Abstract
Members of the fungal genus Fusarium are capable of manifesting in a multitude of clinical infections, most commonly in immunocompromised patients. In order to better understand the interaction between the fungus and host, we have developed the larvae of the greater wax moth, Galleria mellonella, as a heterologous host for fusaria. When conidia are injected into the hemocoel of this Lepidopteran system, both clinical and environmental isolates of the fungus are able to kill the larvae at 37°C, although killing occurs more rapidly when incubated at 30°C. This killing was dependent on several other factors besides temperature, including the Fusarium strain, the number of conidia injected, and the conidia morphology, where macroconidia are more virulent than their microconidia counterpart. There was a correlation in the killing rate of Fusarium spp. when evaluated in G. mellonella and a murine model. In vivo studies indicated G. mellonella hemocytes were capable of initially phagocytosing both conidial morphologies. The G. mellonella system was also used to evaluate antifungal agents, and amphotericin B was able to confer a significant increase in survival to Fusarium infected-larvae. The G. mellonella-Fusarium pathogenicity system revealed that virulence of Fusarium spp. is similar, regardless of the origin of the isolate, and that mammalian endothermy is a major deterrent for Fusarium infection and therefore provides a suitable alternative to mammalian models to investigate the interaction between the host and this increasingly important fungal pathogen.
Keywords: Fusarium, heterologous host, fusariosis, thermotolerance, Galleria, antifungal agent
Introduction
Fusarium species are ubiquitous in nature, commonly found throughout soils and capable of growth on a wide range of substrates (Nelson et al. 1994). They have been isolated from diverse climatic conditions but are prevalent in temperate and tropical environments (Nelson et al. 1994). These fungi represent an important genus of plant pathogens capable of causing disease such as blights, scabs, and root rots on several agriculturally important crops (Desjardins 2003). Fusarium spp. are well known for the production of a vast number of harmful secondary metabolites termed mycotoxins that are responsible for several maladies in livestock and humans after consuming contaminated food sources (Bennett and Klich 2003; Desjardins 2003; Nelson et al. 1994).
Members of the genus Fusarium cause a wide breath of clinical manifestations, most commonly as superficial infections of the skin and/or nail(s), however they are also able to cause locally invasive and disseminated infections and are currently one of the most common etiological agents of filamentous fungal infections (Nucci and Anaissie 2007; Walsh et al. 2004). Fusariosis in immunocompromised patients, particularly those with neutropenia, has a poor prognosis and a corresponding high mortality rate (Nucci and Anaissie 2007).
There are numerous members of the anamorphic genus of Fusarium, however members of the Fusarium solani species complex (FSSC) and the Fusarium oxysporum species complex (FOSC) are the most frequently isolated from clinical laboratory specimens (Nucci and Anaissie 2007). There are ~50 phylogenetically distinct species within the FSSC, including at least 20 which have been isolated from mycoses (O’Donnell et al. 2008). Two of the less common species involved in infections are F. verticillioides and F. proliferatum (Nucci and Anaissie 2007), however these species may be the most prevalent in certain geographical locations (Tortorano et al. 2008).
One limitation in studying the pathogenesis of Fusarium spp. is the availability of suitable hosts to study fungal virulence. Two mouse models have been developed for Fusarium spp. (Legrand et al. 1991; Mayayo et al. 1999) however the ethical issues and expense associated to utilize these models have hindered their use in the laboratory. To circumvent these issues, the larvae of the greater wax moth, Galleria mellonella, have been developed as a model host to investigate virulence of Fusarium spp. This organism has served as a model host for several other fungal pathogens of clinical importance (Cotter et al. 2000; Mylonakis et al. 2005), including the aspergilli (Leger et al. 2000; Reeves et al. 2004). Importantly, many of the virulence factors of these medically important fungi required to infect a mammalian host are also necessary for G. mellonella infection (Brennan et al. 2002; Mylonakis et al. 2005). Through the use of this pathosystem, we have uncovered that Fusarium spp. are more virulent at lower temperatures, and Fusarium isolates from clinical specimens and isolates identified as plant pathogens are both able to kill G. mellonella.
Materials and methods
Strains and media
Fusarium isolates were obtained from the clinical mycology laboratory at Massachusetts General Hospital (MGH) or the Fungal Genetics Stock Center (McCluskey et al. 2010) (Kansas City, MO) (Table 1) and were maintained on potato dextrose agar (PDA) plates at room temperature. To obtain conidia for assays, Fusarium isolates were inoculated to V8 agar plates (10% V8 juice, 2.2% agar) containing 45μg/mL kanamycin, 100 μg/mL ampicillin, and 100 μg/mL streptomycin to prevent potential bacterial contamination and kept at room temperature for 6 days. To harvest the conidia, phosphate buffered saline (PBS) was added to the V8 plates and agitated, the suspended conidia was filtered through two layers of cheese cloth, and the conidia collected by centrifugation. Conidia were washed twice with PBS, suspended in 2 mL of PBS, the concentration determined by counting on a hemocytometer, and adjusted to the desired final concentration by diluting in PBS. To obtain microconidia from isolates producing macroconidia on V8 medium (Fs CI-1 and FGSC 9935), the isolates were inoculated into 50 mL potato dextrose broth (PDB) and allowed to grow for 5 days on a shaker at room temperature. Conidia were then filtered through two layers of cheese cloth to remove mycelia, washed twice with PBS, and suspended in 2 mL of PBS. The number of microconidia was determined by counting on a hemocytometer and adjusted to the desired final concentration by diluting in PBS. This method produced suspensions of >95% microconidia.
Table 1.
List of Fusarium isolates used in this study.
| Isolate abbreviation | Identification | Details | Source | Reference(s) |
|---|---|---|---|---|
| Fo CI-1 | F. oxysporum MLST 33 | Clinical isolate | Groin tissue culture | this study |
| Fs CI-1 | F. solani MLST 2 | Clinical isolate | Blood culture | this study |
| Fs CI-2 | F. solani MLST (3+4) | Clinical isolate | Bronchial lavage | this study |
| Fp CI-1 | F. proliferatum | Clinical isolate | Bronchial wash | this study |
| Fp CI-2 | F. proliferatum | Clinical isolate | Sputum | this study |
| Fs CI-3 | F. solani MLST 2 | Clinical isolate | Nail | this study |
| Fp CI-3 | F. proliferatum | Clinical isolate | Nail | this study |
| F. solani f.sp. pisi | Plant pathogen | FGSC 9596 | (Coleman et al. 2009; Kistler and VanEtten 1984) | |
| F. oxysporum f.sp. lycopersici | Plant pathogen | FGSC 9935 | (Ma et al. 2010) | |
| F. culmorum | Plant pathogen | FGSC 9094 | (Bottalico and Perrone 2002; O’Donnell et al. 1998; Parry et al. 1995) | |
| F. cerealis | Plant pathogen | FGSC 9093 | (Bottalico and Perrone 2002; O’Donnell et al. 1998) | |
| F. verticillioides | Plant pathogen | FGSC 7600 | (Ma et al. 2010; Xu et al. 1995) |
Abbreviations are as follows: Fo = F. oxysporum, Fs = F. solani, Fp = F. proliferatum, FGSC = Fungal Genetics Stock Center
Species identification of clinical Fusarium spp. isolates
The Fusarium isolates obtained from the clinical mycology laboratory at MGH were initially identified by sequencing of the translation elongation factor (TEF) 1α coding region. This was accomplished by PCR using genomic DNA of the Fusarium isolate as template using the primers EF1 and EF2 (O’Donnell et al. 1998) which was then cloned into pGEM-T Easy (Promega, Madison, WI) and bi-directionally sequenced at the MGH DNA sequencing core facilities.
