Summary
Avoidance of and relapse to palatable foods is a qualitative aspect of dieting, a putative risk factor for eating disorders or obesity. The present studies tested the hypotheses that rats with alternating access to highly preferred foods would show: (1) hypophagia, a function of the relative hedonic value of the underaccepted diet, (2) increased anxiety-like behavior and psychomotor ar006Fusal when preferred diet was unavailable, (3) obesity-like changes, and (4) stable individual differences in diet-switch-induced hypophagia. Preferences among three high-carbohydrate diets were determined in female Wistar rats (n = 16). Adolescent rats (n = 162) received the following weekly diet schedules: (1) continuous regular chow (7 days/week), (2) chow (5 days/week) followed by a more preferred diet (2 days/week), or (3) chow (5 days/week) followed by a less preferred chow (2 days/week). Some animals were yoke-restricted (75% calories) when provided chow to increase its rewarding properties. Diurnal locomotor activity was measured in a familiar environment, and anxiety-like behavior was assessed in the elevated plus-maze and defensive withdrawal tests. Rats withdrawn from the preferred diet showed hypophagia, anxiogenic-like behavior, increased locomotion, and weight loss. Chow hypophagia was progressive, individual-specific in magnitude, (partly) non-homeostatic in nature, and blunted by previous chow restriction. Despite eating less, rats cycled with the preferred diet became heavier, fatter, and diurnally less active, with greater feed efficiency and proinflammatory
Keywords: Food intake OR feeding, Visceral obesity OR obese, Palatability, Hedonic evaluation, Negative contrast, Bulimia OR bulimic, Eating disorders, Anxiety, Preferred food dependence, Female rats
1. Introduction
Access to tasty, calorie-rich foods, promotes overeating (Yach et al., 2006). Paradoxically, individuals who restrict the types or quantities of foods that they eat putatively are at greater risk for eating disorders or obesity, although data in this regard are mixed (Polivy and Herman, 1985; Stice et al., 2005). Restrained eaters often limit themselves to “safe” foods, typically less palatable than energy-dense “forbidden” foods (Gonzalez and Vitousek, 2004; Stirling and Yeomans, 2004), to which they often return. Avoiding “forbidden” foods may thereby incidentally, but systematically, vary food palatability across time, experiences that might alter the control of feeding.
Indeed, when animals are switched from preferred to less preferred diets, exaggerated reductions in food intake occur (Archer et al., 2005; Corwin, 2004). Time-limiting access to palatable foods also alters the short-term control of feeding; restricted daily access (≤2 h/day) leads to binge-like intake of preferred food and reduced intake of less preferred foods between palatable food access (Bello and Hajnal, 2006; Corwin, 2006; Cottone et al., 2008). Yet, the effects of repeated, sustained alternations in food palatability, a schedule more like what occurs when humans avoid and relapse to “forbidden” foods, on the control of daily food intake are less known.
In this context, Boggiano (formerly Hagan) and colleagues developed a binge eating model that involves cycles of 2-day dietary supplementation with high-fat, palatable cookies, separated by multiple days of chow access only. In this model, rats with a joint history of caloric restriction during chow access followed by refeeding with cookies show increased intake of palatable food after footshock (Hagan et al., 2002). The control group in this model is ad libitum-fed rats that receive intermittent, supplementary access to cookies (Boggiano et al., 2007). However, research with children suggests that intermittent access to palatable foods itself may alter the acceptance (1-choice intake) and preferredness (multiple-choice relative intake) of foods (Birch and Davidson, 2001; Fisher and Birch, 1999). Consistent with this possibility, control subjects in the binge model show long-lasting reductions in deprivation-induced chow intake compared with rats that never tasted cookies (Hagan and Moss, 1997). The present studies therefore sought to test explicitly the hypothesis that short-term food intake by rats receiving alternating access to differently preferred high-carbohydrate foods becomes more controlled by relative diet palatability over cycles of access. This was predicted to be seen as increasing hyperphagia of the preferred food and increasing hypophagia of the otherwise acceptable, but less preferred, diet compared with chow-fed controls.
Intermittent access to rewarding drugs of abuse leads to negative emotional states when the rewarding substance is no longer available, perhaps due to allostatic, opponent-process shifts in brain reward circuitry (Koob and Le Moal, 2001; Solomon and Corbit, 1974). Such negative emotional states putatively motivate substance use via negative reinforcement mechanisms. Perhaps analogously, negative mood, anxiety, and tension are associated with intermittent dieting (Laessle et al., 1989) or switching to a low-fat diet (Wells et al., 1998) in humans and are suspected triggers of overeating palatable food (Hagan et al., 2002; Waters et al., 2001). Moreover, cessation of exposure to ethanol (Taylor et al., 2006) or opiates (Stinus et al., 1998), as well as stressors (Sabino et al., 2005), also is known to increase sleep-phase locomotor activity in familiar environments. Thus, the present study also tested the hypothesis that rats with alternating access to highly preferred foods would show increased anxiety-like behavior and psychomotor arousal when preferred diet was unavailable.
Voluntarily (Polivy and Herman, 1985; Stice et al., 2005) or involuntarily (Birch and Davidson, 2001) receiving intermittent access to palatable food has been proposed to promote obesity. However, an alternative explanation is that the behavior of restricting access to palatable food is a risk marker for individuals who otherwise would overeat or gain weight (Lowe and Kral, 2006). Thus, the present study tested the hypothesis that rats with alternating access to highly preferred foods would show obesity-like changes.
Finally, previous studies have reported individual differences in the propensity to overeat palatable food in a binge-like manner (Boggiano et al., 2007; Cottone et al., 2008) and conversely, in the magnitude of undereating an alternative tastant after prior access to a more palatable (Freet et al., 2006) and/or energy-dense food option (Levin and Dunn-Meynell, 2002). Thus, the present study tested the hypothesis that rats showed stable individual differences in the propensity to undereat chow after prior access to a more preferred food of similar energy density and macronutrient proportions.
In summary, the present studies tested the independent hypotheses that rats with alternating access to highly preferred foods would show: (1) progressively greater hypophagia of the less preferred diet, (2) increased anxiety-like behavior and psychomotor arousal when preferred diet was unavailable, (3) obesity-like changes, and (4) stable individual differences in diet-switch-induced hypophagia. To test the role of non-nutritional (e.g., hedonic) factors in food intake adaptations, as opposed to increased adiposity or other energy homeostatic factors, several procedures were used. First, diets were chosen to differ in preferredness, and less so in energy density or macronutrient proportions. Second, relationships between undereating of chow on the one hand and body weight and adiposity on the other were examined. Third, some animals were offered alternating access to a less preferred diet that would not promote weight gain. Finally, the reward associated with chow diet was increased by calorically restricting rats during chow access. This experiment followed from the premise that if hypophagia of chow was due to a negative hedonic comparison of the chow to the preferred food, then increasing the rewarding properties of chow via concurrent food restriction should reduce underacceptance of chow selectively in the diet-cycled group. Conversely, if food restriction was increasing relative chow intake for energy homeostatic reasons, then one would not predict its effects to differ per diet schedule. To test the second, “anxiety” hypothesis, diet-alternated rats were studied in the elevated plus-maze, defensive withdrawal test, and familiar locomotor activity chambers after withdrawing access to preferred food. To test the third, “obesity measures” hypothesis, the feed efficiency, body weight, adiposity, and several adipose tissue-derived proinflammatory factors of diet-cycled rats were compared with those of chow-fed subjects. To test the fourth “individual differences” hypothesis, intraclass correlations on chow intake after being switched from preferred diet to chow were performed (Shrout and Fleiss, 1979).
