can stop the clonogenic growth potential of Jak2-V617F transformed cells because this would be crucial to any therapeutic possibility. For this, cells were harvested from the bone marrow of Jak2-V617F MPN mice and cultured ex vivo in the presence of 25 µM of G6 for 0, 12, or 24 hours. The cells were then washed extensively to remove drug and plated in medium lacking EPO and TPO. Five days later, the numbers of granulocyte-macrophage colony-forming units (Figure 7A) and erythroid burst-forming units (Figure 7B) were counted and plotted as a function of time. We found that G6 significantly suppressed the clonogenic growth potential of Jak2-V617F cells in a time-dependent manner; 12 hours of drug exposure resulted in ∼50% growth inhibition and 24 hours of drug exposure virtually eliminated all subsequent clonogenic growth. As such, these results indicate that brief exposures of Jak2-V617F cells to G6 prevent subsequent clonogenic growth.
Figure 7.
G6 treatment eliminates Jak2-V617F-mediated clonogenic growth potential. Bone marrow cells from Jak2-V617F MPN mice were exposed to G6 for the indicated periods. Clonogenic growth potential as assessed by the subsequent number of CFU-GM (A) and BFU-E (B) was plotted as a function of time. *P < .05.
Discussion
Since the discovery of the Jak2-V617F mutation in most patients with MPN, a number of molecularly targeted Jak2 inhibitors have been developed. However, the clinical benefits provided by these inhibitors so far have largely been palliative due to the inability of many to significantly reduce malignant clones in the bone marrow. Thus, the identification of Jak2 inhibitors that can provide significant bone marrow efficacy is highly desirable. We present here preclinical data demonstrating that the Jak2 inhibitor, G6, provides exceptional therapeutic efficacy against Jak2-V617F-mediated myeloproliferative neoplasia. The drug significantly reduced the Jak2-V617F allele burden in the bone marrow. This reduction of the mutant burden in the marrow was concomitant with the elimination of myeloid hyperplasia, correction of the M/E ratio, normalization of the levels of phospho-Jak2 and phospho-STAT5, and an elimination of the Jak2-V617F-dependent clonogenic growth potential. Overall, these results indicate that G6 is highly efficacious in the bone marrow.
In addition to providing exceptional bone marrow efficacy, G6 also corrected virtually every pathologic MPN indicator in the peripheral blood including the red blood cell count, hematocrit, mean corpuscular volume, red blood cell distribution width, hemoglobin, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, mean platelet volume, platelet distribution width, white blood cell count, neutrophil count, lymphocyte count, monocyte count, and the levels of IL-6 (Table 1 and Figure 1). It also eliminated the EMH in the liver that was being driven by the Jak2-V617F transgene (Figure 2). Lastly, within the spleen, G6 alleviated splenomegaly, significantly reduced the megakaryocytic hyperplasia, and restored the normal architecture to this tissue (Figures 3 and 4). As such, G6 significantly ameliorates or eliminates the pathogenesis of nearly every indicator of the MPN phenotype. Whether these observed changes result in increased animal survival is a question that is currently being addressed experimentally.
We recently reported that G6 eliminates the Jak2-V617F mutant burden from the bone marrow using a HEL cell xenograft model of Jak2-V617F-mediated hyperplasia [17]. This xenograft model has the advantage of closely replicating some aspects of human disease including a low tumor burden in the context of the endogenous marrow niche. Limitations of this model, however, include the overall lack of the associated MPN phenotype including myeloid hyperplasia and an increased M/E ratio. Our work here is significant in that we show that G6 is also highly effective in the bone marrow using a mouse model of Jak2-V617F-mediated human myeloproliferative neoplasia. Furthermore, G6 alleviates a number of MPN phenotypes in the peripheral blood, spleen, and liver, which are not found in the xenograft model. As such, the reduction/elimination of the mixed PV/ET phenotype from these Jak2-V617F mice suggests that G6 may have therapeutic potential for the treatment of MPN.
Potential Jak2 small-molecule inhibitor therapy is perhaps most relevant to PMF and less so for PV/ET because PV/ET patients can manage their disease relatively well with cytoreductive therapies. However, this is not to suggest that the results contained herein are insignificant. To the contrary, in a recent review article, it was noted that current limitations to any Jak2 inhibitor therapy include their potential costs and unknown adverse effects in the long term [20]. Therefore, it was argued that for diseases that are managed relatively well using conventional therapies (i.e., PV and ET), there must be clear benefit over the status quo if Jak2 inhibitors are to be used instead. Our preclinical data here, in the form of repeated measures of bone marrow efficacy in a mouse model of PV/ET, are indicative of demonstrable efficacy over the status quo, and hence, G6 could be relevant to PV and ET. Also, Jak2 inhibitors are being tested in clinical studies for use in a number of indicators including PMF, PV, ET, multiple myeloma, acute leukemia, rheumatoid arthritis, psoriasis, and others. Therefore, indicators for possible Jak2 therapy are both numerous and diverse. Finally, in a separate work, we have tested G6 in a mouse model of Jak2-mediated, PMF. We found that, within the bone marrow, the drug significantly reduced pathogenic Jak/STAT signaling, significantly reduced the Jak2 mutant burden, significantly improved the M/E ratio, and significantly reversed the myelofibrosis (unpublished observations). As such, these data indicate that G6 is also efficacious in PMF.