Isolates belonging to the FSSC or FOSC were further subjected to multilocus sequence typing. DNA fragments of the FSSC isolates encoding the internal transcribed spacer and portions of the nuclear large-subunit rRNA (rRNA) and the second-largest subunit of RNA polymerase (RPB2) were amplified as previously described (O’Donnell et al. 2008). A DNA fragment of the FOSC isolate containing the nuclear ribosomal DNA intergenic spacer region (FoITS) were amplified as described in O’Donnell et al (2009). PCR products were cloned in pGEM-T Easy and sequencing was conducted at the MGH sequencing core facilities using vector primers (O’Donnell et al. 2009; O’Donnell et al. 2008), and MLST group determined using the results of a blastn analysis at NCBI or the FUSARIUM-ID database (O’Donnell et al. 2010).
G. mellonella killing assays
G. mellonella caterpillars in the final instar larval stage of development (Vanderhorst, Inc., St. Marys, OH) were stored in the dark and used within seven days from receipt of the shipment. Sixteen randomly chosen G. mellonella larvae (330 ± 25 mg body weight) were used per condition in all assays. All experiments included two control groups: the first group of larvae was mock inoculated with 10 μL of PBS to observe for killing due to physical trauma from the injection, and the second group did not receive any injection. A 10 μl Hamilton syringe was used to inject 10 μl of the inoculum into the hemocoel of each larva via the last left proleg. Before injection the area was cleaned using an alcohol swab. After injection, caterpillars were incubated in plastic containers at 30 or 37°C, and the larval survival scored daily where larvae were considered dead by an absence of movement in response to touch. Experiments that had more than two dead caterpillars in either control group were discarded and repeated. All G. mellonella killing experiments were performed at least twice, and representative experiments are presented. In order to simplify some of the figures, the PBS control group may have been omitted.
Radial growth assay
In order to determine if the ability of the Fusarium strains to grow at physiologically relevant temperatures had a role in Galleria killing, radial growth assays were conducted at 30°C and 37°C. Fusarium isolates were inoculated to 2% water agar plates and allowed to grow for three days at room temperature. Agar plugs (~5mm) were removed from the growing colony margins and inoculated to 10% V8 juice plates with the mycelial side of the plug directly on the V8 agar surface. Inoculated plates were placed in the dark in 30°C or 37°C incubators and scored after seven days of growth. The growth assay is based on two independent replicates. The percent inhibition of radial growth was calculated and standard deviation determined.
Murine model of infection
The murine virulence assays were conducted as on immunosuppressed mice using three clinical Fusarium strains (Fs CI-1, Fs CI-2, and Fo CI-1). Briefly, neutropenia was induced in male CF-1 mice (Charles River Laboratories, Wilmington, MA) weighing ~30g one day prior to inoculation with Fusarium spores. Immunosuppression was induced by a single intraperitoneal dose of 200 mg/kg of cyclophosphamide (Sigma). Groups of eight mice were inoculated by a lateral tail vein injection with 100 μL of a 107 conidial suspension (106 conidia inoculated per mouse). Animal survival was monitored twice daily, and moribund animals or those in distress were sacrificed by CO2 asphyxiation.
Fusarium surface fluorophore labeling
Fusarium conidia were fluorescently labeled by selectively linking Alexa Fluor 647 succinimidyl ester to primary amines located on the conidial surface. Alexa Fluor 647 succinimidyl ester was reconstituted in dimethylformamide (100 mg/mL). Prior to fluorescent labeling, conidia were washed in phosphate buffered saline (PBS) 3 times at 4000 g for 1 minute. After the PBS washes, conidia were suspended in 500 μL of PBS, and 3 μg of dye was mixed with the pathogen at 37°C for 30 minutes. During the labeling/incubation, the spores were shaken for 10 sec every 10 min to prevent them from settling in the bottom of the tube. The dye-conidia mixture was then washed again 3 times in PBS (4000 rpm for 1 minute) and kept on ice and protected from light until use in the imaging experiments.
Fusarium-infected G. mellonella hemolymph
Alexa Flor 647-labeled Fusarium conidia were inoculated into G. mellonella as described above and incubated at 37°C. Twelve hours post-inoculation, hemolymph was collected from the larvae into cold insect physiologic saline as previously described (Fuchs et al. 2010). After centrifugation, the hemocytes were washed twice in 500 μL of Grace’s insect medium (Lonza, Walkersville, MD) and suspended in 300 μL Grace’s medium. The hemocytes were then permitted to adhere to the slide chamber for 1 hr.
Confocal microscopy
Spinning-disk confocal microscopy was performed on a Nikon Ti-E inverted microscope equipped with a CSU-X1 confocal head (Yokogawa) that provides scan speeds of up to 2,000 frames per second. A Coherent, 4 W, continuous-wave laser was used as an excitation light source to produce excitation wavelengths of 647 nm. To acquire high quality fluorescence images, a high magnification, high numerical aperture (NA) objective, used for Total Internal Reflection Imaging (TIRF), was used (Nikon, 100X, 1.49 NA, oil immersion). A piezo stage (Prior Instruments, Rockland, MA) capable of X, Y, and Z movement was used for z-stack acquisition. A halogen light source and an air condenser (0.52 NA) were used for bright field illumination and a polarizer (Nikon, MEN51941) and Wollaston prisms (Nikon, MBH76190) were used to acquire differential interference contrast (DIC) images. Images were acquired using an EM-CCD camera (Hamamatsu, C9100-13) capable of acquiring high-resolution images under low light levels with high quantum efficiency. Emission light from the sample was collected after passage through the appropriate emission filters (Semrock, Rochester, NY). Image acquisition was performed using MetaMorph software (Molecular Devices, Downingtown, PA).
Determination of the minimal inhibitory concentration of antifungal agents
The minimial inhibitory concentration (MIC) for fluconazole, amphotericin B, and mancozeb, against the Fusarium spp. was determined spectrophotometrically using RPMI 1640 media (Mediatech, Inc., Manassas, VA) following the established broth microdilution method of the Clinical and Laboratory Standards Institute document M38-A (National Committee for Clinical Laboratory Standards 2002). All antifungal compounds were obtained from Sigma-Aldrich (St. Louis, MO).
Evaluation of antifungal agents in the G. mellonella-Fusarium system
The same technique of inoculation for the experiments that include antifungal efficacy evaluation were conducted as described above. Administration of the antifungal agent to be evaluated was accomplished by an injection consisting of 10 μL of the compound into the right last proleg of the larvae. Antifungal agents (amphotericin B, fluconazole, and mancozeb) were diluted in PBS, and each larva was administered a single compound by a separate injection. Amphotericin B (1.5 mg/kg), fluconazole (14 mg/kg), or mancozeb (0.25 mg/kg) treatments were administered a single time on the same day of inoculation. G. mellonella caterpillars that were injected twice with PBS were used as a control group for these experiments.