2. Materials and methods
2.1. Subjects
Wistar rats (total n = 139; Charles River, Raleigh, NC) were single-housed on arrival in wire-topped, plastic cages (19 in. × 10.5 in. × 8 in.) in a 12:12 h lit (08:00 h lights off), humidity- (60%) and temperature-controlled (22 °C) vivarium. Adolescent females (126–150 g, 41–47 days old) were studied because of the greater prevalence of dietary restraint behavior in young women than men (George and Johnson, 2001) and because of the putative longitudinal relationship between dieting in adolescent women and subsequent binge eating or obesity (Neumark-Sztainer et al., 2007; Reas and Grilo, 2007; Stice et al., 2002). Independent cohorts participated in a diet preference study and in each of four subsequent experiments. Rats had access to corn-based chow (LM-485 7012: 65% [kcal] carbohydrate, 13% fat, 21% protein, metabolizable energy 3.41 kcal/g; Harlan, Indianapolis, IN) and water ad libitum for 1 week before experiments. Food was provided in GPF20 “J”-feeders (Ancare, Bellmore, NY). Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996) and the “Principles of laboratory animal care” (http://www.nap.edu/readingroom/bookslabrats) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2. Diet preferences
Rats were provided choice access to chow diet and to one of two alternative diets (n = 8/test), comparable in energy density and macronutrient proportions to the chow diet. Once intake stabilized, preference was calculated as the % of daily intake (kcal) comprised by the alternative diet. One diet, preferred over chow by each rat (M ± S.E.M. preference: 92.2 ± 1.1%), is a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A based diet (chocolate-flavored Formula PJPPP, Research Diets, Inc., New Brunswick, NJ: 69.1% [kcal] carbohydrate, 11.8% fat, 19.1% protein, metabolizable energy 3.70 kcal/g; formulated as 45 mg pellets to increase its preferredness; Laboure et al., 2001). The other diet, slightly less preferred to regular chow (33.5 ± 18.5%), was a fixed-nutrition, corn-based diet (Purina 5012; LabDiet, Richmond, IN: 62.7% [kcal] carbohydrate, 10.7% fat, 26.7% protein, metabolizable energy 3.11 kcal/g) formulated in 4– 5 g pellets. The alternative diets will be referred to as “Preferred” and “Non-Preferred,” respectively.
Hypothesis 1
Rats with alternating access to highly preferred food would show progressive hypophagia of the less preferred diet.
2.2.1. Alternating access to a more preferred diet
Rats were divided into two ad libitum-fed groups matched for chow intake, body weight and feed efficiency from the previous 3 days. Each week, one group received chow daily (Chow/Chow, n = 16), whereas the second group received chow for 5 days followed by 2 days of access to the preferred diet (Chow/Preferred, n = 14). For brevity, the first 5 days (chow only) of weekly diet cycles will be referred to as C phases and the last 2 days (chow or preferred per experimental group) will be referred to as P phases. The length of diet cycles (7 days) reduces the likelihood that the estrous cycle (4–5 days) accounts for observed effects and resembles designs used in previous diet cycling studies of female rats (Hagan and Moss, 1997).
2.2.2. Alternating access to a less preferred diet
Rats were divided into two ad libitum-fed groups matched for chow intake, body weight and feed efficiency from the previous 3 days. Each week, one group received chow daily (Chow/Chow, n = 12), a second group (Chow/Preferred, n = 14) received chow for 5 days followed by 2 days of access to the preferred diet, and a third group (Chow/Non-Preferred, n = 8) (referred to as NP phases) received chow for 5 days followed by 2 days of access to the less preferred diet.
2.2.3. Increasing chow’s rewarding properties by concurrent caloric restriction
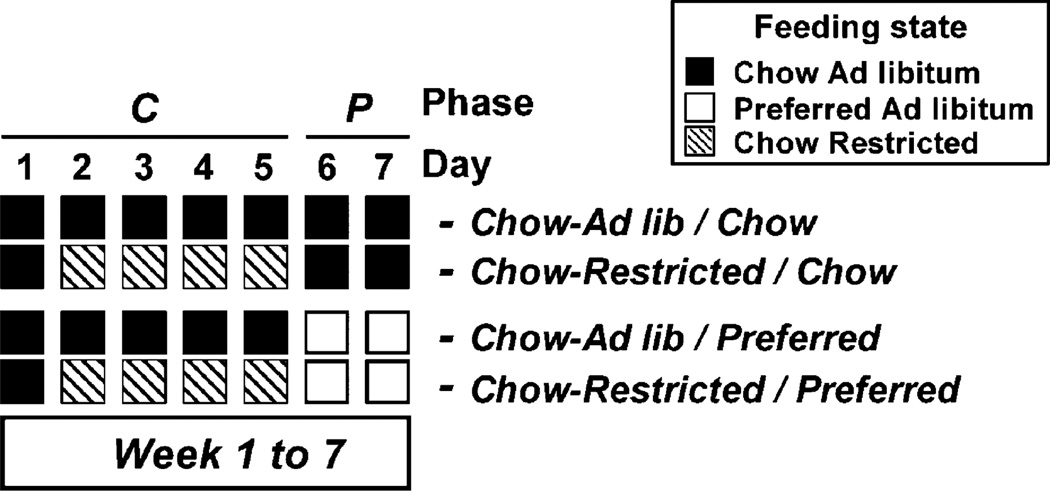
Subjects (n = 60) were divided into 4 groups, matched for baseline weight and chow intake, per a 2 (diet schedule) × 2 2 (caloric restriction) factorial design (see Figure 1). Diet schedule (Chow/Chow vs. Chow/Preferred) referred to which diet rats received during the first 5 days vs. last 2 days of each week. Caloric restriction (Ad lib vs. restricted) referred to whether rats had ad libitum or calorically restricted access to chow during Days 2–5 of each week (the last 4 days of the C [“Chow”] phase). Thus, the 4 experimental groups were: Chow-ad lib/Chow, Chow-restricted/Chow, Chow-ad lib/Preferred, and Chow-restricted/Preferred. Restricted groups received 75% of the mean daily intake of the Chow-Ad lib/Preferred group, which was the reference group for yoking so that all groups would receive a uniform daily ration that was smaller than either ad lib-fed group would otherwise consume. Yoking intake to the Chow-ad lib/Chow group would not have guaranteed restriction below voluntary intake because of the spontaneous hypophagia of the Chow-ad lib/Preferred group. Food was otherwise available ad libitum. The daily ration during restriction ranged from 82 to 49% (grand mean = 62%) of the caloric intake of Chow-ad lib/Chow rats. For testing, intake was compared on Day 1 of each week when subjects had not been food-restricted for at least 48 h and were eating the same chow diet, but with a different feeding history. Those in restricted, but not ad libitum, groups had eaten chow while food restricted.
Figure 1.
Study design of Experiment 3.
Hypothesis 2
Rats with alternating access to highly preferred food would show increased anxiety-like behavior and psychomotor arousal when preferred diet was unavailable.
2.2.4. Diurnal motor activity in a familiar environment
Diurnal motor activity was assessed in previously described wire-mesh photocell cages (20 cm × 25 cm × 36 cm) (Zorrilla et al., 2002) to which rats had been acclimated on the previous day for the duration of the light cycle. On test days, rats were further acclimated to the cages beginning 1.5 h prior to the light cycle onset. White noise (70 dB) was present during acclimation and testing. Random subsets of Chow/Chow and Chow/Preferred rats from Experiment 1 (n = 6–8/group) were tested on Days 1 and 4 (C phase) and Day 6 (P phase) of experimental Week 10, with their diet and water freely available. Photocell interruptions were recorded by computer across the 12-h light cycle.
2.2.5. Anxiety-like behavior
2.2.5.1. Elevated plus-maze test
The black Plexiglas plus-maze apparatus (Cottone et al., 2007, 2008; Zorrilla et al., 2002) consisted of four arms (10 cm × 50 cm) positioned at right angles, 50 cm above the floor. Two “closed” arms had 40-cm high walls; two “open” arms had 0.5-cm high ledges. Testing occurred in a dim room with 1.5–2.0 lx of open arm illumination and <1 lx in the closed arms. Rats were habituated to the anteroom during handling the day before testing. Rats remained in the dark anteroom for ≥2 h before testing. White noise (70 dB) was present during habituation and testing. To begin the 5-min test, rats were placed individually onto the center of the maze facing a closed arm. The apparatus was cleaned with a water-dampened cloth after each subject (File et al., 2004; Schulteis et al., 1998; Shah et al., 2004). The primary measures were the percent of total arm time directed towards the open arms (i.e., 100 × open arm/[open arm + closed arm]), a validated inverse index of anxiety-related behavior (Fernandes and File, 1996), and the number of closed arm entries, a specific index of locomotor activity in a novel environment (Cruz et al., 1994). During the 8th week of diet cycling, a random subset of rats from Experiment 1 were tested in a between-subjects design 5–9 h after being switched from the preferred diet to chow or from chow to the preferred diet. Chow-only controls were tested concurrently (n = 8–16/group/diet conditions). Food was available ad libitum until testing.