Given the causative role of Jak2 kinase in human disorders, Jak2 small molecules may have significant therapeutic potential. Accordingly, within the past several years, a number of groups have developed Jak2 inhibitors. One problem with virtually all these compounds, however, is that, although they demonstrated excellent efficacy in vitro, they have little to no efficacy in vivo [21–24]. This critical inability to reduce the mutant Jak2 burden in the bone marrow was the focus of a recent and sobering review describing current obstacles and limitations in this area of research [20]. Our work here is significant because, in addition to having in vitro efficacy [15,16], we now show that G6 has exceptional in vivo efficacy using a second independent model of Jak2-V617F-mediated pathogenesis.
Perhaps the single greatest problem with current-generation Jak2 inhibitors is that they are largely palliative and not curative in any way [14,20]. In other words, although they alleviate a number of MPN-associated symptoms, they do not alter the burden of mutant Jak2 clones in the bone marrow and, hence, cannot change the natural progression of the disease. The efficacy observed in the bone marrow with G6 treatment (Figures 4–6) suggests that the drug may have curative potential. Furthermore, our observation that brief exposures of Jak2-V617F cells to G6 completely eliminate all subsequent Jak2-V617F-dependent clonogenic growth (Figure 7) suggests that the bone marrow efficacy may be permanent. Studies that will determine this experimentally are currently in progress.
G6 was identified using structure-based virtual screening [15]. It belongs to a group of diarylethene compounds known as stilbenes. Previously, we demonstrated that the stilbenoid core element of G6 is essential for its therapeutic potential [16]. Stilbenes have been reported to exhibit therapeutic efficacy in a wide variety of disease conditions including cancer, stress, cardiovascular, and viral diseases [25–30]. Stilbenes have also been reported to possess tyrosine kinase inhibitory activity [29,30]. Given that stilbenes such as resveratrol and piceatannol are naturally occurring [25–27], they are likely to have limited adverse effects in vivo. In the current study, we did not observe any apparent gross morphology or histologic adverse effects associated with G6 treatment, suggesting that it may be suitable and safe for administration to humans with MPN.
In conclusion, the results in this study demonstrate that the small-molecule Jak2 inhibitor, G6, provides therapeutic benefit to the peripheral blood, liver, spleen, and, most notably, the bone marrow using a mouse model of Jak2-V617F-mediated myeloproliferative neoplasia. Given that the bone marrow is the predilection site for MPN disease pathogenesis, this work is significant in that G6 may be a promising candidate for progression into clinical trials for the treatment of MPN.
Acknowledgments
The authors thank the University of Florida Pathology Core Laboratory for their assistance with sample preparation.
Abbreviations
- Jak2
Janus kinase 2
- STAT
signal transducer and activator of transcription
- PV
polycythemia vera
- ET
essential thrombocythemia
- PMF
primary myelofibrosis
- MPN
myeloproliferative neoplasm
- IL-6
interleukin 6
Footnotes
This work was supported by National Institutes of Health Award R01-HL67277, an American Heart Association Greater Southeast Affiliate Grant in Aid (0855361E), a University of Florida Opportunity Fund Award, and a University of Florida/Moffitt Cancer Center Collaborative Initiative Award. C.R.C. was supported by the Leukemia Lymphoma Society and National Institutes of Health Award K08-DK067359.