Statistical analysis
Kaplan-Meier survival curves were plotted using STATA 6, and a P value of <0.05 was considered to be statistically significant. For the Fusarium-Galleria experiments conducted at different temperatures, the differences in survival and the hazard ratio were calculated by running a Cox proportional hazards regression model (Cox regression) considering the treatment or intervention (Fusarium isolates), the source of the isolates (clinical and plant isolates), and the incubation temperature (30°C and 37°C) as independent variables. The hazard function was considered as the dependent variable. The Cox regression procedure allows the comparison between clinical versus plant isolates and the incubation temperatures of 30°C versus 37°C on larval survival while simultaneously taking into account the effect of each individual Fusarium isolate on Galleria survival.
Results
Killing of G. mellonella by Fusarium conidia
When Fusarium conidia are inoculated into the last proleg of the larvae, the time of survival of the infected insect was dependent on the number of spores inoculated, regardless if the Fusarium isolate was from a clinical (Fig 1A) or an environmental source (Fig 1B). After G. mellonella were injected with 106 conidia and incubated at 37°C, the larvae began dying after 2 days and were usually all dead within 4–6 days. Inoculating 107 conidia/larvae into the hemocoel resulted in an increased rate of G. mellonella killing and usually no larvae survived longer than two days (data not shown). By the time of death, larvae were deeply melanized as observed with other fungal pathogens (Cotter et al. 2000).
Fig 1.
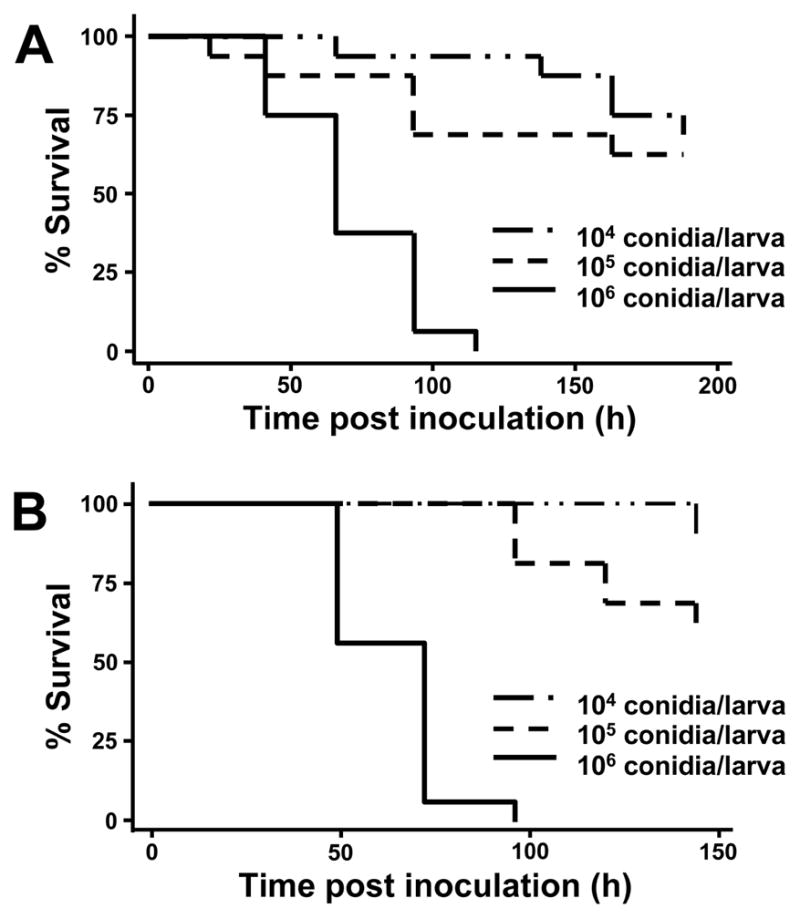
Killing of G. mellonella larvae is dependent on the number of conida inoculated. Kaplan-Meier survival of larvae inoculated with number of a mixture of predominately microconidia indicated for Fo CI-1 (A) and F. solani FGSC 9596 (B).
Killing of G. mellonella by Fusarium spp. is temperature dependent
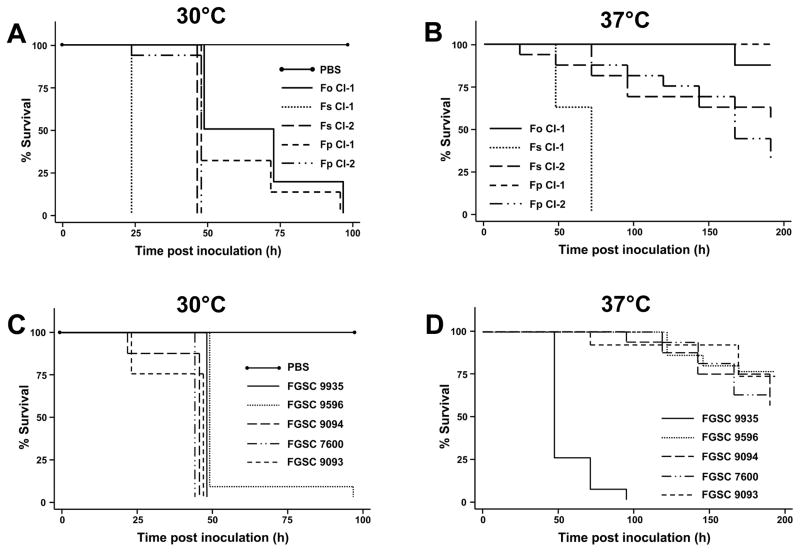
This study is the first instance where virulence of Fusarium spp. has been evaluated at two temperatures in a single host system, and reveals that Fusarium spp. are more pathogenic at temperatures lower than 37°C. Isolates from clinical sources were capable of killing G. mellonella within 3–4 days post inoculation when incubated at 30°C (Fig 2A), where the time necessary to result in 50% killing (TD50%) ranged from ~1–2 days for all clinical isolates included in the assay. However, most of these clinical isolates were unable to cause significant mortality one week or more after inoculation with 1 × 105 conidia and incubated at 37°C (Fig 2B). The exception to this observation was an isolate of F. solani (Fs CI-1) which was collected from a blood culture of a patient with invasive fusariosis. G. mellonella inoculated with Fs CI-1 consistently had a TD50% ~1–3 days, whereas the other clinical isolates were unable to yield a TD50% of less than one week.
Fig 2.
Killing of G. mellonella by clinical and established environmental Fusarium isolates. Kaplan-Meier survival of larvae following injection with 1 × 105 of conidia (Fs CI-1, FGSC 9093, FGSC 9094, FGSC 9935 were predominately macroconidia while the other isolates were predominately microconidia) and incubation at 30°C (A and C) and 37°C (B and D). In some instances, lines were offset slightly to allow them to be visible.
Virulence of a collection of environmental Fusarium isolates was also evaluated in G. melonella (Fig 2C; Table 1). As seen with the clinical isolates, there was a marked decrease in G. mellonella survival when the larvae were incubated at 30°C with a TD50% ranging from ~1–2 days (Fig 2C). Interestingly, all isolates included in this study were capable of killing G. mellonella, including F. cerealis and F. culmorum which are not normally associated with clinical cases of fusariosis (Fig 2C). Conversely, when larvae were incubated at 37°C post-inoculation, most of the isolates were unable to cause any significant degree of killing, although F. oxysporum f.sp. lycopersici, a pathogen from tomato, was highly virulent in G. mellonella (a TD50% of 1.5–3 days) where all the larvae succumb to the fungal infection within four days post-inoculation (Fig 2D).