2.2.5.2. Defensive withdrawal test
The defensive withdrawal test (Zorrilla et al., 2002) apparatus was a walled, black polyvinylchloride open field (106 cm × 92 cm × 77 cm) containing a cylindrical “withdrawal” chamber (2-L Pyrex beaker wrapped in brown tape). The chamber was located 15 cm from a corner facing the open arena. Rats were kept in the dark anteroom for ≥2 h before testing. During the 9th week of diet cycling, a random subset of rats from Experiment 1 were tested 5–10 h after being switched from the preferred diet to chow. Chow-only controls were tested concurrently (n = 5–16/group). Food was available ad libitum until testing. For the 10-min test, rats were placed into the withdrawal chamber facing the rear, and behavior was video recorded. Two reliable, treatment-naive raters scored the latency to first emerge (all four paws in the open field) and the total duration of subsequent withdrawals. Testing occurred under room light (~300 lx).
Hypothesis 3
Rats with alternating access to highly preferred food would show obesity-like changes.
2.2.6. Intake, body weight and feed efficiency
Food and water intake were measured daily during the C phase and at the end of the P (or NP) phase. Body weight was recorded at the end of diet phases. Measures were obtained using a scale of 0.1 g precision. Feed efficiency was calculated as mg body weight gained/kcal energy intake.
2.2.7. Circulating proinflammatory factors
Beginning on experimental Day 92, a random subset of rats from Experiment 1 (n = 6–7/group) were fed chow diet only for 10 consecutive days to control for acute diet effects. Then, on Day 102, overnight fasted (18–21 h) rats were decapitated 2–5 h into the dark cycle, and trunk blood was collected in chilled tubes containing 0.5 M EDTA (~1:10, v/v) and a commercial protease inhibitor cocktail (~1:100, v/v; Sigma Catalog P8340). Plasma was isolated by centrifugation (4 °C, 3000 × g, 15 min) and stored at −80 °C until analysis for levels of rat interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α, total plasminogen activator inhibitor-1 (tPAI-1), andmonocyte chemoattractant protein-1 (MCP-1), by an xMAP technology-based multiplex panel immunoassay using Luminex® instrumentation (Linco, St. Charles, MO). The typical intra-assay coefficient of variation is <4%. The typical minimum detectable concentration is 4.9 pg/ml for IL-1β, TNF-α, and MCP-1 and 12.2 pg/ml for IL-6 and tPAI-1.
2.2.8. Fat pad weights and body composition analysis
Following blood collection, carcasses were frozen (−80 °C). Inguinal (subcutaneous) and gonadal (intra-abdominal/visceral) white fat pads were later dissected and returned to the thawed (25 °C) carcass. Carcasses were then dried (60 °C) to constant mass to determine water content and extracted with petroleum ether in a Soxhlet apparatus to determine fat mass and fat-free dry mass (FFDM; protein and ash) (Harris and Martin, 1984).
Hypothesis 4
Rats with alternating access to highly preferred food would show stable individual differences in diet-switch-induced hypophagia.
To determine whether rats with alternating access to highly preferred food showed stable individual differences in diet-switch-induced hypophagia, individual differences in the tendency to undereat chow in the 24 h after being switched from preferred diet to chow were quantified by two-way, random effect intraclass correlations (Shrout and Fleiss, 1979) using data from Weeks 2–7. Rats were classified as being “sensitive” or “resistant” to diet switches by median split analysis of average normalized chow intake on Day 1 of Weeks 2–7.
2.3. Statistical analysis
Group comparisons used Student’s t-tests (2-group comparisons) or analysis of variance (ANOVA) (≥3-group comparisons), the latter interpreted by simple main effect analysis or Newman–Keuls comparisons after significant omnibus effects (p < 0.05). Average daily intake, feed efficiency, and changes in body weight were calculated for each diet phase, but day-by-day results are also presented in the Supplementary Results, where indicated. Data from Experiments 1 and 2 were analyzed by three-way mixed ANOVAs with diet schedule as a between-subjects factor and week and diet phase as within-subject factors. Chow intakes on Day 1 of each week in Experiment 3 were compared by three-way ANOVA with week as a within-subjects factor and diet schedule and caloric restriction as between-subjects factors. Cumulative food intake, weight gain and feed efficiency after 7 weeks were compared by t-test. Differences in motor activity, adipocytokine levels, adiposity or anxiety-like behavior were assessed by t-test. Due to in homogeneity and skewness of variance, adipocytokine levels were log-transformed for statistical analysis. The statistical packages used were Instat 3.0 and GraphPad Prism4.0 (GraphPad, San Diego, CA, USA), Systat 11.0 and SPSS 11.5 (SPSS, Chicago, IL, USA).
3. Results
Hypothesis 1
Rats with alternating access to highly preferred food would show progressive hypophagia as a function of the relative hedonic value of the under-accepted diet.
3.1. Alternating access to a more preferred diet
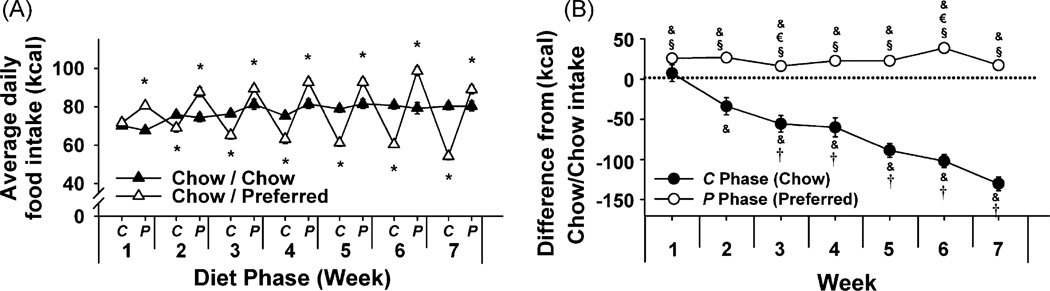
As shown in Figure 2A, Chow/Preferred rats in Experiment 1 overate during the P (preferred) phase and underate during the C (chow) phase, as compared with Chow/Chow controls (diet phase × diet schedule: F[1, 28] = 113.4, p < 0.001). This interaction reflects that when the Chow/Chow and Chow/Preferred groups are considered separately, a very strong, linear effect of week is found in Chow/Chow animals (week: F[6, 90] = 18.5, p = 6.4 × 10−14), reflecting that food intake of Chow/Chow subjects monophasically and progressively increased with age. In contrast, chow intake of Chow/Preferred rats decreased progressively, whereas their overeating of preferred food remained stable (Figure 1B; week × diet phase: F[6, 78] = 30.0, p = 4.2 × 10−19). Day-by-day analysis showed that chow hypophagia progressively increased because both the magnitude and persistence of chow hypophagia increased across diet cycles (Supplementary Figure 1). As a result, total energy intake of Chow/Preferred rats across the entire study was less than that of Chow/Chow controls (M ± S.E.M.: 3779.1 ± 75.9 kcal vs. 3486.4 ± 55.6 kcal, t[28] = 3.03, p < 0.005). Chow/Preferred rats also showed cyclic drinking behavior (week × diet diet phase × diet schedule: F[6, 168] = 8.02, p < 0.001). However, relative to Chow/Chow controls, water intake of Chow/Preferred rats decreased during access to the preferred diet, and was normal or increased during chow access (Supplementary Figure 2).
Figure 2.
Effects of repeated, alternating 5-day access to chow and 2-day access to either chow (Chow/Chow, n = 16) or highly preferred chocolate-flavored sugary diet (Chow/Preferred, n = 14) in female Wistar rats. Panels show (M ± S.E.M.) (A) average daily food intake, and (B) food intake of Chow/Preferred rats within C (Chow) and P (Preferred) diet phases relative to chow-fed controls. Symbols indicate significant differences (p < 0.05) from *Chow/Chow, &0, †C (Chow) phase of Week 2, €P (Preferred) phase of Week 2, or §respective C (Chow) phase.
