Abstract
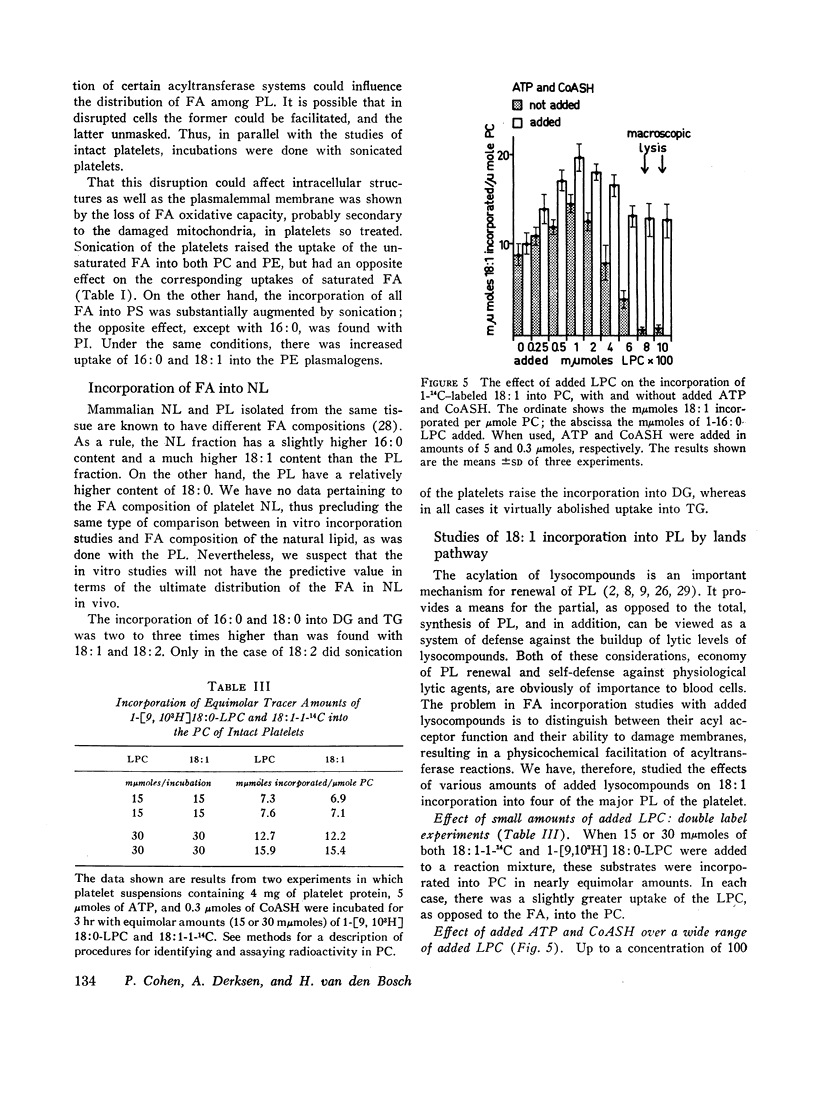
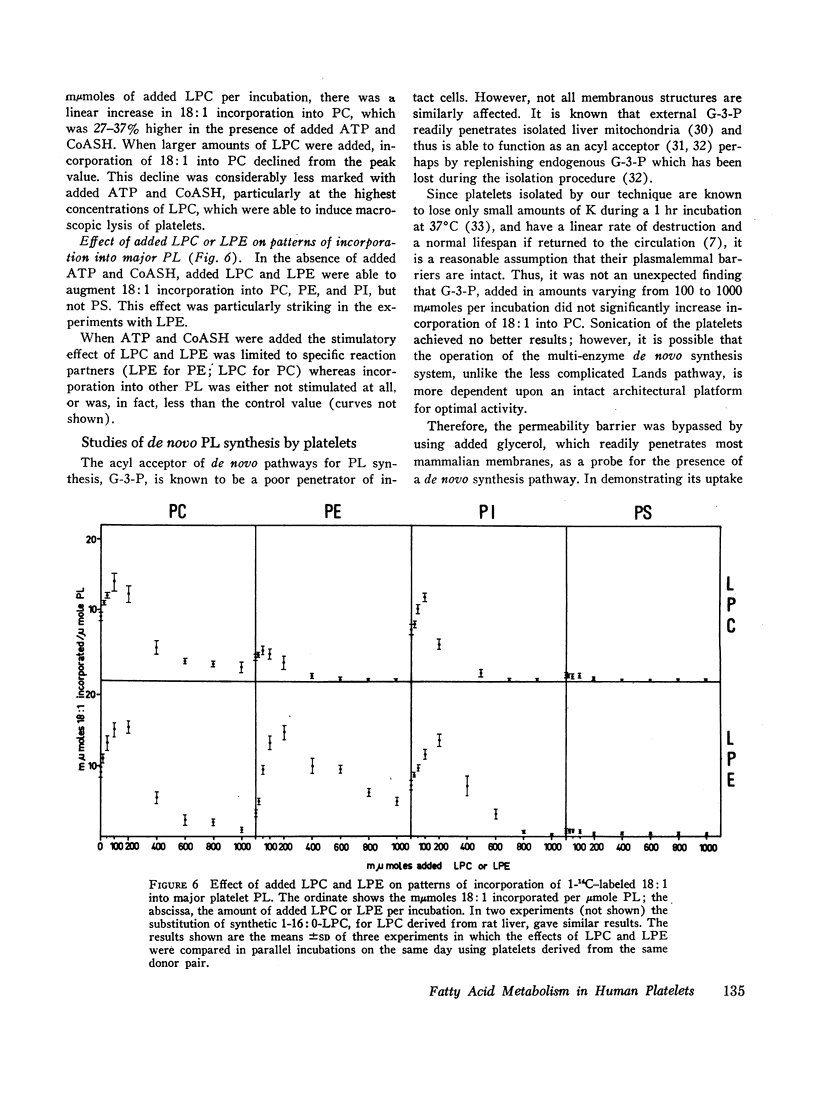
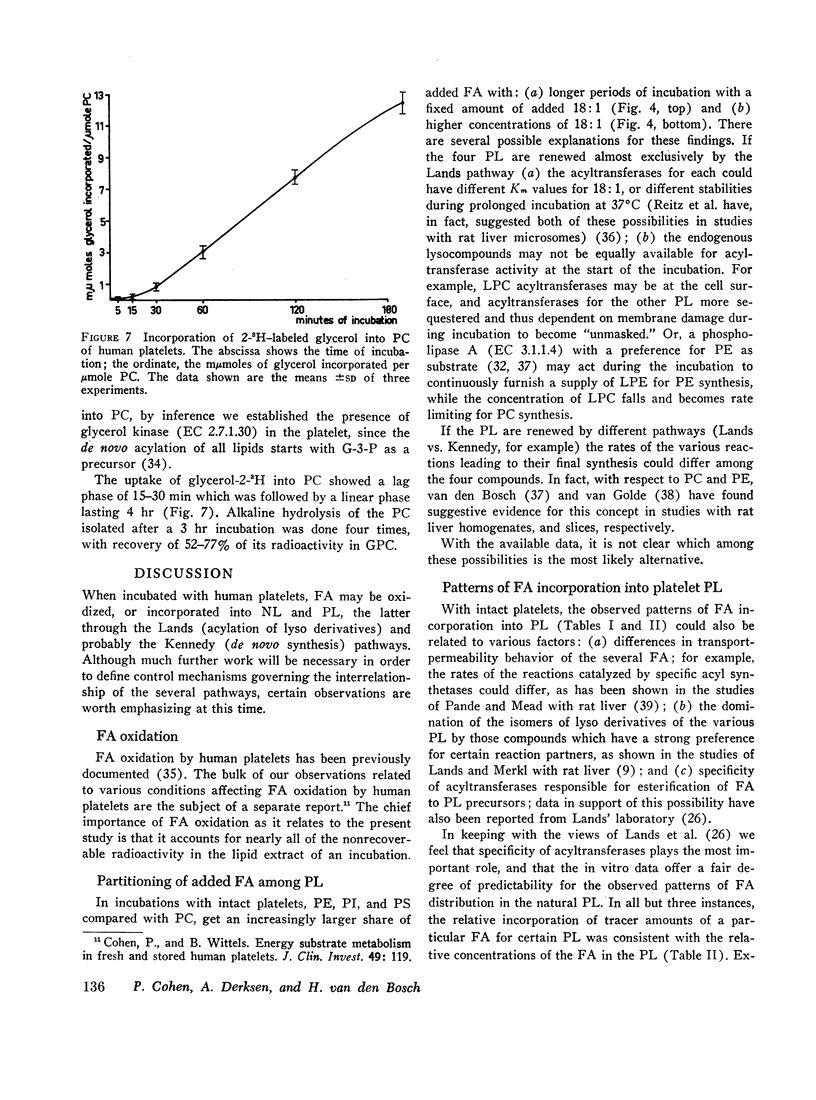
The metabolic fate of 14C-labeled fatty acids which have been incubated with human platelets, has been traced. The following has been shown. (a) Intact platelets have a considerable capacity to oxidize fatty acids. (b) When tracer amounts of four of the most common fatty acids in normal plasma were incubated with platelets, each showed a distinctive pattern of uptake among neutral lipids and phospholipids. With regard to the latter, it was shown that these distribution patterns were, in most cases, similar to those of the fatty acids found in natural platelet phospholipids. (c) By increasing the time of incubation or the amount of added oleic acid, the distribution of oleic acid uptake between lecithin and other phosphoglycerides was altered so that a larger share was incorporated into the latter. (d) The effects of added lysolecithin or lysoethanolamine phosphoglycerides on oleic acid incorporation into platelet phosphoglycerides are quite variable. At low concentrations, added lysolecithin functions chiefly as a reaction partner for oleic acid. Added adenosine triphosphate and CoASH augment the incorporation of oleic acid into lecithin over a wide range of added lysolecithin (12.5-500 μmoles/liter). At higher concentrations of added lysolecithin, in the absence of ATP and CoASH, oleic acid incorporation into lecithin is considerably reduced. Also, added lysolecithin and lysoethanolamine phosphoglycerides, in the absence of ATP and CoASH, are able, at certain concentrations, to stimulate oleic acid incorporation into all except the serine phosphoglycerides. (e) Platelets appear to have a de novo pathway for renewal of lecithin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAER E., BUCHNEA D. Synthesis of saturated and unsaturated L-alpha-lecithins; acylation of the cadmium chloride compound of L-alpha-glycerylphosphorylcholine. Can J Biochem Physiol. 