There was no significant difference between the virulence of Fusarium isolates from clinical sources and environmental isolates when the G. mellonella were incubated at either 30°C (P=0.267, hazard ratio = 0.68, CI (0.34–1.34)) or 37°C (P=0.57, hazard ratio =0.77, CI (0.31–1.87)) post inoculation. However, there is a significant difference in the survival of the larvae when placed at 30°C versus 37°C after inoculation with the Fusarium spp., which was evident regardless if the fungus was from a clinical (P= 0.002, hazard ratio = 4.03, CI(1.65–9.80)) or environmental origin (P=0.00, hazard ratio =5.86, CI (2.58–13.27)).
The difference in virulence of the Fusarium spp. on G. mellonella when incubated at 30 and 37°C could be due to various reasons, including host factors and the ability of the fungus to germinate and grow within the larvae. Radial growth assays were conducted to assess the thermotolerance of the Fusarium isolates at higher temperatures. There was a distinct correlation between the inhibition of radial growth at 37°C and the ability of the fungus to kill G. mellonella larvae at 37°C. All of the Fusarium isolates displayed reduced growth at 37°C, including those isolated from invasive clinical specimens (Table 2); however, Fs CI-1 was the most thermotolerant of the invasive clinical isolates (58.5±4.6% inhibition) and FGSC 9935 was the most thermotolerant of the environmental isolates (64.2±6.7% inhibition), both of which were able to kill G. mellonella at 37°C.
Table 2.
Comparison of radial growth of Fusarium isolates at 30 and 37°C.
| Percent inhibition of radial growth at 37°C relative to radial growth at 30°Ca | |
|---|---|
| Fo CI-1 | 90.7±4.9 |
| Fs CI-1 | 58.5±4.6 |
| Fs CI-2 | 67.7±5.8 |
| Fs CI-3 | 47.4±0.2 |
| Fp CI-1 | 84.5±3.8 |
| Fp CI-2 | 89.6±0.6 |
| Fp CI-3 | 89.0±3.1 |
| FGSC 9596 | 68.6±5.5 |
| FGSC 9935 | 64.2±6.7 |
| FGSC 9094 | 100b |
| FGSC 9093 | 100b |
| FGSC 7600 | 82.5±2.0 |
Average and standard deviation of two independent assays.
FGSC 9093 and FGSC 9094 were unable to grow when incubated at 37°C.
A positive correlation exists between G. mellonella and a murine model to assess the virulence of Fusarium spp
Three clinical Fusarium isolates (Fs CI-1, Fs CI-2, and Fo CI-1) were selected to evaluate their pathogenicity in a murine model to determine if their virulence is correlated to the results observed in G. mellonella. Both of the F. solani isolates were highly virulent in a mouse model while Fo CI-1 did not cause any morbidity within the experimental timeframe (Fig 3), a result consistent with previous findings (Mayayo et al. 1999). Importantly, a positive correlation was present between the rate of killing of these Fusarium isolates. Fs CI-1 was the most virulent in a murine model, and Fo CI-1 the least (Fig 3), similar to the results observed using G. mellonella followed by incubation at 37°C (Fig. 2B). The median time of death of mice inoculated with Fs CI-1 was 72 h whereas those inoculated with Fs CI-2 was 120 h (Fig 3).
Fig 3.
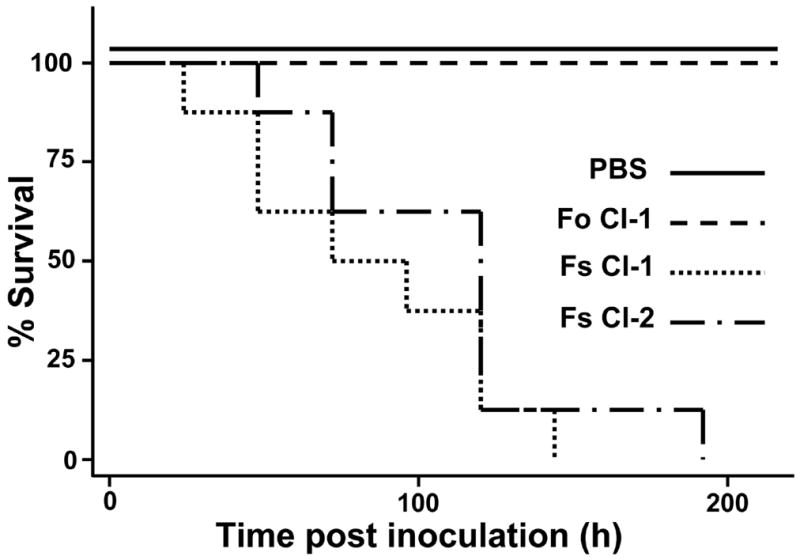
Clinical isolates of Fusarium spp. evaluated in a murine model. Kaplan-Meier survival of neutropenic mice inoculated with 106 conidia of two F. solani isolates (Fs CI-1 and Fs CI-2) and one F. oxysporum isolate (Fo CI-1).
Morphology of the conidia is involved in G. mellonella killing
Many Fusarium spp. are capable of producing two types of conidia, microconidia and macroconidia. In our inoculation assays, we observed isolates producing either predominantly microconidia or a mixed population of these conidial morphologies. Interestingly, the clinical isolate which displayed the highest G. mellonella killing at 37°C, Fs CI-1, produced a greater number of macroconidia than microconidia suggesting conidial morphology may play a role in establishing disease.
In order to investigate the role the spore type has in killing, Fs CI-1 and the environmental isolate F. oxysporum f.sp. lycopersici were selected for additional assays, as each of the conidia morphologies could be separated to >90% of the desired conidia morphology by culturing on different media. There was a distinct difference in survival between larvae inoculated with the different conidia for both Fusarium isolates, where larvae inoculated with macroconidia died within three days while the majority of larvae challenged with microconidia survived (P value <0.0001; Fig 4).
Fig 4.