3.2. Alternating access to a less preferred diet
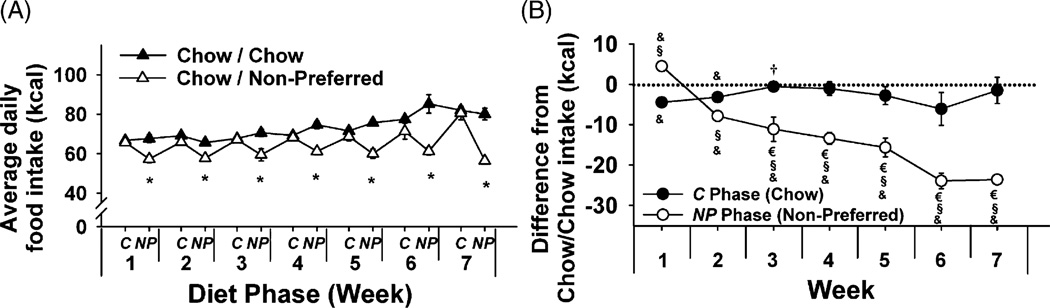
When cycled with preferred food in Experiment 2, Chow/Preferred rats again showed stable hyperphagia of the preferred diet and increasingly underate standard chow relative to Chow/Chow controls (not shown). Chow/Non-Preferred rats stably accepted, but did not overeat, their more preferred food, which in their case was the standard chow (C phase), while progressively undereating their less preferred food, the non-preferred diet (NP phase) (week × diet phase × diet schedule interaction, F[12, 150] = 3.99, p < 0.001) (see Figure 3A and B). This interaction reflects that when the Chow/Chow and Chow/Non-Preferred groups are considered separately, a strong, linear effect of week is found in Chow/Chow animals (week: F[6, 66] = 25.2, p = 2.4 × 10−15; diet phase: F[1, 11] = 3.28, p = 0.097), reflecting that food intake of Chow/Chow subjects monophasically and progressively increased with age. In contrast, Chow/Non-Preferred rats maintained control-like levels of regular chow food intake with relative and progressive reductions in intake of the non-preferred diet (Figure 1C; week × diet phase: F[6, 42] = 5.55, p = 0.00026; diet phase: F[1, 7] = 37.79, p = 0.000469). By the end of Week 7, Chow/Non-Preferred rats weighed less than Chow/Chow rats (251.4 × 5.4 g vs. 261.8 × 2.5 g, respectively; t[18] = 1.95, p < 0.05). Thus, progressive hypophagia of a less preferred diet was not specific to a given diet or schedule of access (5 days vs. 2 days of access to the preferred food) and did not require excess body weight. Rather, diets were stably eaten or increasingly undereaten in relation to their relative preferredness.
Figure 3.
Effects of repeated, alternating 5-day access to chow and 2-day access to either regular chow (Chow/Chow, n = 12) or a slightly less preferred chow (Chow/Non-Preferred, n = 8) in female Wistar rats. Panels show (M ± S.E.M.) (A) average daily food intake, and (B) food intake of Chow/Non-Preferred rats within C (Chow) and NP (Non-Preferred) diet phases relative to chow-fed controls. Symbols indicate significant differences (p < 0.05) from *Chow/Chow, &0, †C (Chow) phase of Week 2, or €NP (Non-Preferred) phase of Week 2, or §respective C (Chow) phase.
3.3. Food restriction during chow access blunts development of chow hypophagia
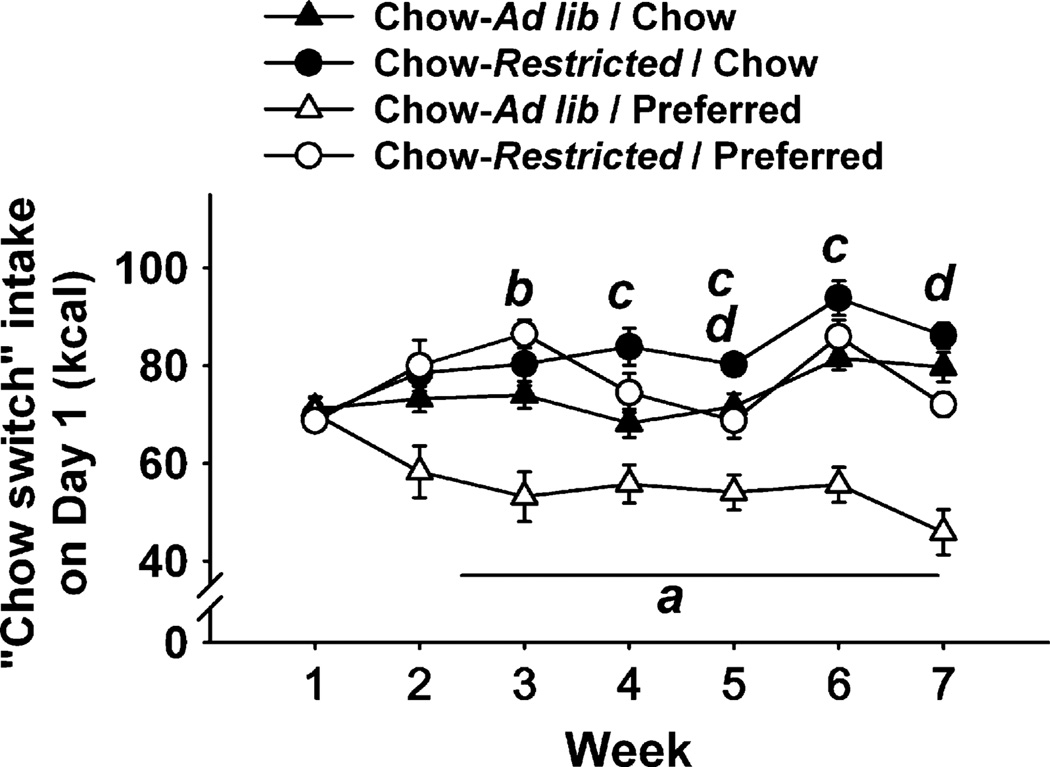
In Experiment 3, Chow-ad lib/Preferred rats ate less chow than all other groups during the probe tests on Day 1 of each weekly cycle (Figure 4), yielding a week × diet schedule × experimental restriction interaction (F[6,336] = 2.71, p < 0.001). As predicted, Chow-restricted/Preferred rats, which were calorically restricted during chow feeding (see Figure 1 for experimental design), ate more chow each week in a non-deprived state than did Chow-ad lib/Preferred rats. Indeed, Chow-restricted/Preferred rats initially even overate chow compared to Chow-ad lib/Chow conditions (e.g., Week 3). The blunted chow hypophagia of Chow-restricted/Preferred rats did not appear to be attributable to: (1) food restriction history per se because Chow-restricted/Chow rats did not show a comparable increment relative to Chow-ad lib/Chow rats (Figure 4); (2) continued recovery from restriction-induced weight loss because Chow-restricted/Preferred rats weighed the same as Chow-ad lib/Preferred rats prior to chow switches (see Supplementary Figure 3), or (3) greater refeeding because Chow-restricted/Preferred rats had not eaten more than Chow-ad lib/Preferred rats during the preceding 48-h P phase (Supplementary Figure 3). By Weeks 6 and 7, Chow-restricted/Preferred rats finally began to undereat chow relative to Chow-restricted/Chow rats but still not to the same degree as Chow-ad lib/Preferred rats. Thus, concurrent food restriction attenuated, but did not altogether block, the development of chow hypophagia following alternating access to a more preferred diet.
Figure 4.
Effects of diet alternation with or without caloric restriction during chow access on “chow switch” intake, or chow intake after preferred diet access on Day 1 of each weekly cycle (see Figure 1). Letters denote significant difference (p < 0.05) of: (a) Chow-Ad lib/Preferred (n = 16) from all groups; (b) Chow-Restricted/Preferred (n = 16) from Chow-Ad lib/Chow (n = 14); (c) Chow-Restricted/Chow (n = 14) from Chow-Ad lib/Chow; (d) Chow-Restricted/Preferred from Chow-Restricted/Chow. Panels show M ± S.E.M.
