Abstract
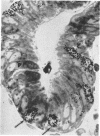
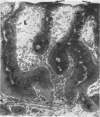
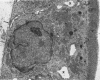
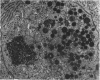
In vitro experiments of small intestinal mucosal function and metabolism utilizing excised tissue have been limited to a few hours by rapid epithelial cell necrosis which occurs with current incubation methods. We describe a method for culturing human mucosal biopsies for up to 24 hr employing organ culture methodology and demonstrate its potential application to studies of mucosal function. Peroral biopsies were placed in organ culture plates and maintained with modified Trowell's medium in 95% O2-5% CO2 at 37°C for 6-24 hr. To study cell proliferation, 2 μc of thymidine-3H was added per ml of medium. To study fat absorption, biopsies were exposed to micellar solutions of linolenic acid, monoolein, and taurodeoxycholate in Krebs-Ringer buffer for 15 min after culture in vitro for 24 hr. After 24 hr of culture, villi were shorter and wider. Cells in the lamina were reduced in number. Light and electron microscopic morphology of epithelial cells compared favorably to those of control biopsies except in occasional areas of partial necrosis. Some absorptive cells were more cuboidal and contained more lysosomes; many appeared entirely normal. Most crypt cells appeared normal; some contained increased glycogen and lysosomes. Mitoses were present, and labeled cells were abundant in crypts of biopsies after 6 hr of incubation with thymidine-3H-containing medium. By 24 hr. labeled cells migrated to the base of the villi. When biopsies cultured in vitro were subsequently exposed to micellar lipid, numerous lipid droplets were identified in the cytoplasm of absorptive cells. Thus, after 24 hr in vitro under these culture conditions, many human small intestinal epithelial cells maintain near normal morphology, epithelial cell proliferation proceeds, and fat absorption occurs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDBORG L. L., RUBIN G. E., QUINTON W. E. A multipurpose instrument for suction biopsy of the esophagus, stomach, small bowel, and colon. Gastroenterology. 1959 Jul;37(1):1–16. [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESCHNER E., LEWIS C. M., LIPKIN M. IN VITRO STUDY OF HUMAN RECTAL EPITHELIAL CELLS. I. ATYPICAL ZONE OF H3 THYMIDINE INCORPORATION IN MUCOSA OF MULTIPLE POLYPOSIS. J Clin Invest. 1963 Dec;42:1922–1928. doi: 10.1172/JCI104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins W. O., 3rd, Hijmans J. C., McCarty K. S. A light and electron microscopic study of duodenal epithelium of chick embryos cultured in the presence and absence of hydrocortisone. Gastroenterology. 1967 Oct;53(4):557–574. [PubMed] [Google Scholar]
- HARRER D. S., STERN B. K., REILLY R. W. REMOVAL AND DISSOCIATION OF EPITHELIAL CELLS FROM THE RODENT GASTROINTESTINAL TRACT. Nature. 1964 Jul 18;203:319–320. doi: 10.1038/203319a0. [DOI] [PubMed] [Google Scholar]
- Kopp W. L., Trier J. S., Mackenzie I. L., Donaldson R. M., Jr Antibodies to intestinal microvillous membranes. I. Characterization and morphologic localization. J Exp Med. 1968 Aug 1;128(2):357–373. doi: 10.1084/jem.128.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb L. M., Lisco H. In vitro uptake of tritiated thymidine by carcinoma of the human colon. Cancer Res. 1966 Apr;26(4):733–740. [PubMed] [Google Scholar]
- MACDONALD W. C., TRIER J. S., EVERETT N. B. CELL PROLIFERATION AND MIGRATION IN THE STOMACH, DUODENUM, AND RECTUM OF MAN: RADIOAUTOGRAPHIC STUDIES. Gastroenterology. 1964 Apr;46:405–417. [PubMed] [Google Scholar]
- MANAX W. G., BLOCH J. H., EYAL Z., LILLEHEI R. C. EXPERIMENTAL PRESERVATION OF THE SMALL BOWEL. Am J Surg. 1965 Jan;109:26–31. doi: 10.1016/s0002-9610(65)80098-8. [DOI] [PubMed] [Google Scholar]
- MOOG F., NEHARI V. The influence of hydrocortisone on the epithelial phosphatase of embryonic intestine in vitro. Science. 1954 Jun 4;119(3101):809–810. doi: 10.1126/science.119.3101.809. [DOI] [PubMed] [Google Scholar]
- Momose K., Salerno R. A. Intestinal preservation by hypothermia and hyperbaric oxygen. Ann Surg. 1968 Jul;168(1):157–162. doi: 10.1097/00000658-196807000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS E. W. The absorption of fat by intestine of golden hamster in vitro. J Cell Biol. 1963 Jun;17:597–607. doi: 10.1083/jcb.17.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B. K., Jensen W. E. Active transport of glucose by suspensions of isolated rat intestinal epithelial cells. Nature. 1966 Feb 19;209(5025):789–790. doi: 10.1038/209789a0. [DOI] [PubMed] [Google Scholar]
- Strauss E. W. Electron microscopic study of intestinal fat absorption in vitro from mixed micelles containing linolenic acid, monoolein, and bile salt. J Lipid Res. 1966 Mar;7(2):307–323. [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]