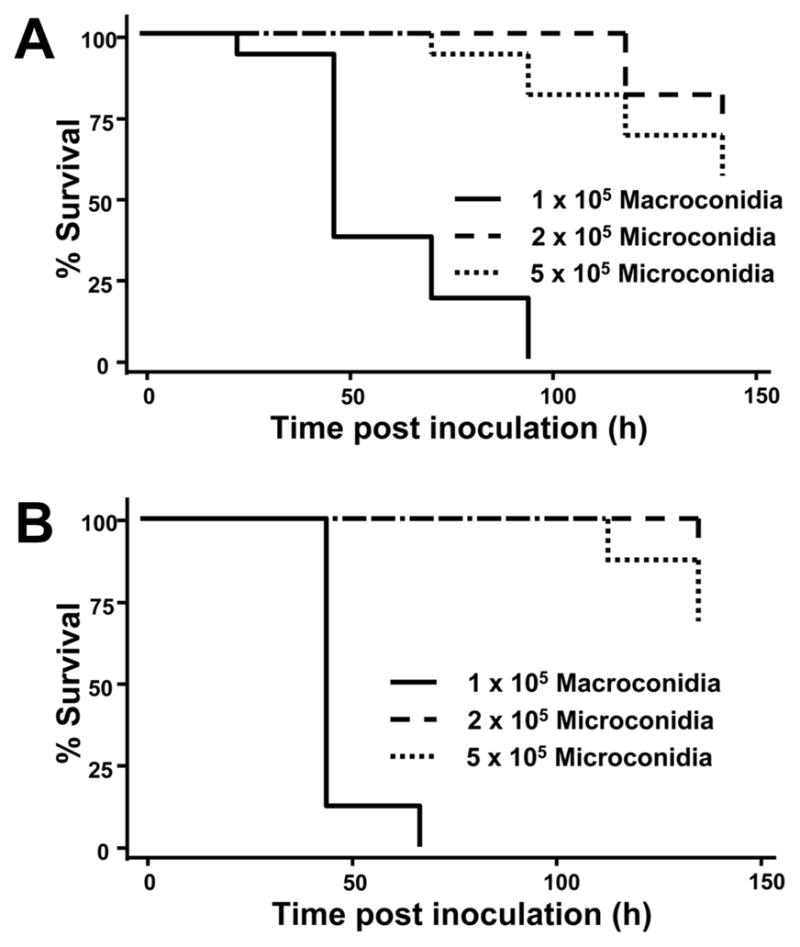
Fusarium conidia morphology is involved in virulence on G. mellonella. Survival of larvae was dependent on the morphology of the inoculated F. solani conidia of the clinical isolate Fs CI-1 (A) and the environmental reference isolate FGSC 9935 (B) and placed at 37°C. Statistical analysis of larval survival after injection with 1 × 105 macroconidia compared to 2 × 105 or 5 × 105 microconidia resulted in a P value < 0.0001 for either isolate. The differences in survival of larvae injected with the two microconidia concentrations resulted in P values = 0.22 and 0.069 for Fs CI-1 (A) and FGSC 9935 (B), respectively.
As Fusarium spp. macroconidia are multicellular (having ~4–5 cells per conidia), the bias of macroconidia virulence could be due to increased cell germination within this inoculum. Fusarium macroconidia typically germinate from either one or, more commonly, both terminal spore cells (Harris 2005), resulting in approximately twice the amount of germination tubes produced. However, when twice the amount of microconidia compared to macroconidia were injected into the G. mellonella larvae (2 × 105 microconidia vs. 1 × 105 macroconidia), the macroconidia were significantly more virulent (P= <0.0001; Fig 4). Additionally, the amount of fungal biomass between microconida and macroconidia could have a role in the observed difference in G. mellonella killing. When microconidia were inoculated at a concentration five times that of macroconidia (5 × 105 microconidia vs. 1 × 105 macroconidia), the larvae inoculated with macroconidia still died more rapidly than the higher concentration of microconidia (P= <0.0001; Fig 4).
Superficial and invasive clinical Fusarium isolates are virulent in G. mellonella
As previously mentioned, Fusarium spp. are capable of causing superficial localized infections at the extremities of immunocompetent patients. The G. mellonella-Fusarium spp. pathosystem was utilized to investigate if fungal isolates associated with these superficial infections were as virulent as isolates from an invasive infection. When G. mellonella larvae were challenged with 1 × 105 conidia from fusaria isolated from superficial infections, killing occurred regardless of the severity of the clinical infection from where the strain was isolated (Fig 5). An isolate of F. solani (Fs CI-3) isolated from a nail culture was capable of killing 100% of the infected larvae within 72 hrs, a rate that is comparable to the most virulent systemic Fusarium isolate obtained from a blood culture (the TD50% for Fs CI-3 was ~1.5 days versus a TD50% for Fs CI-1 of ~1–2 days). Despite being isolated from a superficial infection site, Fs CI-3 also exhibited a relatively high degree of thermotolerance (47.4±0.2% inhibition; Table 2).
Fig 5.
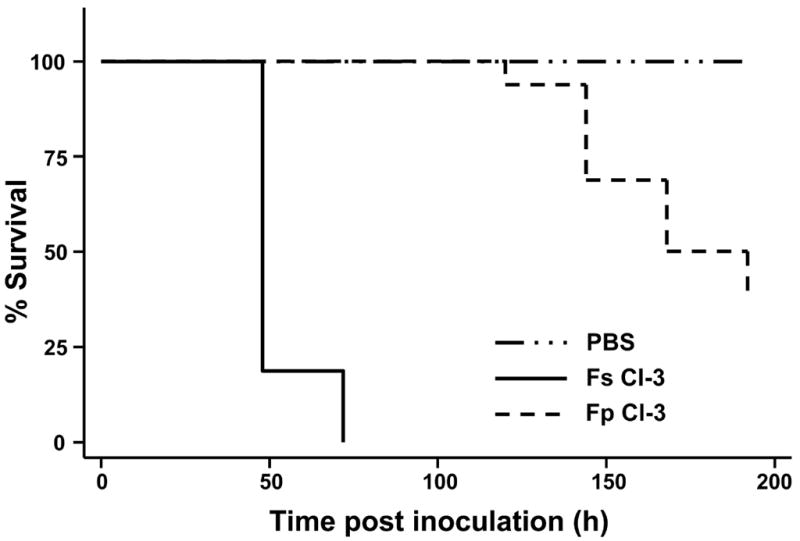
Clinical Fusarium isolates from superficial infections are capable of killing G. mellonella. Kaplan-Meier survival of larvae following injection with 1 × 105 conidia and incubation at 37°C. Fs CI-3 produced mostly macroconidia.
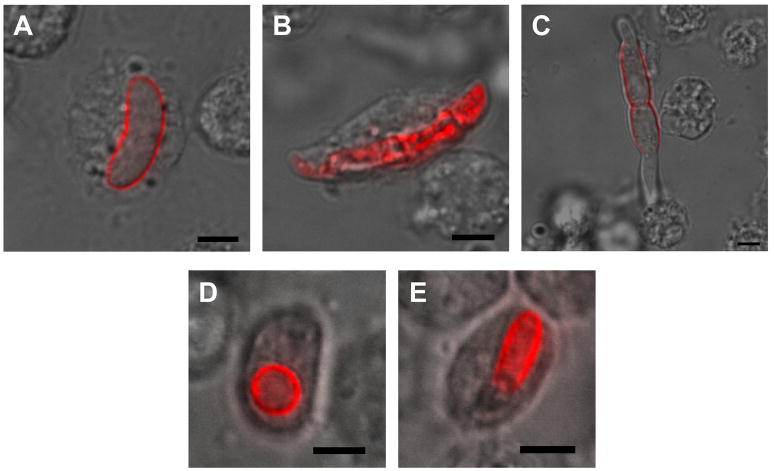
Interaction of Fusarium conidia and hemocytes
The hemolymph of G. mellonella larvae challenged with Fusarium spp. was collected and examined by confocal microscopy 16 hours after injection with conidia (Fig 6). Macroconidia of the fungus are able to be phagocytosed despite their size (Fig 6A and B). Large obstructions in the hemolymph are sequestered through a process known as nodulation where multiple hemocytes adhere and surround the object (Lavine and Strand 2002), in this case a germinating Fusarium conidium (Fig 6C). Hemocyte phagocytosis also occurs in response to encountering microconidia in the hemolymph (Fig 6D and E).
Fig 6.