Hypothesis 2
Rats with alternating access to highly preferred food would show increased anxiety-like behavior and psychomotor arousal when preferred diet was unavailable.
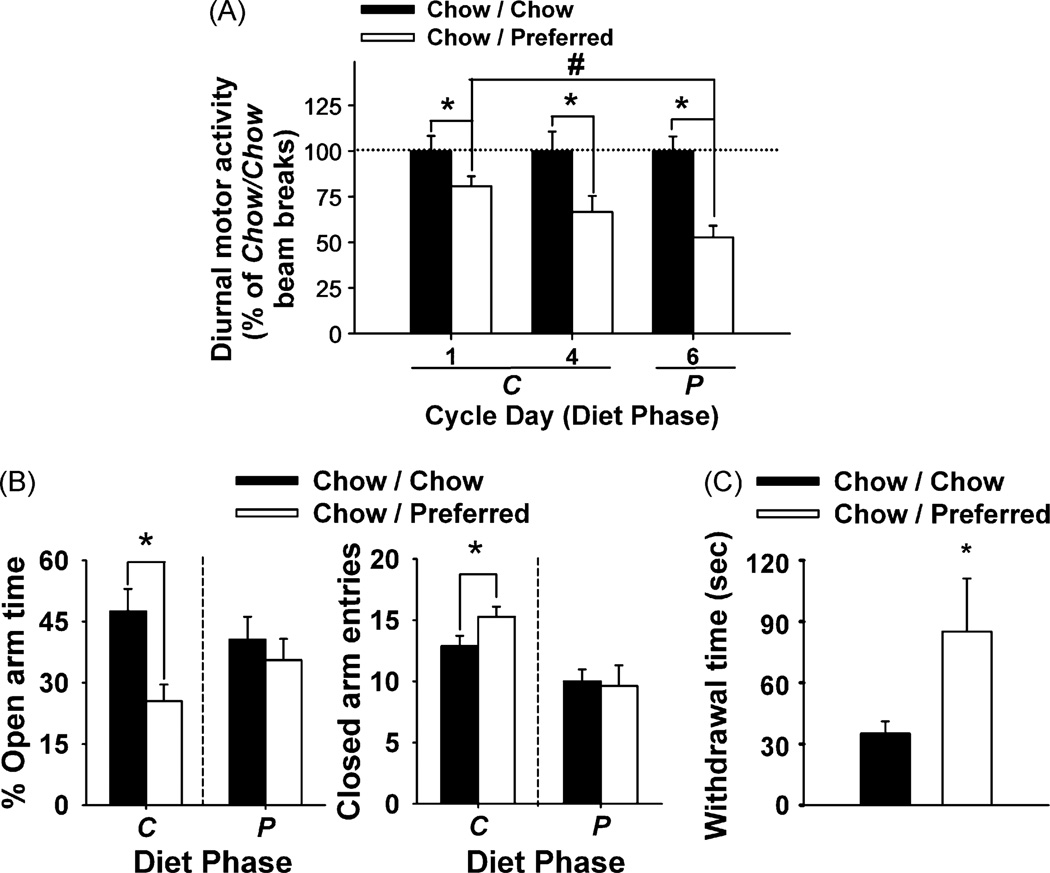
By Week 10, Chow/Preferred rats showed less 12-h diurnal motor activity than Chow/Chow rats regardless of what diet they were currently receiving (diet schedule: F(1,35) = 25.29, p < 0.001). However, Chow/Preferred rats were significantly more active on the first day that they were switched to the chow diet (C-Day 1) compared with when they had access to the preferred diet (P-Day 6) (Figure 5A). Chow/Preferred rats also exhibited reduced open arm time, an anxiogenic-like effect, and increased closed arm entries, a measure of hyperactivity, following switches to chow diet (C phase), but not following switches to preferred diet (P phase) (Figure 5B). Finally, after being switched to chow diet, Chow/Preferred rats also withdrew more into the sheltered chamber of the defensive withdrawal test than did Chow/Chow rats, another anxiogenic-like effect (Figure 5C), with the two groups showing similar initial latencies to emerge (26.0 ± 4.2 s vs. 22.0 ± 7.7 s, respectively).
Figure 5.
Long-term effects of diet schedule on motor activity and anxiety-like behavior in female Wistar rats. Panels show (M ± S.E.M.) (A) 12-h diurnal motor activity, represented as percent of Chow/Chow beam breaks. Rats were tested on Days 1, 4 (C phase) and 6 (P phase) of experimental Week 10 (n = 6–8/group), (B) elevated plus-maze behavior (left) percent of total arm time directed towards the open arms (lower % open arm time signifies more anxiogenic-like behavior), and (right) number of closed arm entries, an index of locomotor activity. Rats were tested during Week 8, 5–9 h after switches from preferred diet (B → A phase) or from chow diet (A → B phase), in a between-subjects design (n = 8–16/group), or (C) time spent in the withdrawal chamber during the defensive withdrawal test. Rats were tested during Week 8, 5–10 h after switches from palatable diet to chow diet (B → A phase) (n = 5–16/group). In all studies, the phase-appropriate diet and water were available ad libitum. Symbols indicate significant differences (p < 0.05) from *Chow/Chow; #Chow/Preferred Day 1 motor activity.
Hypothesis 3
Rats with alternating access to highly preferred food would show obesity-like changes.
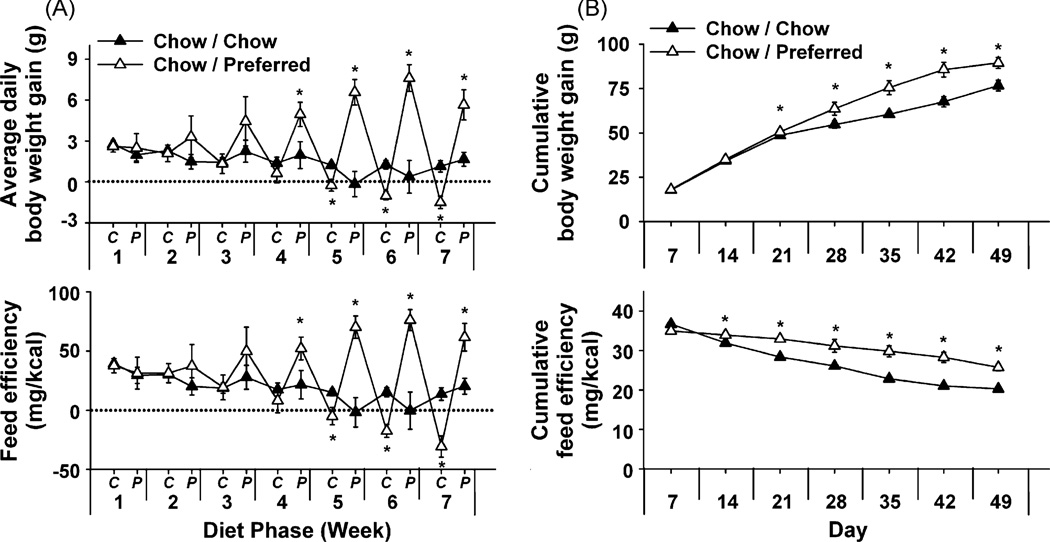
With diet cycling, Chow/Preferred rats also showed amplifying cycling of body weight (Figure 6A, top) in contrast to the neutral weight trajectory of Chow/Chow rats (week × diet phase × diet schedule: F[6, 168] = 3.44, p < 0.005). Chow/Preferred rats lost body weight during each chow phase (up to 8 g, or 3% total body weight) and regained more weight with each refeeding. Body weight changes were not fully explained by feeding changes, indicated by cycling feed efficiency (Figure 6A, bottom) (week × diet phase × diet schedule: F[6, 168] = 3.37, p < 0.001). Diet history-related differences in weight gain emerged later than those in food intake and became asymmetrically positive (Figure 6A, top). Accordingly, despite eating less than controls, Chow/Preferred rats showed increased cumulative weight gain and feed efficiency (Figure 6B).
Figure 6.