References
- 1.Talarico L, Wolf BC, Kumar A, Weintraub LR. Reversal of bone marrow fibrosis and subsequent development of polycythemia in patients with myeloproliferative disorders. Am J Hematol. 1989;30:248–253. doi: 10.1002/ajh.2830300411. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, Marilus R, Villegas A, Tognoni G, Barbui T. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, van der Walt JD, Reilly JT, Grigg AP, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 5.Cervantes F, Alvarez-Larran A, Arellano-Rodrigo E, Granell M, Domingo A, Montserrat E. Frequency and risk factors for thrombosis in idiopathic myelofibrosis: analysis in a series of 155 patients from a single institution. Leukemia. 2006;20:55–60. doi: 10.1038/sj.leu.2404048. [DOI] [PubMed] [Google Scholar]
- 6.De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, Mico C, Tieghi A, Cacciola RR, Santoro C, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372–380. doi: 10.3324/haematol.12053. [DOI] [PubMed] [Google Scholar]
- 7.Kiladjian JJ, Cervantes F, Leebeek FW, Marzac C, Cassinat B, Chevret S, Cazals-Hatem D, Plessier A, Garcia-Pagan JC, Darwish Murad S, et al. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood. 2008;111:4922–4929. doi: 10.1182/blood-2007-11-125328. [DOI] [PubMed] [Google Scholar]
- 8.Wang JC, Chang TH, Goldberg A, Novetsky AD, Lichter S, Lipton J. Quantitative analysis of growth factor production in the mechanism of fibrosis in agnogenic myeloid metaplasia. Exp Hematol. 2006;34:1617–1623. doi: 10.1016/j.exphem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. [PubMed] [Google Scholar]
- 10.Xu M, Bruno E, Chao J, Huang S, Finazzi G, Fruchtman SM, Popat U, Prchal JT, Barosi G, Hoffman R. Constitutive mobilization of CD34+ cells into the peripheral blood in idiopathic myelofibrosis may be due to the action of a number of proteases. Blood. 2005;105:4508–4515. doi: 10.1182/blood-2004-08-3238. [DOI] [PubMed] [Google Scholar]
- 11.Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J, Bourantas KL. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol. 2005;130:709–715. doi: 10.1111/j.1365-2141.2005.05674.x. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 13.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Santos FP, Verstovsek S. JAK2 inhibitors: what's the true therapeutic potential? Blood Rev. 2011;25:53–63. doi: 10.1016/j.blre.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiss R, Polgar T, Kirabo A, Sayyah J, Figueroa NC, List AF, Sokol L, Zuckerman KS, Gali M, Bisht KS, et al. Identification of a novel inhibitor of JAK2 tyrosine kinase by structure-based virtual screening. Bioorg Med Chem Lett. 2009;19:3598–3601. doi: 10.1016/j.bmcl.2009.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder A, Govindasamy L, Magis A, Kiss R, Polgar T, Baskin R, Allan RW, Agbandje-McKenna M, Reuther GW, Keseru GM, et al. Structure-function correlation of G6, a novel small molecule inhibitor of Jak2: indispensability of the stilbenoid core. J Biol Chem. 2010;285:31399–31407. doi: 10.1074/jbc.M110.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirabo A, Embury J, Kiss R, Polgar T, Gali M, Majumder A, Bisht KS, Cogle CR, Keseru GM, Sayeski PP. The stilbenoid tyrosine kinase inhibitor, G6, suppresses Jak2-V617F-mediated human pathological cell growth in vitro and in vivo. J Biol Chem. 2011;286:4280–4291. doi: 10.1074/jbc.M110.200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, Li Q, Fu X, Xu M, Zhao ZJ. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmore SA. Enhanced histopathology of the bone marrow. Toxicol Pathol. 2006;34:666–686. doi: 10.1080/01926230600939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood Rev. 2011;25:229–237. doi: 10.1016/j.blre.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G, Kennedy D, Estrov Z, Cortes J, Verstovsek S. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115:1131–1136. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppikar P, Abdel-Wahab O, Hedvat C, Marubayashi S, Patel J, Goel A, Kucine N, Gardner JR, Combs AP, Vaddi K, et al. Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood. 2010;115:2919–2927. doi: 10.1182/blood-2009-04-218842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 24.Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM, Druker BJ, Burns CJ, Fantino E, Deininger MW. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115:5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrosa M, Tomas-Barberan FA, Espin JC. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E, and B1 in human SK-Mel-28 melanoma cells. J Agric Food Chem. 2003;51:4576–4584. doi: 10.1021/jf030073c. [DOI] [PubMed] [Google Scholar]
- 26.Le Corre L, Chalabi N, Delort L, Bignon YJ, Bernard-Gallon DJ. Resveratrol and breast cancer chemoprevention: molecular mechanisms. Mol Nutr Food Res. 2005;49:462–471. doi: 10.1002/mnfr.200400094. [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y. New anticancer agents: in vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo. 2005;19:37–60. [PubMed] [Google Scholar]
- 28.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 29.Geahlen RL, McLaughlin JL. Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun. 1989;165:241–245. doi: 10.1016/0006-291x(89)91060-7. [DOI] [PubMed] [Google Scholar]
- 30.Swanson-Mungerson M, Ikeda M, Lev L, Longnecker R, Portis T. Identification of latent membrane protein 2A (LMP2A) specific targets for treatment and eradication of Epstein-Barr virus (EBV)-associated diseases. J Antimicrob Chemother. 2003;52:152–154. doi: 10.1093/jac/dkg306. [DOI] [PubMed] [Google Scholar]