G. mellonella hemocytes associate with Fusarium conidia. Macroconidia of Fs CI-1 after phagocytosis by hemocytes (A and B). A germinating Fs CI-1 conidium with multiple hemocytes associated initiating nodulation (C). Microconidia of Fo CI-1 phagocytosed by hemocytes (D and E). Scale bars represent 5 μm.
Assessment of antifungal agents in G. mellonella
Few clinically used antifungal agents have efficacy against Fusarium spp., although the polyene amphotericin B has been shown to be effective against several Fusarium spp. (Al-Abdely 2004; Carneiro et al. 2011; Muhammed et al. 2011; Nucci and Anaissie 2007). The G. mellonella-Fusarium spp. pathosystem was used to evaluate single dose treatments of two clinically relevant antifungal agents; amphotericin B and the azole fluconazole which has limited efficacy against Fusarium spp. Following administration of 14 mg/kg fluconazole there was no significant increase in survival of the larvae, conversely a dose of 1.5 mg/kg of amphotericin B was able to increase survival of Fusarium-infected larvae (Fig 7). No significant difference in larval survival was observed for other assayed concentrations of the antifungal agents (amphotericin B 0.5–2.0 mg/kg; fluconazole 3.5–28 mg/kg; data not shown). There was a correlation between the survival of the infected G. mellonella and the in vitro MIC of the antifungal agents (MIC for isolate Fo CI-1: Flu 256 μg/mL; AmpB 2 μg/mL).
Fig 7.
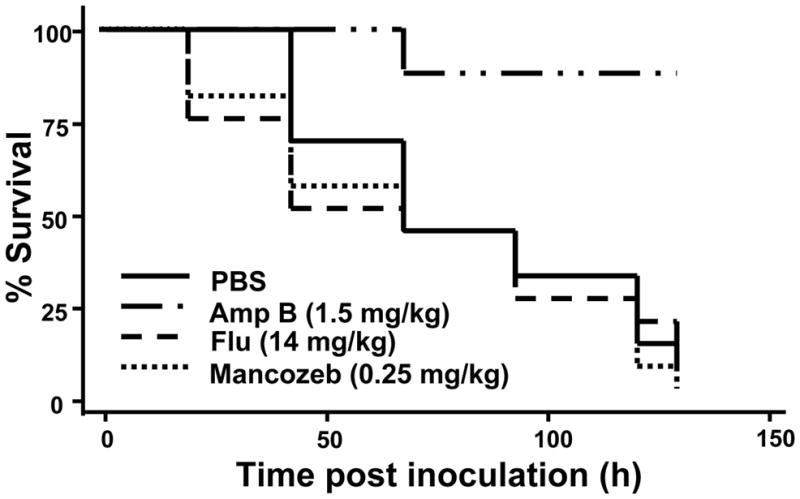
Amphotericin B prolongs survival of Fusarium-infected G. mellonella. Percent survival of larvae challenged with 1.5 × 106 conidia of Fo CI-1 followed by treatment with the indicated antifungal agents. Abbreviations: Amp B, amphotericin B; Flu, fluconazole.
In order to assess the possibility that G. mellonella can be used as an alternative host to detect compounds with antifungal efficacy against Fusarium, the Fusarium-Galleria system was used to evaluate the antifungal efficacy of a compound not used clinically against Fusarium spp. Mancozeb, a fungicide used as a protectant in agriculture against Fusarium spp., was evaluated in G. mellonella (Fig 7). Previously this compound had been reported to inhibit Fusarium spp. growth (Allen et al. 2004; Fravel et al. 2005), which was confirmed having an MIC of 4 μg/mL against isolate Fo CI-1. Despite antifungal activity of the compound, treatment of infected G. mellonella larvae with a single dose of ranging from 0.07–0.53 mg/kg of mancozeb resulted in no significant difference in survival when compared to the PBS control (Fig 7).
Discussion
The diverse host range of Fusarium spp. has been well documented, however these fungi are best known for the ability to cause devastating crop losses as plant pathogens and contaminating feed with mycotoxins (Desjardins 2003; Desjardins and Proctor 2007). Fusarium spp. have been described as entomopathogenic fungi on a wide range of insects, including members of the order Lepidoptera (Hajek et al. 1993; Teetor-Barsch and Roberts 1983). Taking advantage of the diverse host range of these pathogens we show that the greater wax moth provides a suitable alternative host to evaluate virulence of Fusarium spp. isolated from various sources.
G. mellonella has served as a heterologous host for several medically important fungi, including Candida spp., Cryptococcus neoformans, Aspergillus fumigatus, and Aspergillus flavus (Cotter et al. 2000; Jackson et al. 2009; Leger et al. 2000; Mylonakis et al. 2005; Reeves et al. 2004). Fusarium spp. were able to form a lethal infection when injected into the hemocoel and incubated at lower temperatures. Several secondary metabolites from Fusarium spp. have been shown to have insecticidal activity and therefore may play a role in G. mellonella larvae killing (Claydon et al. 1977, 1979; Mule et al. 1992; Strongman et al. 1988). In addition, the morphology of the conidia was important for virulence in this pathosystem (Fig 3), however the precise mechanism conferring the higher virulence of macroconidia is unknown. The observed difference in virulence from the two conidia morphologies compounded with the fact that the environmental condition of the Fusarium isolate may influence the type of conidia the fungus produces, suggests that the surrounding environment may play an indirect role in the virulence of Fusarium on the larvae. It should be noted though that the virulence of the two different conidia morphologies was conducted for only two isolates (Fs CI-1 and FGSC 9935), as it was not possible to obtain inoculums of a single conidial morphology for the other isolates.
The amenability of G. mellonella to various temperatures, including physiological relevant temperatures, has made the arthropod ideally suited as a heterologous host for Fusarium spp. Unlike other clinically relevant fungal pathogens, Fusarium spp. displayed an inversely related temperature-virulence relationship, where there was increased virulence when incubated at 30°C than at 37°C. This increase in virulence at lower temperatures may explain the prevalence of localized skin infections and onychomycosis caused by Fusarium spp. in immunocompetent and immunocompromised patients (Gupta et al. 2000; Nucci and Anaissie 2002). These skin infections usually are located on the patient’s extremities such as the toes (Nucci and Anaissie 2007), a location that would be lower than physiological temperature, therefore potentially favoring Fusarium spp. colonization.
The observed decrease in G. mellonella killing at mammalian physiological temperatures compared to 30°C suggests that many Fusarium spp. have the capability to cause disease, however the frequency of invasive infections by these fungi may be curtailed by the higher internal temperatures of mammals. A study of 4802 fungal isolates found that an additional ~6% of the fungi were unable to grow for each increase of 1°C within the 30–40°C temperature range (Robert and Casadevall 2009). Therefore, thermotolerance could potentially be one of the limiting factors for an increased incidence of invasive fusariosis, and conversely mammalian endothermy a factor in limiting members of this fungal group from causing disease. Although, host endothermy alone can not account for the reduced incidence of Fusarium spp. in the clinic, as many of these isolates can grow at 37°C (Mehl and Epstein 2007; Sugiura et al. 1999), and therefore other factors, in particular the immune system of the host, play a major role in host’s defense against the fungus (Carneiro et al. 2011; Dignani and Anaissie 2004; Nucci and Anaissie 2007).