Effects of repeated, alternating 5-day access to chow and 2-day access to either chow (Chow/Chow, n = 16) or highly preferred chocolate-flavored sugary diet (Chow/Preferred, n = 14) in female Wistar rats. Panels show (M ± S.E.M.) (A) (Top) average daily change in body weight, and (Bottom) feed efficiency (body weight change/energy intake) during each C (Chow) or P (Preferred) diet phase of each week, or (B) cumulative body weight gain (Top) and feed efficiency (Bottom). *Differs from Chow/Chow p *< 0.05.
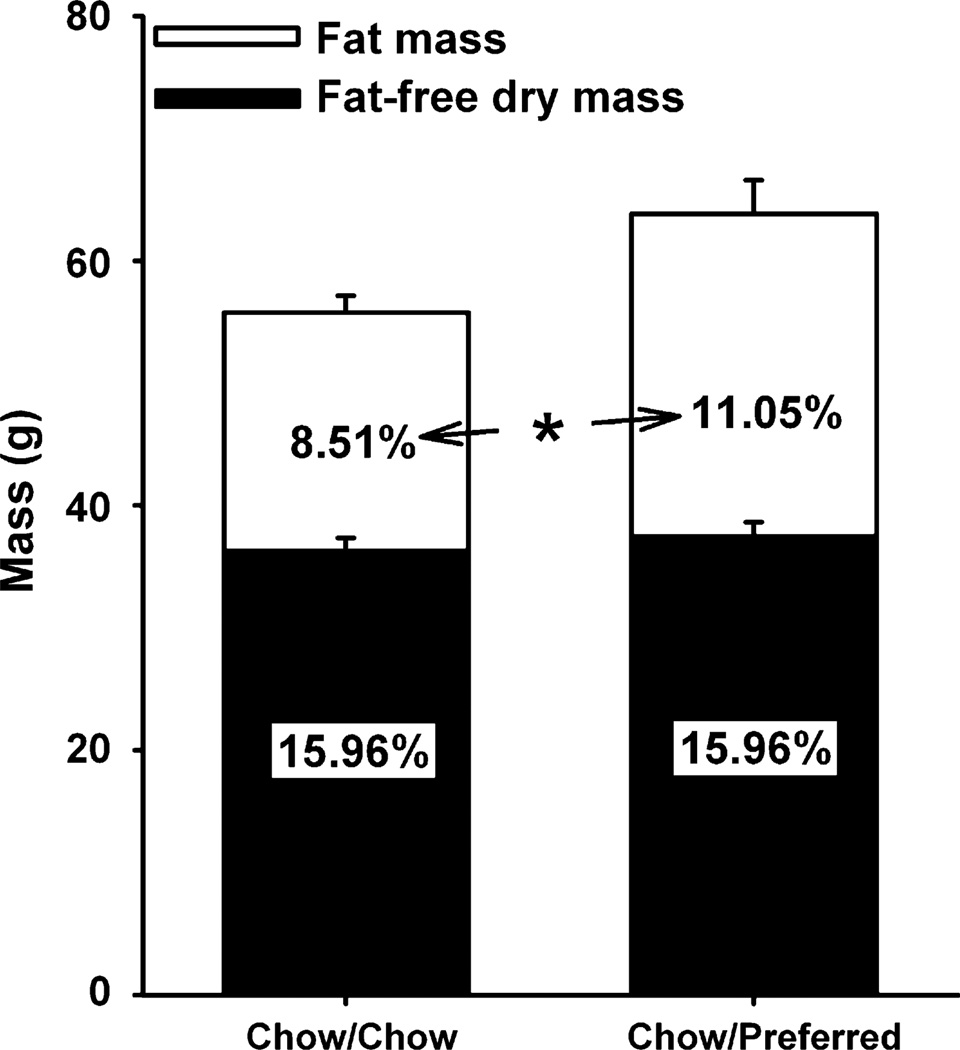
Chow/Preferred rats showed selective gains in terminal fat mass over lean mass (Figure 7), with ~3.9 g increases in gonadal fat pads (10.4 ± 1.2 g vs. 6.5 ± 0.7 g, p < 0.05), approximately 2-fold greater than increases in inguinal fat pads (1.9 g) (6.5 ± 0.7 g vs. 4.6 ± 0.3 g, p < 0.05). Chow/Preferred rats also exhibited greater circulating levels of IL-1β (44.2 + 14.2 pg/ml vs. 8.9 + 1.7 pg/ml, p < 0.05), IL-6 (70.1 + 44.4 pg/ml vs. 3.2 + 0.2 pg/ml, p < 0.05), and tPAI-1 (120.2 + 33.8 pg/ml vs. 43.0 + 23.7 pg/ml, p < 0.05), but not TNF-α or MCP-1 (see Supplementary Figure 6), than chow-fed controls.
Figure 7.
Long-term effects of diet schedule on body composition. Panel shows (M ± S.E.M.) fat mass and fat-free dry mass of a random subset of female Wistar rats from Experiment 1 (n = 15). On Day 92, rats were fed chow diet only for 10 consecutive days to control for acute diet effects. On Day 102, overnight fasted rats were decapitated 2–5 h into the dark cycle. Stacked bars represent absolute weights, and inset numerals indicate percent of total carcass mass. *Both absolute and relative fat mass of Chow/Preferred rats differ from Chow/Chow rats, p < 0.05.
Hypothesis 4
Rats with alternating access to highly preferred food would show stable individual differences in diet-switch-induced hypophagia.
Chow/Preferred rats showed stable individual differences in their degree of undereating chow after being switched from the preferred diet, unlike Chow/Chow rats that showed less regular variations in daily intake (ICC[2,6] = 0.81 vs. 0.31; F[13,65] = 5.30, p < 0.00001 vs. F[15,75] = 1.45, p > 0.14). Median split analysis showed that “switch-sensitive” Chow/Preferred rats only consumed 50% of Chow/Chow daily intake after diet switches (37.5 ± 5.1 kcal less, p < 0.000001). Chow/Preferred rats also ate more preferred diet than their “switch-resistant” counterparts by the end (6th and 7th weeks: 98.8 ± 2.2 kcal vs. 89.6 ± 2.8 kcal, t[12] = 2.35, p < 0.05), but not beginning (1st and 2nd weeks: 85.7 ± 2.8 kcal vs. 82.4 ± 1.6 kcal, t[12] = 1.04, p = 0.31), of the measurement period. Note that rats “resistant” to chow hypophagia following preferred diet access also ate less chow than Chow/Chow rats (10.1 ± 2.4 kcal less, p < 0.01), just not to the same degree as “switch-sensitive” rats (p < 0.005). “Switch-sensitive” rats were significantly less fat than “resistant” rats at study completion defined by both total fat mass (M ± S.E.M.: 23.7 ± 3.0 g vs. 33.0 ± 0.7 g, p < 0.04) and % fat mass (M ± S.E.M.: 10.28 ± 0.95% vs. 12.98 ± 0.01%, p < 0.05).
4. Discussion
Female rats with alternating access to differently preferred foods progressively decreased intake of their less preferred food. When preferred diet access was withdrawn, rats showed increased anxiety-like behavior and diurnal locomotor activation, effects not seen with renewed access to the preferred diet. Rats with cyclic access to the highly preferred diet ultimately became overweight and developed morphometric and endocrine signs of obesity, despite eating fewer calories than chow-fed controls. The present diet cycling procedure may model changes in feeding, anxiety-like behavior, metabolism or adiposity that are associated with intermittent palatable food access that may occur when humans avoid “forbidden” foods or qualitatively diet (Laessle et al., 1989). An implication of the present results is that intermittent consumption of preferred foods may decrease the acceptance of less preferred, perhaps more nutritious, food options. Indeed, children with more restricted access to palatable foods select fewer less palatable options when given a choice (Fisher and Birch, 1999).
4.1. Undereating of less preferred food
Undereating of less preferred food (e.g., chow hypophagia) after preferred diet access might be a corrective energy homeostatic mechanism to oppose the weight gain/adiposity that results from excess energy intake (Archer et al., 2005; Corwin, 2004). Indeed, rats cycled with preferred food weighed more than controls before being switched to chow diet, were fatter, and showed endocrine signs of obesity. Perhaps positive energy balance or excess adiposity increases the palatability threshold for food to maintain intake. Mechanistic mediators might include leptin, ghrelin or other energy homeostasis hormones that directly modulate reward neurocircuitry (Abizaid et al., 2006; Cota et al., 2006).