1959 Aug;37(8):953–959. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BENSON A. A., MARUO B. Piant phospholipids. I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958 Jan;27(1):189–195. doi: 10.1016/0006-3002(58)90308-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BRECHER G., CRONKITE E. P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950 Dec;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- COLLIER H. B. Factors affecting the hemolytic action of "lysolecithin" upon rabbit erythrocytes. J Gen Physiol. 1952 Mar;35(4):617–628. doi: 10.1085/jgp.35.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Cooley M. H., Gardner F. H. Platelet preservation. 3. Comparison of radioactivity yields of platelet concentrates derived from blood anticoagulated with EDTA and ACD. N Engl J Med. 1965 Oct 14;273(16):845–850. doi: 10.1056/NEJM196510142731603. [DOI] [PubMed] [Google Scholar]
- Cooley M. H., Cohen P. Potassium transport in human blood platelets. J Lab Clin Med. 1967 Jul;70(1):69–79. [PubMed] [Google Scholar]
- Deykin D., Desser R. K. The incorporation of acetate and palmitate into lipids by human platelets. J Clin Invest. 1968 Jul;47(7):1590–1602. doi: 10.1172/JCI105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Phospholipid metabolism by phagocytic cells. I. A comparison of conversion of [32P]lysolecithin to lecithin and glycerylphosphorylcholine by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. Biochim Biophys Acta. 1966 Dec 7;125(3):510–524. [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- KOKKE R., HOOGHWINKEL G. J., BOOIJ H. L., van den BOSCH, ZELLES L., MULDER E., van DEENEN L. Metabolism of lysolecithin and lecithin in a yeast supernatant. Biochim Biophys Acta. 1963 Jun 18;70:351–353. doi: 10.1016/0006-3002(63)90763-7. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J Biol Chem. 1953 Sep;204(1):345–357. [PubMed] [Google Scholar]
- LANDS W. E., MERKL I. Metabolism of glycerolipids. III. Reactivity of various acyl esters of coenzyme A with alpha'-acylglycerophosphorylcholine, and positional specificities in lecithin synthesis. J Biol Chem. 1963 Mar;238:898–904. [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- Lands W. E., Blank M. L., Nutter L. J., Privett O. S. A comparison of acyltransferase activities in vitro with the distribution of fatty acids in lecithins and triglycerides in vivo. Lipids. 1966 May;1(3):224–229. doi: 10.1007/BF02531877. [DOI] [PubMed] [Google Scholar]
- Miloszewski K., Walls W. D., Losowsky M. S. Absence of plasma transamidase activity in congenital deficiency of fibrin stabilizing factor (Factor 13). Br J Haematol. 1969 Aug;17(2):159–162. doi: 10.1111/j.1365-2141.1969.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Mulder E., van Deenen L. L. Metabolism of red-cell lipids. 3. Pathways for phospholipid renewal. Biochim Biophys Acta. 1965 Oct 4;106(2):348–356. doi: 10.1016/0005-2760(65)90043-3. [DOI] [PubMed] [Google Scholar]
- Mulder E., van Deenen L. L. Metabolism of red-cell lipids. I. Incorporation in vitro of fatty acids into phospholipids from mature erythrocytes. Biochim Biophys Acta. 1965 Jul 7;106(1):106–117. doi: 10.1016/0005-2760(65)90099-8. [DOI] [PubMed] [Google Scholar]