A decreased number of neutrophils in patients is a major risk factor for fusariosis. The equivalent immune cells in G. mellonella, contained in the hemolymph, are a collection of several different types of hemocytes, of which plasmatocytes and granulocytes are involved in phagocytosis, nodule formation, and encapsulation (Lavine and Strand 2002). Furthermore, studies have shown that the hematocyte density within the hemolymph was inversely correlated with the degree of virulence of the fungal pathogen that was inoculated (Bergin et al. 2003). The hemocytes are capable of phagocytizing Fusarium conidia, and may provide a suitable pathosystem to evaluate the host immune response to Fusarium infection in vivo. Furthermore, G. mellonella is capable of producing a number of antimicrobial peptides (Brown et al. 2009), some of which have been shown to have antifungal activity against F. graminearum and F. oxysporum (Brown et al. 2008). In the study by Brown et al. (2008), the spores of F. oxysporum were found to be more tolerant of the G. mellonella moricin-like antimicrobial peptides than those of F. graminearum (Brown et al. 2008), an observation which may account for the consistent more rapid killing of G. mellonella larvae by both clinical and environmental isolates of F. oxysporum (Fig 2).
Fusarium isolates able to cause disease in humans are prevalent within the surrounding environment (Anaissie et al. 2001; Mehl and Epstein 2008; O’Donnell et al. 2004; Zhang et al. 2006). Multilocus analysis of members of the FSSC from clinical and environmental sources revealed that many isolates from both sources had shared haplotypes which suggests that clinical infections caused by these fusaria originally were obtained from the environment (Zhang et al. 2006). This notion is further supported from studies with F. solani f. sp. cucurbitae race 2 strains which were obtained from clinical specimens and from infected plant tissue. Regardless of the source of the fungus, all isolates were pathogenic on cucurbits and were able to grow at human body temperature. Furthermore, isolates from clinical sources were fertile with those from plants, demonstrating they comprise a single species (Mehl and Epstein 2007). Our results using G. mellonella as a host for several species of Fusarium show a similar trend. Isolates from both clinical and plant/environmental sources were able to kill G. mellonella larvae (Fig 2), further supporting there is little difference in the virulence of Fusarium regardless of the source.
As Fusarium spp. are capable of infecting a diverse array of hosts, their virulence in human hosts could be a result of traits that have been selected by the fungus persisting in the environment, a concept termed “accidental virulence” (Casadevall and Pirofski 2007). All of the Fusarium isolates included in this study were able to kill the larvae of G. melonella. Therefore it possible that passage of Fusarium through insects in the environment has selected for traits which allow the fungus to overcome the immune response of the invertebrate, thereby conferring virulence to other potential animal hosts. The correlation between the killing rate of the Fusarium isolates in the G. mellonella and mice models suggest that virulence factors maybe conserved between these hosts supporting the notion that these factors may have evolved by “accidental virulence” (Fig 3).
Currently, there are a limited number of antifungal agents available to treat fusariosis (Muhammed et al. 2011). Of these compounds, amphotericin B provides the broadest efficacy to multiple Fusarium spp. (Nucci and Anaissie 2007; O’Donnell et al. 2008); however newer azoles, such as posaconazole and voriconazole, have some antifungal efficacy for Fusarium spp. (Nucci and Anaissie 2007; O’Donnell et al. 2008). G. mellonella provides a suitable alternative to identify and evaluate the efficacy of antifungal agents in vivo instead of animal models. Furthermore, since the larvae have significantly less body mass than their murine counterpart, G. mellonella is highly suited for instances where a limited amount of compound is available to evaluate the antifungal efficacy against Fusarium species.
The use of G. mellonella has an alternative host for Fusarium spp. provides a suitable system to further investigate host-pathogen interactions and to evaluate the antifungal efficacy of compounds against Fusarium species. This system demonstrated that Fusarium spp. from both clinical and environmental sources are more virulent at temperatures lower than 37°C and that conidia morphology is involved in establishing disease in G. mellonella.
Research Highlights.
The greater wax moth, Galleria mellonella, is a suitable alternative host for Fusarium spp.
Thermotolerance is important for pathogenicity of Fusarium spp.
Macroconidia of Fusarium are more infectious than microconidia
Acknowledgments
We would like to thank Evan Mojica in the MGH clinical mycology laboratory for kindly providing Fusarium isolates and Beth Burgwyn Fuchs for insightful discussions. This research was supported by a National Institutes of Health R01 award (AI075286) and a R21 award (AI079569) to EM and a T32 award (AI07061) to JJC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey J. Coleman, Email: jjcoleman@partners.org.
Maged Muhammed, Email: mmuhammed@partners.org.
Pia V. Kasperkovitz, Email: kasperkovitz.pia@mgh.harvard.edu.
Jatin M. Vyas, Email: jvyas@partners.org.
Eleftherios Mylonakis, Email: emylonakis@partners.org.
References
- Al-Abdely HM. Management of rare fungal infections. Current Opinion in Infectious Diseases. 2004;17:527–532. doi: 10.1097/00001432-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Allen TW, Enebak SA, Carey WA. Evaluation of fungicides for control of species of Fusarium on longleaf pine seed. Crop Protection. 2004;23:979–982. [Google Scholar]
- Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Muller FMC, Lozano-Chiu M, Summerbell RC, Dignani MC, Chanock SJ, Walsh TJ. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: A new paradigm for the epidemiology of opportunistic mold infections. Clinical Infectious Diseases. 2001;33:1871–1878. doi: 10.1086/324501. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clinical Microbiology Reviews. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes and Infection. 2003;5:1389–1395. doi: 10.1016/j.micinf.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Bottalico A, Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology. 2002;108:611–624. [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochemistry and Molecular Biology. 2008;38:201–212. doi: 10.1016/j.ibmb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochemistry and Molecular Biology. 2009;39:792–800. doi: 10.1016/j.ibmb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Carneiro HA, Coleman JJ, Restrepo A, Mylonakis E. Fusarium infection in lung transplant patients: report of 6 cases and review of the literature. Medicine (Baltimore) 2011;90:69–80. doi: 10.1097/MD.0b013e318207612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L-a. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryotic Cell. 2007;6:2169–2174. doi: 10.1128/EC.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon N, Grove JF, Pople M. Insecticidal secondary metabolic products from the entomogenous fungus Fusarium solani. Journal of Invertebrate Pathology. 1977;30:216–223. [Google Scholar]
- Claydon N, Grove JF, Pople M. Insecticidal secondary metabolic products from the entomogenous fungus Fusarium larvarum. Journal of Invertebrate Pathology. 1979;33:364–367. [Google Scholar]