On the other hand, several findings suggest a non-nutritional, perhaps hedonic, component to the progressive undereating of less preferred food. Such potential interpretations include “negative contrast” (Cottone et al., 2008; Rogers, 1985), due to recent experience with or the prospect of access to a more rewarding alternative (Flaherty et al., 1995; Grigson et al., 1993); “palatable food withdrawal,” analogous to an aversive state of drug withdrawal (Teegarden and Bale, 2007); and opponent-process shifts in brain reward function (Solomon and Corbit, 1974). First, progressive hypophagia also was seen in the context of relative weight loss, and not only weight gain, in rats cycled with the diet less preferred than standard chow. Second, the standard chow diet could be underconsumed or induce hypophagia of another diet, depending on the potential alternative. Thus, differences in the relative rewarding properties of diets, distinct from intrinsic diet properties (e.g., sugar/fat/energy content, “palatability”), may contribute to the progressive undereating of less preferred food. Third, greater fat mass was inversely, not directly, related to greater degrees of chow hypophagia, in comparisons of rats that were “sensitive” vs. “resistant” to undereating chow after preferred diet access. Fourth, the degree to which individual rats overate the preferred diet was unrelated to the degree to which they underate chow. Fifth, cycled rats that had a history of caloric restriction during chow access, which would increase the relative rewarding or incentive motivating properties of chow, showed blunted chow hypophagia. Importantly, restricted, cycled rats weighed the same and had eaten the same amount of food in the preceding 48 h as their freely fed counterparts at the time that chow intake was compared, suggesting a similar ongoing state of energy balance. Restricted, chow-fed controls did not show the same increment, suggesting that increased chow intake was not due to historical energy scarcity per se. Thus, the progressive undereating of less preferred food in the current study might not fully be explained as homeostatic opposition to positive energy balance.
4.2. Anxiety-like behavior and diet cycling
Increased anxiety-like behavior and sleep-phase locomotor activity in a familiar environment were seen when rats did not have access to the preferred diet. Results are unlikely to be attributable to scheduled food access or recent hypophagia because scheduled food deprivation decreases anxiety-like behavior (Inoue et al., 2004) or to obesity because genetic obesity models do not show increased anxiety-like behavior (Chaouloff, 1994; Levin et al., 2000). Increased anxiety-like behavior and diurnal activity in familiar environments also occur following stressors or cessation of exposure/access to ethanol and opiates (Koob and Le Moal, 2001, 2005; Sabino et al., 2005; Stinus et al., 1998; Taylor et al., 2006). Such negative emotional states are thought to motivate the maintenance and resumption of substance use. Consistent with this model, increased anxiety-like behavior was not seen when cycled rats had access to preferred food. Also supporting this hypothesis, a previous study found that the more anxiety-like behavior a rat showed when withdrawn from 10-min daily access to the current preferred diet, the greater its degree of binge-like intake upon renewed access (Cottone et al., 2008). The results suggest a self-perpetuating model by which preferred foods become compulsively selected or overeaten to negate dysphoria that might otherwise emerge (Laessle et al., 1989; Wells et al., 1998).
Unlike the present results, male mice withdrawn from continuous access to preferred diets in a previous study did not show increased anxiety-like behavior (Teegarden and Bale, 2007). Rather, mice withdrawn from a high-fat diet showed only locomotor activation, and those withdrawn from a preferred high-carbohydrate diet showed no behavioral changes. The different species, sex, diets, or post-withdrawal time points may account for the dissimilar results. Alternatively, perhaps intermittent access has different or greater effects than continuous exposure, a phenomenon similar to that observed with several substances of abuse (O’Dell et al., 2004). Intermittent access might lead to greater allostatic shifts in brain reward function (Koob and Le Moal, 2001), sensitization of brain stress circuitry (Becker, 1998; Shekhar et al., 2005), or associative learning (Weiss et al., 2001). Supporting a role for intermittent exposure, as opposed to continuous cumulative exposure, in adaptations, rats in a previous study showed increased anxiety-like behavior after receiving 16 days of highly restricted (10 min/day; 160 min total) access to the current preferred diet (Cottone et al., 2008). Also, intermittent, but not continuous, access to a 25% glucose solution led to increased naloxone-precipitated anxiety-like behavior in rats (Colantuoni et al., 2002). The present findings also clarify that high preferredness may be sufficient and that high dietary fat/energy content, properties often emphasized in the literature (Teegarden and Bale, 2007), are not necessary to promote consummatory and emotional changes upon cessation of food access (see also (Avena et al., 2007). Here, withdrawal from a highly preferred (92%), high-carbohydrate (69.1% kcal), low-fat (11.8% kcal), and only moderately energy-dense (3.7 kcal/g) diet led to increased anxiety-like behavior and sustained hypophagia of alternatives similar in energy density and macronutrient proportions.
4.3. Diet cycling model, feeding efficiency, and obesity-like changes
Despite eating less, rats with alternating access to the preferred diet ultimately gained 12% more weight than chow-fed controls due to 30% greater and selective gains in body fat. This finding resembles claims that a long-term outcome of human dieting is increased weight gain and adiposity despite less energy intake (Bennett and Gurin, 1982). Female rats receiving daily 10-min access to the present high-sucrose diet also gained excess body fat (Cottone et al., 2008) as did female rats provided limited access to a shortening-based high-fat diet three times per week (Dimitriou et al., 2000). The greater thriftiness of diet-cycled rats with the energy they consume may result from their decreased diurnal motor activity or other energy conservation responses to dietary self-restriction. Differences in macronutrient proportions are unlikely to explain findings, but the sucrose content of the preferred diet may have played a role (Kanarek et al., 1987). Also contributing may be the pattern of overeating high-energy food after self-imposed hypophagia, resembling eating practices of many “relapsing” dieters (Calderon et al., 2004), and may promote lipogenesis via well-known “meal-fed” metabolic adaptations related to the frequency of feeding (Batista et al., 1997; Cohn, 1963). Diet-cycled rats showed 60% greater expansion of gonadal (visceral) than inguinal (subcutaneous) fat pads as well as increased circulating levels of the inflammatory adipokines IL-1β, IL-6, and tPAI-1. Plasma TNF-α and MCP-1 levels were normal, consistent with suspected autocrine/paracrine, not endocrine, actions of adipose-derived forms of these molecules. Thus, the diet cycling model mirrored proinflammatory correlates of human obesity putatively relevant to metabolic syndrome disorders (Greenberg and Obin, 2006; Wajchenberg, 2000).
4.4. Individual differences in chow underconsumption
Stable individual differences were seen in the degree of chow underconsumption after preferred food access, resembling previous findings (Boggiano et al., 2007; Cottone et al., 2008). Diet switch-sensitive rats only ate 50% as much chow as controls and ultimately began eating more preferred diet than their diet-switch resistant counterparts. Differences in preferred diet intake followed, rather than initiated, individual differences in chow hypophagia. One interpretation of the individual differences is that perhaps sensitive rats more effectively compensated chow intake to offset recent hyperphagia/weight gain. This hypothesis is consistent with the reduced adiposity of sensitive rats at study completion and may relate to the different ability of rats genetically selected for vulnerability vs. resistance to diet-induced obesity to reduce intake after diet-induced overeating (Levin et al., 1997). Alternatively, sensitive rats may be more sensitive to successive negative contrasts in food reward, which, like the present results, progress with experience, are reversed by anxiolytics, and show heritable individual differences (Flaherty, 1990; Flaherty et al., 1994).
Perceived cultural norms for thinness or health have led many humans to avoid intake of palatable “forbidden” foods, often unsuccessfully (Polivy and Herman, 1985; Stice et al., 2005). The present results suggest that alternating access to differently preferred diets may increase the role of diet preferredness in determining day-to-day intake of a food. Otherwise adequate food became less accepted, an effect blunted by concurrent caloric chow restriction. The absence of preferred food was associated with anxiogenic-like behavior and sleep-phase motor activation. Despite ingesting less energy, diet-cycled female rats developed morphometric and endocrine signs of obesity. The present model may prove useful for understanding the effects of qualitative dieting in humans. Cultural body shape and health norms, in conflict with food reward contrasts, are hypothesized to complete a negatively reinforced, vicious cycle with allostatic states.