- Mulder E., van den Berg J. W., van Deenen L. L. Metabolism of red-cell lipids. II. Conversions of lysophosphoglycerides. Biochim Biophys Acta. 1965 Jul 7;106(1):118–127. doi: 10.1016/0005-2760(65)90100-1. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA M. M., VAUGHAN M. INCORPORATION OF FATTY ACIDS INTO PHOSPHOLIPIDS OF ERYTHROCYTE MEMBRANES. J Lipid Res. 1964 Apr;5:156–162. [PubMed] [Google Scholar]
- Owens K. A two-dimensional thin-layer chromatographic procedure for the estimation of plasmalogens. Biochem J. 1966 Aug;100(2):354–361. doi: 10.1042/bj1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Long chain fatty acid activation in subcellular preparations from rat liver. J Biol Chem. 1968 Jan 25;243(2):352–361. [PubMed] [Google Scholar]
- ROBERTSON A. F., LANDS W. E. METABOLISM OF PHOSPHOLIPIDS IN NORMAL AND SPHEROCYTIC HUMAN ERYTHROCYTES. J Lipid Res. 1964 Jan;5:88–93. [PubMed] [Google Scholar]
- Reitz R. C., el-Sheikh M., Lands W. M., Ismail I. A., Gunstone F. D. Effects of ethylenic bond position upon acyltransferase activity with isomeric cis-octadecenoyl coenzyme A thiol esters. Biochim Biophys Acta. 1969 Apr 29;176(3):480–490. doi: 10.1016/0005-2760(69)90215-x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig A., Ways P. The oxidation of long-chain fatty acids by the formed elements of human blood. Blood. 1966 Jan;27(1):57–64. [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEENENL, DE HAAS G. H. THE SUBSTRATE SPECIFICITY OF PHOSPHOLIPASE A. Biochim Biophys Acta. 1963 Oct 22;70:538–553. doi: 10.1016/0006-3002(63)90792-3. [DOI] [PubMed] [Google Scholar]
- VEERKAMP J. H., MULDER I., van DEENEN L. Comparison of the fatty acid composition of lipids from different animal tissues including some tumours. Biochim Biophys Acta. 1962 Feb 26;57:299–309. doi: 10.1016/0006-3002(62)91123-x. [DOI] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]