- Coleman JJ, Rounsley SD, Rodriguez-Carres M, Kuo A, Wasmann CC, Grimwood J, Schmutz J, Taga M, White GJ, Zhou S, Schwartz DC, Freitag M, Ma L-J, Danchin EGJ, Henrissat B, Coutinho PM, Nelson DR, Straney D, Napoli CA, Barker BM, Gribskov M, Rep M, Kroken S, Molnár I, Rensing C, Kennell JC, Zamora J, Farman ML, Selker EU, Salamov A, Shapiro H, Pangilinan J, Lindquist E, Lamers C, Grigoriev IV, Geiser DM, Covert SF, Temporini E, VanEtten HD. The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genetics. 2009;5:e1000618. doi: 10.1371/journal.pgen.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunology and Medical Microbiology. 2000;27:163–169. doi: 10.1111/j.1574-695X.2000.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Desjardins AE. Gibberella from A (venaceae) to Z (eae) Annual Review of Phytopathology. 2003;41:177–198. doi: 10.1146/annurev.phyto.41.011703.115501. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Proctor RH. Molecular biology of Fusarium mycotoxins. International Journal of Food Microbiology. 2007;119:47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Dignani MC, Anaissie E. Human fusariosis. Clinical Microbiology and Infection. 2004;10:67–75. doi: 10.1111/j.1470-9465.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Fravel DR, Deahl KL, Stommel JR. Compatibility of the biocontrol fungus Fusarium oxysporum strain CS-20 with selected fungicides. Biological Control. 2005;34:165–169. [Google Scholar]
- Fuchs BB, O’Brien E, El Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Baran R, Summerbell RC. Fusarium infections of the skin. Current Opinion in Infectious Diseases. 2000;13:121–128. doi: 10.1097/00001432-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Hajek AE, Nelson PE, Humber RA, Perry JL. Two Fusarium species pathogenic to gypsy moth, Lymantria dispar. Mycologia. 1993;85:937–940. [Google Scholar]
- Harris SD. Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia. 2005;97:880–887. doi: 10.3852/mycologia.97.4.880. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Higgins LA, Lin XR. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One. 2009;4:e4224. doi: 10.1371/journal.pone.0004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler HC, VanEtten HD. Three non-allelic genes for pisatin demethylation in the fungus Nectria haematococca. Journal of General Microbiology. 1984;130:2595–2603. [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Leger RJS, Screen SE, Shams-Pirzadeh B. Lack of host specialization in Aspergillus flavus. Applied and Environmental Microbiology. 2000;66:320–324. doi: 10.1128/aem.66.1.320-324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C, Anaissie E, Hashem R, Nelson P, Bodey GP, Ro J. Experimental Fusarial hyalohyphomycosis in a murine model. Journal of Infectious Diseases. 1991;164:944–948. doi: 10.1093/infdis/164.5.944. [DOI] [PubMed] [Google Scholar]
- Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B, Houterman PM, Kang S, Shim WB, Woloshuk C, Xie XH, Xu JR, Antoniw J, Baker SE, Bluhm BH, Breakspear A, Brown DW, Butchko RAE, Chapman S, Coulson R, Coutinho PM, Danchin EGJ, Diener A, Gale LR, Gardiner DM, Goff S, Hammond-Kosack KE, Hilburn K, Hua-Van A, Jonkers W, Kazan K, Kodira CD, Koehrsen M, Kumar L, Lee YH, Li LD, Manners JM, Miranda-Saavedra D, Mukherjee M, Park G, Park J, Park SY, Proctor RH, Regev A, Ruiz-Roldan MC, Sain D, Sakthikumar S, Sykes S, Schwartz DC, Turgeon BG, Wapinski I, Yoder O, Young S, Zeng QD, Zhou SG, Galagan J, Cuomo CA, Kistler HC, Rep M. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. Journal of Medical Microbiology. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- McCluskey K, Wiest A, Plamann M. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. Journal of Biosciences. 2010;35:119–126. doi: 10.1007/s12038-010-0014-6. [DOI] [PubMed] [Google Scholar]
- Mehl HL, Epstein L. Fusarium solani species complex isolates conspecific with Fusarium solani f. sp cucurbitae race 2 from naturally infected human and plant tissue and environmental sources are equally virulent on plants, grow at 37 degrees C and are interfertile. Environmental Microbiology. 2007;9:2189–2199. doi: 10.1111/j.1462-2920.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- Mehl HL, Epstein L. Sewage and community shower drains are environmental reservoirs of Fusarium solani species complex group 1, a human and plant pathogen. Environmental Microbiology. 2008;10:219–227. doi: 10.1111/j.1462-2920.2007.01446.x. [DOI] [PubMed] [Google Scholar]
- Muhammed M, Coleman JJ, Carneiro HA, Mylonakis E. The challenge of managing fusariosis. Virulence. 2011;2:91–96. doi: 10.4161/viru.2.2.15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mule G, Dambrosio A, Logrieco A, Bottalico A. Toxicity of mycotoxins of Fusarium sambucinum for feeding in Galleria mellonella. Entomologia Experimentalis Et Applicata. 1992;62:17–22. [Google Scholar]
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infection and Immunity. 2005;73:3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved standard. Document M38-A; Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clinical Microbiology Reviews. 1994;7:479. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and Immunocompromised hosts: Implications for diagnosis and management. Clinical Infectious Diseases. 2002;35:909–920. doi: 10.1086/342328. [DOI] [PubMed] [Google Scholar]
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clinical Microbiology Reviews. 2007;20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Cigelnik E, Casper HH. Molecular phylogenetic, morphological, and mycotoxin data support reidentification of the Quorn mycoprotein fungus as Fusarium venenatum. Fungal Genetics and Biology. 1998;23:57–67. doi: 10.1006/fgbi.1997.1018. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. Journal of Clinical Microbiology. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Sutton DA, Rinaldi MG, Magnon KC, Cox PA, Revankar SG, Sanche S, Geiser DM, Juba JH, van Burik J-AH, Padhye A, Anaissie EJ, Francesconi A, Walsh TJ, Robinson JS. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. Journal of Clinical Microbiology. 2004;42:5109–5120. doi: 10.1128/JCM.42.11.5109-5120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, Summerbell RC, Robert VA, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. Journal of Clinical Microbiology. 2010;48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DW, Jenkinson P, McLeod L. Fusarium ear blight (scab) in small-grain cereals - a review. Plant Pathology. 1995;44:207–238. [Google Scholar]
- Reeves EP, Messina CGM, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia. 2004;158:73–79. doi: 10.1023/b:myco.0000038434.55764.16. [DOI] [PubMed] [Google Scholar]
- Robert VA, Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. Journal of Infectious Diseases. 2009;200:1623–1626. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]
- Strongman DB, Strunz GM, Giguère P, Yu C-M, Calhoun L. Enniatins from Fusarium avenaceum isolated from balsam fir foliage and their toxicity to spruce budworm larvae, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) Journal of Chemical Ecology. 1988;14:753–764. doi: 10.1007/BF01018770. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Barr JR, Barr DB, Brock JW, Elie CM, Ueno Y, Patterson DG, Potter ME, Reiss E. Physiological characteristics and mycotoxins of human clinical isolates of Fusarium species. Mycological Research. 1999;103:1462–1468. [Google Scholar]
- Teetor-Barsch GH, Roberts DW. Entomogenous Fusarium species. Mycopathologia. 1983;84:3–16. doi: 10.1007/BF00436991. [DOI] [PubMed] [Google Scholar]
- Tortorano AM, Prigitano A, Dho G, Esposto MC, Gianni C, Grancini A, Ossi C, Viviani MA. Species distribution and in vitro antifungal susceptibility patterns of 75 clinical isolates of Fusarium sp. from Northern Italy. Antimicrobial Agents and Chemotherapy. 2008;52:2683–2685. doi: 10.1128/AAC.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JR, Yan KY, Dickman MB, Leslie JF. Electrophoretic karyotypes distinguish the biological species of Gibberella fujikuroi (Fusarium section Liseola) Molecular Plant-Microbe Interactions. 1995;8:74–84. [Google Scholar]
- Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. Journal of Clinical Microbiology. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]