Supplementary Material
Acknowledgments
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute on Drug Abuse (DK64871, P30DK56336, and 1K99DA023680-01A1). We thank Tim Nagy and the NIDDK Program Project-funded University of Alabama-Birmingham Clinical Nutrition Research Unit Small Animal Phenotyping Core for body composition analysis, Dr. Antoine Tabarin and Dr. Donald Coscina for helpful discussions, Mike Arends for editorial assistance, and Molly Brennan and Maegan Mattock for technical assistance. This is manuscript 18320 from The Scripps Research Institute.
Role of funding source
The work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institute on Drug Abuse (NIDA) (DK64871, P30DK56336, and 1K99DA023680-01A1); the NIDDK and NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.psyneuen. 2008.08.010.
Contributor Information
Pietro Cottone, Email: cottone@scripps.edu.
Eric P. Zorrilla, Email: ezorrilla@scripps.edu.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer ZA, Rayner DV, Barrett P, Balik A, Duncan JS, Moar KM, Mercer JG. Hypothalamic energy balance gene responses in the Sprague–Dawley rat to supplementation of high-energy diet with liquid ensure and subsequent transfer to chow. J. Neuroendocrinol. 2005;17:711–719. doi: 10.1111/j.1365-2826.2005.01363.x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2007 doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista MR, Ferraz M, Bazotte RB. Are physiological changes in meal-fed rats determined by the amount of food ingested in the last meal or due to feeding schedule? Physiol. Behav. 1997;62:249–253. doi: 10.1016/s0031-9384(97)00110-8. [DOI] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res. World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res. Bull. 2006;70:422–429. doi: 10.1016/j.brainresbull.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Bennett W, Gurin J. Basic Books. New York: 1982. The Dieter’s Dilemma: Eating Less and Weighing More. [Google Scholar]
- Birch LL, Davidson KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr. Clin. North Am. 2001;48:893–907. doi: 10.1016/s0031-3955(05)70347-3. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int. J. Obes. (Lond.) 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Calderon LL, Yu CK, Jambazian P. Dieting practices in high school students. J. Am. Diet. Assoc. 2004;104:1369–1374. doi: 10.1016/j.jada.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Failure to find behavioural differences between lean and obese Zucker rats exposed to novel environments. Int. J. Obes. Relat. Metab. Disord. 1994;18:780–782. [PubMed] [Google Scholar]
- Cohn C. Feeding frequency and body composition. Ann. N. Y. Acad. Sci. 1963;110:395–409. doi: 10.1111/j.1749-6632.1963.tb17104.x. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes. Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Bingeing rats: a model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Barrera JG, Seeley RJ. Leptin in energy balance and reward: two faces of the same coin? Neuron. 2006;51:678–680. doi: 10.1016/j.neuron.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:525–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int. J. Eat. Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol. Biochem. Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety current protocols in neuroscience. Hoboken, NJ: John Wiley & Sons; 2004. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. AJCN. 1999;69:1264–1272. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Effect of anxiolytics and antidepressants on extinction and negative contrast. Pharmacol. Ther. 1990;46:309–320. doi: 10.1016/0163-7258(90)90097-l. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J. Exp. Psychol. Anim. Behav. Process. 1995;21:229–247. doi: 10.1037//0097-7403.21.3.229. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Krauss KL, Rowan GA, Grigson PS. Selective breeding for negative contrast in consummatory behavior. J. Exp. Psychol. Anim. Behav. Process. 1994;20:3–19. [PubMed] [Google Scholar]
- Freet CS, Tesche JD, Tompers DM, Riegel KE, Grigson PS. Lewis rats are more sensitive than Fischer rats to successive negative contrast, but less sensitive to the anxiolytic and appetite-stimulating effects of chlordiazepoxide. Pharmacol. Biochem. Behav. 2006;85:378–384. doi: 10.1016/j.pbb.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George VA, Johnson P. Weight loss behaviors and smoking in college students of diverse ethnicity. Am. J. Health Behav. 2001;25:115–124. doi: 10.5993/ajhb.25.2.4. [DOI] [PubMed] [Google Scholar]
- Gonzalez VM, Vitousek KM. Feared food in dieting and non-dieting young women: a preliminary validation of the food phobia survey. Appetite. 2004;43:155–173. doi: 10.1016/j.appet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Spector AC, Norgren R. Microstructural analysis of successive negative contrast in free-feeding and deprived rats. Physiol. Behav. 1993;54:909–916. doi: 10.1016/0031-9384(93)90301-u. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int. J. Eat. Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol. Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Harris RB, Martin RJ. Recovery of body weight from below “set point” in mature female rats. J. Nutr. 1984;114:1143–1150. doi: 10.1093/jn/114.6.1143. [DOI] [PubMed] [Google Scholar]
- Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, Koob GF. Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol. Psychiatry. 2004;55:1075–1081. doi: 10.1016/j.biopsych.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Aprille JR, Hirsch E, Gualtiere L, Brown CA. Sucrose-induced obesity: effect of diet on obesity and brown adipose tissue. Am. J. Physiol. 1987;253:R158–R166. doi: 10.1152/ajpregu.1987.253.1.R158. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Laboure H, Saux S, Nicolaidis S. Effects of food texture change on metabolic parameters: short- and long-term feeding patterns and body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R780–R789. doi: 10.1152/ajpregu.2001.280.3.R780. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite. 1989;12:83–94. doi: 10.1016/0195-6663(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R46–R54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague–Dawley rats. Am. J. Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin BE, Richard D, Michel C, Servatius R. Differential stress responsivity in diet-induced obese and resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1357–R1364. doi: 10.1152/ajpregu.2000.279.4.R1357. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46:16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Haines J, Story M, Eisenberg ME. Why does dieting predict weight gain in adolescents? Findings from project EAT-II: a 5-year longitudinal study. J. Am. Diet Assoc. 2007;107:448–455. doi: 10.1016/j.jada.2006.12.013. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin. Exp. Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Dieting and binging. a causal analysis. Am. Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Reas DL, Grilo CM. Timing and sequence of the onset of overweight, dieting, and binge eating in overweight patients with binge eating disorder. Int. J. Eat. Disord. 2007;40:165–170. doi: 10.1002/eat.20353. [DOI] [PubMed] [Google Scholar]
- Rogers PJ. Returning ‘cafeteria-fed’ rats to a chow diet: negative contrast and effects of obesity on feeding behaviour. Physiol. Behav. 1985;35:493–499. doi: 10.1016/0031-9384(85)90129-5. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Zorrilla EP. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2005; 2005. Repeated exposure to social defeat threat produces persistent post traumatic stress-like behavior in male rats Program No. 307.20. Online. [Google Scholar]
- Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol. Biochem. Behav. 1998;60:727–731. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–115. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol. Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. J. Consult. Clin. Psychol. 2005;73:195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychol. 2002;21:131–138. [PubMed] [Google Scholar]
- Stinus L, Robert C, Karasinski P, Limoge A. Continuous quantitative monitoring of spontaneous opiate withdrawal: loco-motor activity and sleep disorders. Pharmacol. Biochem. Behav. 1998;59:83–89. doi: 10.1016/s0091-3057(97)00319-5. [DOI] [PubMed] [Google Scholar]
- Stirling LJ, Yeomans MR. Effect of exposure to a forbidden food on eating in restrained and unrestrained women. Int. J. Eat. Disord. 2004;35:59–68. doi: 10.1002/eat.10232. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Bando JK, Romeo HE, Prolo P. Differential effects of alcohol consumption and withdrawal on circadian temperature and activity rhythms in Sprague–Dawley, Lewis, and Fischer male and female rats. Alcohol Clin. Exp. Res. 2006;30:438–447. doi: 10.1111/j.1530-0277.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Waters A, Hill A, Waller G. Internal and external antecedents of binge eating episodes in a group of women with bulimia nervosa. Int. J. Eat. Disord. 2001;29:17–22. doi: 10.1002/1098-108x(200101)29:1<17::aid-eat3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wells AS, Read N, Laugharne JD, Ahluwalia NS. Alterations inmood after changing to a lowfat diet. Br. J.Nutr. 1998;79:23–30. doi: 10.1079/bjn19980005. [DOI] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.